Natural Colloids in Soil: Effect of Storage and Extraction Conditions on Colloid Amount and Composition
Academic editor: Balwant Singh
Funding: This study received funding from German Research Foundation.
ABSTRACT
Background
Natural soil colloids (1–1000 nm) play a crucial role in the mobility and cycling of nutrients. In many studies, colloids are analyzed mainly as so-called water-dispersible colloids (WDCs), with varying storage and extraction steps. However, it is still unknown how these different treatments affect the amount and composition of colloids.
Aim
We hypothesize that (1) in particular the storage conditions of the soil, such as freezing + thawing or lyophilization, influence the amount and properties of WDCs and that (2) these are altered depending on the shaking time used for extraction.
Methods
Topsoil samples (0–20 cm) from two different soils (dystric Leptosol and stagnic Luvisol) were extracted after storing in three different ways, including (1) direct extraction from fresh soil, (2) freezing + thawing, and (3) lyophilization. After extraction with different shaking times (1, 2, 6, 12, and 24 h), WDCs (1.2– 450 nm) were analyzed via flow field-flow fractionation coupled to an inductively coupled plasma mass spectrometer and an organic carbon detector.
Results
The results indicate that both freezing + thawing and lyophilization significantly altered the properties (size distribution and chemical composition) of WDCs and decreased the portion of WDC bound elements. This reduction was the greatest for phosphorus (P) (27%–38% and 71%–80%, respectively) and more pronounced for lyophilization. Varying shaking times also affected the prevalence of colloids, but this effect was less pronounced than that of storage conditions, as revealed by cluster analysis.
Conclusion
This study particularly highlighted that storage as well as extraction conditions affect significantly the extractability of WDCs. This effect can even override differences in the colloidal loading between different reference soil groups. Consistent sample handling is therefore a prerequisite for obtaining comparable results.
1 INTRODUCTION
Natural soil colloids (particles ranging between 1 and 1000 nm; Lead and Wilkinson 2006) are part of the particulate phase of soils, and play a decisive role in the mobility and cycling of nutrients (e.g., Bol et al. 2016; Gottselig et al. 2017; Gu et al. 2020; Montalvo et al. 2015). Accordingly, colloids are analyzed in many studies, using different storage and extraction conditions. However, the effect of these differences in sample handling on the amount and composition of colloids is not fully understood.
The chemical, mineralogical, and organic composition of colloids is highly dependent on soil properties and environmental conditions. The mineral part of colloids often includes alumino-silicates, iron and aluminum (hydr)oxides and carbonates, while the organic part consists mainly of humic substances, microbial exudates and decomposed plant material (Siebers et al. 2024; Totsche et al. 2018). Colloids exist in two primary forms: free colloids, which are water-dispersible and mobile, and occluded colloids, which are encapsulated within soil aggregates. Free colloids are often rich in less decomposed organic matter, such as lipids and fatty acids, contributing to nutrient transport and loss. In contrast, occluded colloids are stabilized within aggregates and consist of more decomposed organic matter, such as carbohydrates and amides, and are less susceptible to degradation (Krause et al. 2020; Tang et al. 2022). These forms play distinct roles in nutrient cycling and soil aggregation (Siebers et al. 2018; Totsche et al. 2018).
Up to 85% of nutrients as well as pollutants such as pharmaceuticals in soils and surface waters occur primarily in the <450 nm size fraction (e.g., Rick and Arai 2011; Siemens et al. 2004; Yan et al. 2015). Elements in samples filtered to <450 nm are considered dissolved (Greenberg 1985). However, the definition of the dissolved phase overlaps with those of nanoparticles (NPs; 1–100 nm) and fine colloids (1–450 nm) (Jarvie et al. 2012). Several studies showed that around 50% of P in the dissolved phase are bound to such colloids, like in forest soils (Missong et al. 2018) or in stream waters (Baken et al. 2016; Gottselig et al. 2017, Siebers et al. 2023), which is also expected for other nutrients. Furthermore, colloids are important for the transport of environmental pollutants, for example, up to 84% of the total amount of pharmaceutical residues are bound to such small particles (Cheng et al. 2017; Duan et al. 2013; Maskaoui and Zhou 2010). Accordingly, colloids and colloidal-bound elements gained more and more attention in recent years when analyzing the dissolved phase.
Numerous studies have analyzed colloids, with different sample handling procedures. The pretreatment of soil samples can significantly impact the colloidal content and composition compared to fresh soil samples. For example, freezing and thawing cycles can break soil aggregates due to the expansion of water upon freezing, leading to an increased release of colloids. This mechanical stress induces the redistribution of elements within different size fractions, potentially altering the elemental composition of the colloids, while lyophilization may cause the deformation of particles and affect their chemical properties (Siebers et al. 2018). Nguyen et al. (2020) showed that for dam reservoir sediment, storage conditions led to uncertainties in colloidal determination by a factor of 40, with the highest deviations from colloidal abundance found in fresh sediment observed for lyophilization. Additionally, lyophilization increased the release of certain elements like P and Ca in the colloidal fractions by factors of 3 and 2.6, respectively (Nguyen et al. 2020, and references therein). The observed effects of storage conditions on colloidal content and composition may vary for different soils and elements. Thus, while these pretreatment methods are useful for preserving certain soil properties, they also introduce changes that must be considered when analyzing soil colloids. Based on the findings of past studies, the authors recommend using fresh sediment or soil for colloid analyzes, which, however, may not be possible when dealing with large sets of samples. Accordingly, storage conditions such as freezing and thawing must be tested as a potential method to store soil samples before colloid analyses.
Besides storage conditions, extraction techniques may also alter the extractability of colloids. In soil science, colloids are in most studies extracted from bulk soil or sediment by shaking the soil/sediment with deionized water, gaining the fraction of so-called water-dispersible colloids (WDCs). However, the time of extraction, that is, the shaking time used in different studies to extract WDCs, varies widely, from 1 h (Mills et al. 2017), 2 h (Buettner et al. 2014; Kretzschmar et al. 1995; Luo et al. 2017), 6 h (Missong et al. 2016; Séquaris et al. 2013), 12 h (Klitzke et al. 2008), 16 h (Zirkler et al. 2012), 17 h (Sinaj et al. 1998), 24 h (Gu et al. 2018; Liang et al. 2010; Liu et al. 2014; Yan et al. 2017), to even 48 h (VandeVoort et al. 2013). Bergendahl and Grasso (1998) already showed that with increasing agitation time, the total colloidal surface area also increased. Especially for long shaking times of ≥24 h microbial-induced processes, such as degradation of organic carbon (Corg) and resulting fragmentation into smaller WDCs or even total loss of such colloids may occur when not working under sterile conditions. How differences in agitation time affect the amount and composition of extracted colloids is not understood though, but needs to be elucidated to evaluate the comparability of different studies.
Consequently, this study was aimed to establish a recommendation for the extraction of WDCs from soil, that is, finding the most suited approach for analyzing the natural prevalence of WDCs in soils without causing artifacts. In detail, we wanted to determine how storage conditions (freezing + thawing and lyophilization compared to fresh soil) and different extraction conditions, that is, shaking times, alter the amount and composition of WDCs extracted from soil and if these alterations are soil and/or element-specific. To do so, topsoils of two different soil reference groups were sampled (classified as Luvisol and Leptosol according to World Reference Base, WRB). The samples underwent different storage conditions (fresh soil vs. frozen + thawed soil vs. freeze-dried soil), and then WDCs were extracted using varying shaking times. After extraction, the amount and properties of WDCs were analyzed via flow field-flow fractionation (AF4) coupled to inductively coupled plasma mass spectrometry (ICP-MS) and an organic carbon detector (OCD).
2 MATERIALS AND METHODS
2.1 Soil Samples
Topsoil (0–20 cm) was sampled from two different reference soil groups, classified according to the WRB as dystric Leptosol and stagnic Luvisol. The sampling area is located in the Lower Rhine Embayment near Selhausen, Germany (50°52′ 09.34″ N; 6°27′00.58″ E) and has been cultivated for over 100 years. Due to their pedogenesis, soil texture is highly variable ranging from slightly gravelly silt loam to very gravelly loam (Soil Survey Staff 1999) (detailed information in Bornemann et al. 2011). The texture of both soils is classified as silt loam; however, the Luvisol had higher contents of clay and silt (17%–18% clay and >70% silt) than the Leptosol (12%–13% clay and 55%–64% silt) (Table S1). The soil organic carbon (SOC) contents were higher in the Leptosol (SOC, 14.1–16.9 g SOC kg−1) than in the Luvisol (10.1–11 g SOC kg−1). After sampling, fresh soil was sieved <2 mm.
2.2 Storage of Soil Samples and Extraction of Water-Dispersible Colloids
Samples of both soil groups were stored in three different ways, including (1) no storage (fresh soil was extracted directly the day after sampling), (2) freezing and thawing (soil was stored in a freezer at –20°C for 3 days and afterward thawed in a cooling room), and (3) lyophilization (soil was stored in a freezer at –20°C for 3 days and then dried via lyophilization). Each treatment was repeated three times. For each repetition of each treatment, 20 g of fresh soil was weighted in glass vessels, and then separately handled as described above.
For all treatments, WDCs were extracted after a modified method of Missong et al. (2018). In detail, 20 g of soil was mixed with 160 mL of distilled water (soil:water ratio 1:8) and shaken end-over-end for 6 h. To detect a potential effect of the shaking time on colloid extraction, varying shaking times of 1, 2, 6, 12, or 24 h were tested for the fresh soil (each in threefold repetition). To isolate the dissolved fraction, including WDCs, after a sedimentation time of 10 min, the supernatant of the samples was centrifuged (5 min, 4000 g; Heraeus Multifuge 4KR, Thermo Fisher Scientific) and afterward analyzed via AF4 coupled to ICP-MS and OCD.
2.3 AF4 Analyses
The extracted colloids were size-separated using AF4 (Postnova Analytics, Landsberg, Germany). The Corg concentration in the different colloidal size fractions was measured using an OCD (DOC-Labor GmbH, Germany) coupled to AF4. The concentrations of Al, Ca, Fe, Mg, Mn, P, and Si in each size fraction were determined by AF4 coupled to an ICP-MS (Agilent 7500, Agilent Technologies, Japan) using post-channel calibration with rhodium (Rh) as the internal standard (Nischwitz et al. 2016). Details of the AF4 separation method are presented in Table 1.
| Soil samples | |
|---|---|
| Membrane | PES (1 kDa) |
| Spacer | 500 µm |
| Carrier solution | 25 µM NaCl |
| Detector flow | 0.5 mL min−1 |
| Focus step | |
| Injection volume | 2.5 mL |
| Injection flow | 0.2 mL min−1 |
| Injection time | 20 min |
| Cross flow | 3.0 mL min−1 |
| Focus flow | 3.3 mL min−1 |
| Transition time | 1 min |
| Elution step | |
| Constant | 15 min |
| mode 1 | 3.0 mL min−1 |
| Power mode 1 | 20 min |
| (exponent: 0.2) | 2.0 down to 0.15 mL min−1 |
| Power mode 2 | 10 min |
| (exponent: 0.8) | 0.15 down to 0.0 mL min−1 |
| Constant | 60 min |
| mode 2 | 0.0 mL min−1 |
The lowest size limit of 0.66 nm was established by the cutoff of the used membrane (1 kDa). Reference materials (Sulfate Latex Standards, 8% w/v, 21–630 nm; Postnova Analytics, Landsberg, Germany) were used with the same AF4 conditions as the samples to calibrate the particle diameters included in each size fraction. The specified hydrodynamic diameters of the particles are equivalent sizes based on the elution time of the reference materials. The first size fraction was estimated to be between 0.66 (membrane molecular weight cutoff) and 20 nm, the second fraction includes nanoparticles >20–30 nm, the third size fraction ranges from >30 to 60 nm, the fourth size fraction from >60 to 220 nm, and the fifth size fraction from >220 to 450 nm which was the size cutoff set for centrifugation. Because the AF4 technique does not recover dissolved species, the total element recovery of aqueous phases was not assessed in the same run. However, by measuring without AF4 fractionation (zero crossflow), it is possible to determine total concentrations in the soil extract using the AF4 system. By subtracting particulate concentrations (with cross-flow) from total concentration (without cross-flow), the truly dissolved concentrations were obtained. In the centrifuged and unfractionated samples, the total elemental concentrations of the dissolved fraction Al, Ca, Fe, Mg, Mn, P, and Si were determined using ICP-MS (Agilent 7500, Agilent Technologies) and OCD (DOC-Labor GmbH, Germany) for C, as described above.
2.4 Statistics
Testing for significant differences was conducted using a one-way analysis of variance (ANOVA), after passing the test regarding normal distribution (Shapiro–Wilk) and equal variance (Brown–Forsythe). If data were not normally distributed, a one-way ANOVA on ranks was applied with a following Turkey test if significant differences were detected. All statistical analyses were conducted using SigmaPlot 13.0 (Systat Software GmbH). Hierarchical tree cluster analysis as described in Gottselig et al. (2017) was performed using IBM SPSS Statistics 29 (IBM, Armonk, USA). We have used 1–Pearson´s r as the distance measure (calculated as 1 minus the absolute value of the Pearson correlation coefficient), with the complete linkage rule and without normalization for the element concentration in the WDC fraction and the single size fractions.
3 RESULTS
3.1 Effect of the Storage Conditions on the Elemental Composition of WDCs
To analyze the effect of different storage conditions on the amount and composition of natural soil colloids, we compared field-fresh soil without pre-treatment to soil that underwent either freezing + thawing or lyophilization (each extracted with an agitation duration of 6 h). In the Leptosol, the elemental content of WDCs for fresh soil was the highest for Corg (21,256 ± 1,278 µg kg−1 and the lowest for Mn (278 ± 30 µg kg−1; Table S2). For most elements, there were no differences in the elemental content of WDCs between the two soils; however, the Luvisol contained 1.5 times more C than the Leptosol, while the Leptosol contained 2.0 times more Mn than the Luvisol.
For both soils and all elements, the elemental content of WDCs was significantly higher in the fresh soil compared to the variants freezing + thawing and lyophilization, except for Corg in both soils and Ca in the Leptosol (Figure 1; Table S2). For all elements and soils, significantly lower elemental contents of WDCs were detected in freeze-dried soils compared to soils that underwent freezing + thawing (Figure 1; Table S2).
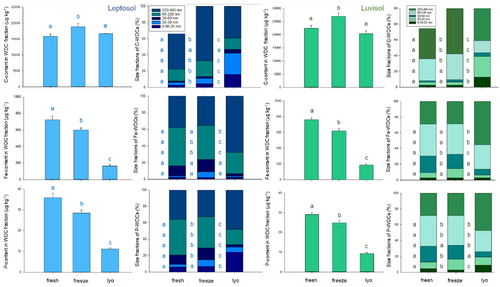
For both soils and all analyzed elements (except Ca in Luvisol for the pre-treatment freezing + thawing), the portion of the WDC fraction of the dissolved phase (<450 nm) decreased with both pre-treatments compared to fresh soil, whereas the portion of the truly dissolved fraction increased to the same extent. The reduction of colloidal-bound elements caused by both pre-treatments was more pronounced in the Leptosol than in the Luvisol (except for Corg, Mn, and Fe; Table S3). In general, the portion of the WDC fraction within the dissolved phase decreased significantly when freezing + thawing was applied (except for Fe in the Leptosol and Ca in the Luvisol). In both soils, P was most strongly reduced by freezing + thawing compared to fresh (reduction of 37.7 ± 2.0% and 27.3% ± 2% for Leptosol and Luvisol, respectively). In contrast, Ca showed the second strongest reduction in the Leptosol (35.4 ± 2.0% reduction), (Figure 1; Table S2), while in the Luvisol, colloidal-bound Ca was not reduced at all (–0.1 ± 0.3%, Figure 1; Table S3).
When comparing freeze-dried soil to fresh soil, a significant decrease in the portion of the WDC fraction within the dissolved phase was observed for all elements, except for Ca in the Luvisol. Here for both, Leptosol and Luvisol, P showed the highest reduction (80.4 ± 1.2% and 71.3 ± 2.3%, respectively), while Mn was least affected (23.5 ± 1.2% and 14.4 ± 2.0%, respectively). Notably, for both soils and all tested elements (exception for Corg and for Mn in Luvisol), the reduction of the WDC fraction within the dissolved phase was substantially smaller for freezing + thawing compared to lyophilization, being significant for Mg, P, and Si in both soils (Figure 1; Table S3).
Besides the elemental content of WDCs, the association of elements to specific WDC size fractions was also affected by the pre-treatment. In general, freezing + thawing had fewer effects on the association of elements to the size distribution of WDCs than lyophilization. Comparing all five WDC size fractions for all elements, for the treatment freezing + thawing 41 ± 23% were significantly different compared to fresh soil, while for lyophilization, 79 ± 2% of size fractions showed significant differences (Table S4). In contrast to fresh soil, elements in freeze-dried samples were either significantly more associated with small WDCs and less to medium or large WDCs (Corg and Ca; Figure 1; Table S4), associated fewer to medium but mainly to large WDCs (Al, Fe, Mg, Mn, Si, and P) or in the case of P also to small WDCs (Figure 1; Table S4).
Considering the percentage of single elements to the total element content of WDCs, in fresh Leptosol samples, Corg had the highest portion (35.4%), and Mn the lowest (0.45%). For the frozen soil, the portion of Corg and Ca increased to 44.7% and 1.95%, respectively, whereas the portion of the other elements decreased. The increase observed for Corg and Ca was even more pronounced when lyophilization was applied (71.7% and 2.45% of total elemental content of WDCs), while other elements contributed less to the elemental load of WDCs. The portion of elements to the total element content of WDCs for the Luvisol displays a comparable pattern (Figure S1, Table S2).
3.2 Effect of Agitation Time on the Prevalence of WDCs
For all elements and both soils, the total elemental content of WDCs increased with increasing agitation time (Figure 2; Table S2). For the Leptosol, the agitation time affected mainly colloidal Corg and Ca, here the amount increased from 1 to 24 h by a factor of 1.9 and 1.6, respectively, while the other elements showed only a slight increase. The Luvisol showed a stronger response. Here, colloidal-bound Corg was most strongly affected and increased from 1 to 24 h by a factor 2.0, followed by Al and P (factor 1.9; Table S2).
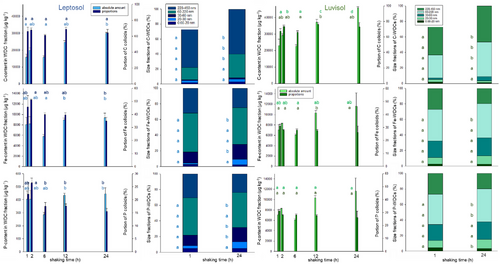
In contrast to total elemental amounts of WDCs, in the Leptosol for all elements except Corg, the portion of WDCs in the dissolved phase decreased with increasing agitation time (Table S3). However, significant differences were only detected for Al and Mn; for all other elements only, the agitation time of 2 h showed significantly more elements in the WDCs than after 24 h of shaking. For the Luvisol, the effect of agitation time on the portion of WDCs was less pronounced (only visible for Fe and Mn), and no significant differences were detected at all (except Corg, Table S3). For both soils and all elements (except Ca), we observed a decrease in large WDCs with increasing shaking time (Figure 2).
The size distribution of WDCs was substantially affected by the agitation time. Most common significant differences were observed between 1 and 12 h and 1 and 24 h of agitation. For all elements and size fractions, for the Leptosol, 15% of size fractions differed significantly between 1 and 12 h of agitation, while between 1 and 24 h, such significant differences were even more frequent (58%; Table S4). For the Luvisol, the same trend was apparent. Here, for both, 1 versus 12 h and 1 versus 24 h of agitation, for 38% of size fractions significant differences were observed (Figure 2; Table S4).
The contribution of single elements to the total elemental load of WDCs in the Leptosol after 1 h shaking time was the highest for Si with 34.0% and Corg with 28.4%. After 24 h of shaking, the portion of Corg increased to 40.3%, while the portion of Si decreased (27.5%). In the Luvisol with 1 h shaking time, Corg represents the largest portion (41.0 %), followed by Si (27.7%). After a shaking time of 24 h, the portions of the elements in the WDC fraction are comparable to the Leptosol (Figure S2).
3.3 Impact of Storage Condition and Agitation Time on WDCs
Hierarchical tree cluster analysis indicated the formation of three clusters for the Luvisol and four clusters for the Leptosol (Figure 3). For the Luvisol, both pre-treatments formed a separate cluster. In the Leptosol, this was only the case for lyophilization. Here, the pre-treatment freezing + thawing formed a separate cluster with a shaking duration of 24 h. For both soils, there is a clear grouping of the shaking durations, and hence of the fresh soil samples. While for the Luvisol all shaking durations (1, 2, 6, 12, and 24 h) cluster together, for the Leptosol, only medium-term shaking durations from 2 to 12 h built one cluster (Figure 3).
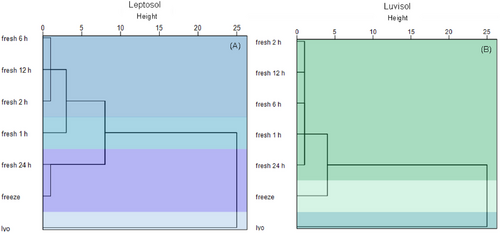
Conducting a combined hierarchical tree cluster analysis of WDC fractions for both soils, five clusters could be detected (Figure S3). Lyophilization pre-treatment formed a separate cluster for both soils as well as freezing + thawing for the Luvisol. Furthermore, a cluster for the medium shaking durations (2, 6, and 12 h) was found for the Leptosol. The shaking time of 1 h for the Leptosol formed a separate cluster here. Within the Luvisol, the fresh soil, that is, all shaking durations, clustered together; however, the pre-treatment freezing + thawing for the Leptosol was included here (Figure S3).
4 DISCUSSION
4.1 Composition of Colloids
The composition and amount of colloids differ between different soils, like organic and mineral soils (Huang et al. 2016; Missong et al. 2018; Séquaris et al. 2013;) but also within one soil profile between different soil horizons (Missong et al. 2018). In detail, Missong et al. (2018) found that WDCs of organic horizons were dominated by Corg and Al, whereas WDCs of the mineral horizons mainly consisted of Fe, Mn and Si. The formation but also the potential mobilization and stability of natural colloids in soil are related to soil's physical (e.g., moisture and temperature) and chemical properties (e.g., pH and ionic strength) (Jiang et al. 2013; Klitzke et al. 2008; Tsao et al. 2011). The composition of the Luvisol colloids observed in this study is largely comparable to the findings of Tang et al. (2022) and Siebers et al. (2024), who analyzed soils from the same site. The dominance of aluminum (hydr)oxides and the presence of silicates, as reflected in the Si/Al ratios (0.07–0.11, data not shown), align closely with their findings. Tang et al. (2022) identified fine colloids (<220 nm) enriched in Al, Si and Fe, while Siebers et al. (2023) reported colloidal fractions primarily composed of Al- and Fe(hydr)oxides, alongside alumino-silicate minerals and organic matter. Interestingly, the elemental composition of WDCs did not mirror that of the bulk soil, as, for example, the Leptosol contained more SOC than the Luvisol, while for WDCs it was the other way around.
4.2 Effect of Pre-Treatments on WDCs
Comparability between studies analyzing the prevalence of natural soil colloids may be compromised if samples are stored at different conditions before WDC extraction and analysis. Before the extraction of colloids, samples often undergo some pre-treatment, like freezing and thawing or lyophilization, especially when large numbers of samples are processed or samples need to be stored. However, the results of this study show that both, freezing + thawing and lyophilization, significantly alter the amount as well as the composition of WDCs. Lyophilization had the strongest effect and led to a decline in elements associated with WDCs of 14–80%. This concords with the results reported by Nguyen et al. (2020); here, lyophilization had a stronger effect than air drying in comparison to extraction from wet sediment and led to a reduction of colloidal mass of 82%. With freezing + thawing reduction in the concentrations of all elements being substantially lower than for lyophilization, we recommend using freezing + thawing as a storage option.
Drying, including lyophilization, in general, affects the mobilization of soil colloids, and this effect is time-dependent. In the initial phase of re-wetting, an increased mobility of WDCs, including dissolved organic matter, is observed, followed by a decrease in the mobilization (Kjaergaard et al. 2004; Münch et al. 2002). This decrease might be attributed to an increase in the hydrophobicity of WDCs, caused by an increase in water repellency on solid surfaces during drying (Dekker et al. 2001). Indeed, Klitzke and Lang (2007) found an increase in the hydrophobicity of the solid phase after drying and re-wetting, but not for the WDCs, where the physicochemical properties were not changed by drying. The observed decrease of colloids after lyophilization might also be caused by aggregation, especially for the elements Fe, Mn, Mg, Si and P, which were associated with WDCs of a larger size fraction after lyophilization compared to fresh soil. Similar observations were made by Ngyuen et al. (2020). Here, lyophilization led to an increase in contents of P, Corg and Ca associated with large colloids. Higher aggregate stability in the course of lyophilization has been reported by several studies (e.g., Dagesse 2011) and could lead to decreased colloidal mobility as the cohesion of previously weakened particles by drying increases again. If the water film is getting thinner than the colloidal size, trapped colloids will be immobilized by the attachment to the surface water interface. Furthermore, the increasing concentration and resulting precipitation of clay, salts and minerals in the remaining soil solution caused by evapotranspiration will strengthen inter-particle bridges (Majdalani et al. 2008).
In contrast, the increase in Corg and Ca associated with small WDCs but decreased association with large WDCs after freezing + thawing and lyophilization points to the destruction of large colloids. Interestingly, Nguyen et al. (2020) also found more Ca associated with small colloids, while Corg showed no clear trend. The destruction of large colloids might be caused by the growth of ice crystals, resulting in crushing of larger structures, similar to the findings for soil aggregates (e.g., Bullock et al. 1999; Lehrsch et al. 1991).
Also, an increase in shaking time probably led to the destruction of soil aggregates and simultaneously also of large colloids, as the amount of extracted WDCs increased with increasing shaking time while simultaneously large colloids were reduced (Figure 2). Although it is known that varying shaking times have been applied to extract WDCs (Nguyen et al. 2020), to the best of our knowledge, this has never been tested before, neither for colloids nor for aggregates. As the results of this study highlight that the shaking time affects the amount and composition of WDCs, it should be considered an important factor when comparing different studies.
Observed significant shifts in the size distribution of C-WDCs, that is, the fragmentation of large colloids (220–460 nm) into medium-sized colloids (60–220 nm and 30–60 nm) with increasing shaking time, could be caused by mechanical stress during shaking but could also be the result of microbial degradation processes. Both processes do not apply only to C-WDCs but may also explain shifts in the size distribution of other elements (like observed for all elements except for Ca) if C structures in organo-mineral complexes are degraded. For microbial degradation of Corg, a decrease in total C amounts would be expected. Even if the C will be taken up by microbes and contribute to their biomass, a part of the C will be respired. Such decrease was, however, not observed as the increased mobilization of C-WDCs during prolonged shaking presumably overlaid a potential C loss (Figure 2). Hence, the underlying mechanism of the observed fragmentation cannot be clarified based on the recent results but should be elucidated in future studies. Nevertheless, to avoid a potential falsification by microbial processes, we recommend shaking durations shorter than 12 h.
In addition to the shaking time, other parameters such as shaking speed, type of shaking, soil/water ratio, ionic strength, and the type of extraction of WDCs (sedimentation, centrifugation, filtration) may also be important factors influencing colloid content and composition. This makes it particularly important to analyze these parameters in future research.
The effects of sample handling were element-specific, and for some elements also soil-specific. The found shift in the contribution of specific elements to the total WDC load during pre-treatments can hinder the evaluation of elemental stoichiometric relationships (like C, N, P, S) if these elements respond differently to the chosen pre-treatment. For example, in the Leptosol, the P content of colloids was reduced by 38% when freezing + thawing was applied, while Corg was only reduced by 14%. Similarly, for lyophilization, the reduction was elemental-specific, with up to 80% and 43% for P and Corg, respectively. Further testing is required if other elements like N or S are affected. Soil elemental stoichiometry is a major driver of microbial processes in soil, such as organic matter decomposition and nutrient cycling (e.g., Zechmeister-Boltenstern et al. 2015). If the stoichiometry, e.g., the C:N:P ratio, is altered by sample handling and extraction, the evaluation of such ratios as indicators of ecosystem function will be falsified.
In addition, the soil-specific response of WDCs to sample handling can hinder the comparison of WDC loads from different soil reference groups. For most elements, the reduction in element content associated with WDCs due to the pre-treatments was more pronounced for the Leptosol, while the increase of elements associated with WDCs with increasing shaking time was stronger for the Luvisol. This may in part be explained by the different soil properties, as the Leptosol had a higher Corg content, and especially aggregates in soils with higher Corg contents were shown to be more stable after freezing. The same was reported for soils with more than 17% clay (Lehrsch et al. 1991). Here, the clay content of the Luvisol with 17%–18% together with lower Corg content was presumably not high enough to form more stable aggregates.
Summarizing, hierarchical tree cluster analysis (Figure 3) revealed that the pre-treatment of samples, especially lyophilization, had the strongest effect on the amount of extracted WDCs, i.e., reduced these, similar to short shaking durations (Figure 3). The combined tree cluster analysis (Figure S3) revealed that especially lyophilization affects the prevalence of WDCs, and this effect was even stronger than that of the soil reference group. In addition, it can be concluded that both particularly short and long shaking times (1 or 24 h) influence the WDC fraction. These effects can be enhanced if other extraction conditions like sonification, filtration, or centrifugation techniques differ (Nguyen et al. 2020, 2021). Our results, hence, confirm that a direct comparison of the WDC amount and properties from different studies are hampered when different sample handling is applied. Consequently, using fresh soil should be the preferred option. If storage is needed, we strongly suggest avoiding lyophilization but using freezing + thawing or air drying instead. Furthermore, a shaking duration of 2–12 hours is recommended.
5 CONCLUSION
In the present study, we analyzed the effect of different pre-treatments, that is, sample storage and the shaking time during extraction on the amount and composition of extracted WDCs. Both tested treatments (freezing + thawing and lyophilization), significantly reduced the prevalence of WDCs and altered their composition. This effect was significantly stronger for lyophilization than for freezing + thawing. At the same time, observed effects were element and soil-specific. Further, an increase in the shaking time led to an increase in the amount of extracted colloids with a simultaneous reduction of large WDCs.
Based on the results, we highly recommend avoiding lyophilization as pretreatment, as well as short or long shaking times (<2 to >12 h), and considering different pre-treatments as well as shaking times when comparing results from different studies.
Acknowledgments
This study was founded by German Research Foundation as part of the research unit 5095, conducted by subproject 1 (AM 134/28–1) and the project Go 2899/1–1. We also thank two anonymous reviewers and the editor for constructive comments.
Open access funding enabled and organized by Projekt DEAL.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




