Boron deficiency responses in maize (Zea mays L.) roots
This article has been edited by Monika Wimmer.
Abstract
Background
Boron (B) is an essential micronutrient for plants. Dicot plants respond to insufficient B supply by altering root architecture and root hair growth. How root systems of rather low-B demanding monocot species such as maize (Zea mays L.) respond to B deficiency in terra has not been experimentally resolved, yet.
Aims
The study aims to investigate root responses and their physiological consequences under B deficiency during the vegetative growth of maize.
Methods
B73 wild-type (WT) maize and its root hairless rth3 mutant were grown under varying B supply conditions in soil columns and in an automated root phenotyping facility. Biomass data, root system architecture traits, the mineral elemental composition and molecular B-deficiency responses were quantified.
Results
Though having very low leaf B concentrations, no major growth deficit, apart from chlorotic stripes on leaves, was recorded on maize root and shoot development, with or without root hairs, on B-deficient conditions. Although leaf B concentration of the rth3 mutant is significantly lower under B-deficient and under B-surplus conditions compared to the WT, the rth3 mutant neither developed a larger total root length, more fine roots nor displayed a higher expression of B uptake transporters as compensatory adaptations.
Conclusions
Strikingly, maize plants did neither react with an inhibited root growth nor by a compensatory root foraging behaviour to severe B-deficient in terra growth conditions. This is rather atypical for plants. The performance and altered leaf B concentrations of rth3 mutants may be biased by secondary effects, such as an overall reduced root growth.
1 INTRODUCTION
Boron (B) is an essential micronutrient for both, monocotyledonous and dicotyledonous plants (Warington, 1923). The essential biological function of B is the bonding of two rhamnogalacturonan-II monomers by borate esters in the pectin fraction of primary cell walls (O'Neill et al., 2001; Wimmer et al., 2020). This crosslinking of apoplastic building blocks is physiologically crucial to sustain growth and development by simultaneously assuring the stability and elasticity of plant cell walls and to sustain meristem activity (Matthes et al., 2020). Though B is only required in trace amounts, B deficiency has tremendous effects on plant performance and has been reported to be one of the most widespread deficiencies worldwide (Shorrocks, 1997). Dicotyledonous plants respond to B limitation by growth retardation first effecting primary root growth but also shoot and most importantly holistic meristem performance. On the root level, the reduction in primary and lateral root elongation is accompanied by an increased formation and elongation of root hairs (Bienert et al., 2021; Gruber et al., 2013; Liu et al., 2021; Martín-Rejano et al., 2011). Monocotyledonous plants generally have a lower B requirement than dicotyledonous plants supposedly based on the lower pectin content in their cell wall (Hu et al., 1996). For maize (Zea mays L.), symptoms caused by B deficiency were already described in 1934 (van Overbeek, 1934). Like for other monocots, maize develops typical white stripes between leaf veins when grown on B-deficient medium (Tollenaar, 1969). On the field, B deficiency impacts on maize performance by abnormal development of ears, silks, tassels and anthers leading to a reduction in grain yield and corn quality (Lordkaew et al., 2011). Although it is known that B fertilization does significantly increase yield in maize, many maize fields/stands are undersupplied with B (Lordkaew et al., 2011 and references therein). For instance, >40% of maize stands (n = 182) have been undersupplied with B in Germany (Yara Megalab Analysen 2012–2015; www.yara.de). Another reference reports that more than 62% of maize tissue samples in 21 states of the USA (600,000 samples) were deficient in B or could have benefitted from a B application (https://www.winfieldunited.com/news-and-insights/don-t-let-a-boron-deficiency-compromise-your-corn). Plant B uptake decreases under drought conditions due to a reduced soil solution mobility and thereby inhibited delivery of B to the roots. Many corn-growing regions have concomitant mild-to-moderate drought conditions during peak demand periods for B (Shorrocks, 1997). Maize yield losses due to B deficiency are thought to occur more frequently in the context of a changing climate in which maize production will be increasingly challenged with drought (Yin et al., 2021).
Nutrient uptake in general is taking place below ground being dependent on its specific content and availability in the soil, the root system architecture and transport systems that facilitate or drive the uptake of specific nutrients into the plant. Molecular adaptations to increase B uptake efficiency have been described for various plant species (Matthes et al., 2020). In Arabidopsis thaliana, AtNIP5;1, a membrane spanning channel protein, enhances B fluxes over the plasma membrane under B-deficient growth conditions (Takano et al., 2006). This channel works in close collaboration with AtBOR1 a secondary active B transporter shuffling B into the direction of the vasculature (Takano et al., 2002). In maize, tassel-less1 (TLS1)/ZmNIP3;1 has homologous characteristics to AtNIP5;1 resulting in B-deficient plants when mutated (Durbak et al., 2014; Leonard et al., 2014). In this context, ZmRTE (ROTTEN EAR) functions as the secondary active B transporter alike AtBOR1 (Chatterjee et al., 2017). Boron uptake transporters are localized in epidermal root cells being in contact with the rhizosphere. Adapting root system architecture in response to specific nutrient availabilities is frequently observed in various plant species. An arrest of primary root growth and an increase in root hair length and density are seen for Arabidopsis plants grown under B-deficient growth conditions (Liu et al., 2021). Apart from the increase in root surface area and the extended outreach into the rhizosphere by root hair modulation and therewith improving the soil exploration capacity, it is not known whether this morphological adaptation has physiological importance on B uptake under limited supply or whether this is mainly a freak of nature. Surprisingly, though the detrimental consequences of B deficiency on maize shoots and yield are known (Lordkaew et al., 2011; Figure S1), information on how the root system of maize, the cereal with the highest B demand, is physiologically responding at the morphological or the molecular level to B deficiency is very scarce or even absent. During vegetative growth stages, B concentrations of >8 mg B kg−1 leaf dry weight did not result in visible B-deficiency symptoms in maize. In contrast, B concentrations <4 mg B kg−1 leaf dry weight are usually reported when visible B-deficiency symptoms occur (Lordkaew et al., 2011; Quiroga et al., 2020; https://extension.umn.edu/micro-and-secondary-macronutrients/boron-minnesota-soils). The lack of research aiming to identify B-deficiency-related symptoms in maize is mostly due to experimental obstacles hampering the generation of defined and repeatable B nutrient regimes in a soil substrate. Therefore, effects of B deficiency on maize roots were mostly tested in non-natural substrate such as washed quartz sand which was flushed with nutrient solution twice per day, in hydroponics, or in soil substrates where, however, the B content and its availability was not controlled (Durbak et al., 2014; Lordkaew et al., 2011).
Here, we investigate the impact of B deficiency on maize root and shoot growth parameters as well as on B uptake under B-deficient growth conditions, using the root hairless mutant rth3 and its corresponding wild-type (WT) B73. A physiological comparison of maize B73 plants and its root hairless rth3 mutants (Hochholdinger et al., 2008) grown either in the field or in soil columns suggested that under standard nutrient-sufficient growth conditions, the existence of root hairs does, with the exception of P, not impact significantly on the nutritional status of maize (Lippold et al. 2021; Vetterlein et al., 2022). Both lines were cultivated on various substrates allowing to generate physiologically relevant B-deficient, B-sufficient or B-surplus conditions either in soil column experiments or in rhizotrons of a fully automated root phenotyping facility. Remarkably, we succeeded in generating growth conditions, in which maize developed B-deficiency symptoms on leaf blades. Though B73 did not differ substantially from the rth3 mutant in respect to leaf number or shoot fresh weight, the leaf B concentration was significantly lower for the mutant under B-deficient and B-surplus growth conditions. In contrast to our expectations, (1) B deficiency did not reduce root growth and length neither in the rth3 mutant nor the WT, and (2) below ground compensatory adaptations such as a larger root system, more fine roots or a higher expression of B uptake transporters was lacking under B deficiency in the rth3 mutant compared to B73.
2 MATERIALS AND METHODS
2.1 Plant material and seed preparation
All experiments were carried out using the Z. mays root hair defective mutant rth3 and its corresponding WT B73 (Wen & Schnable, 1994; Hochholdinger et al., 2008). The mutant seeds used were highly homozygous as they were backcrossed to B73 for more than eight generations. Seeds were surface sterilized for 10 min using 10% H2O2 and soaked in saturated CaSO4 for 3 h prior to sowing.
2.2 Soil substrate
Plants were grown in a white-peat volcanic clay mixture (as from now on called FN soil) which has an intrinsic B content below 0.1 mg kg−1 substrate (Eggert & von Wirén, 2017). The substrate was prepared by adding 0.5% CaCO3 and 0.3% CaO as described in Pommerrenig et al. (2018). In the following, the buffered substrate was sieved to a particle size of 2 mm and fertilized with 6× nutrient solutions prepared out of 100× stock solution mixed with Milli-Q water: 100× macro element mix (60 g L−1 NH4NO3, 40 g L−1 KH2PO4, 20 g L−1 MgSO4 and 6 g L−1 K2SO4), 100× micro element mix (4 g L−1 MnCl2, 1.3 g L−1 CuSO4·5H2O, 1.3 g L−1 ZnSO4·7H2O, 8.5 mg L−1 NaMoO4) and 100× NaFeEDTA mix (2.5 g L−1). The B-sufficient and B-surplus growth conditions were applied using a 100× B(OH)3 (1.4 mg L−1) stock solution. No glassware was used in any process during the preparation of the nutrient solution or the irrigation of the plants to prevent additional input of B into the growth system.
The sand substrate is composed of quartz sand (WF 33, Quarzwerke Weferlingen). Details on the fertilization are found in Lippold et al. (2021). Boron contents were adjusted to 0.2, 0.7 and 1.5 mg B kg−1 sand substrate using the described stock solution.
The loam substrate originated from the upper 50 cm of a Haplic Phaeozem soil profile which was sieved to 1 mm particle size prior column packing (Lippold et al., 2021). The loam substrate was fertilized, and columns were filled according to Lippold et al. (2021).
2.3 Plant growth
Maize plants were grown in acrylic glass columns (25 cm height × 7 cm inner diameter) for 22 days until the developmental stage BBCH15 (Meier, 2001). The bulk density in the column was 0.23 g cm−3 for FN soil, 1.47 g cm−3 for sand and 1.26 g cm−3 for loam. Furthermore, the volumetric water content was kept at approximately 30% for FN soil, 18% for sand or 22% for loam by individual watering of the pots. Plants were grown in a growth cabinet at 22/18°C day/night cycle with a 12 h light-period illuminated at 350 µM m−2 s−1 at 65% humidity.
2.4 Microscopy
The comparative images of the root hair zones of Z. mays B73 WT and its root hairless rth3 mutant grown in B-sufficient and B-deficient soil substrate were made with a Keyence VHX-5000 digital microscope (Keyence International) and a VH-Z20R/Z20T 20-200× zoom objective. To reduce reflections, a super-diffuse illumination adapter (Keyence OP-42305) was attached to the objective.
2.5 Harvest parameters, root measurements and element analysis
At the day of harvest, shoot height was measured, and the numbers of leaves were determined. In the following, the shoot was separated from the root. Roots were cleaned from soil particles as thoroughly as possible with deionized water using a root washing device. Roots were stored in 70% ethanol solution until further use. For the determination of root parameters, roots were scanned at 700 dpi with an Expression 11000XL scanner (Epson) and analysed using the software WinRhizo 2019 (Regent Instruments). For the above ground material, the fourth leaf and the remaining shoot material were weighed and dried at 65°C until constant weight. Approximately 30 mg of plant material was subjected to elemental measurements using a high-resolution inductively coupled plasma mass spectrometry (HR-ICP-MS, Element 2, Thermo Fisher Scientific). Plant height was measured with a folding rule to the most elongated point. Shoot fresh weight was measured on a lab scale (Kern), and leaf number was counted manually.
2.6 Automated root and shoot phenotyping
Approximately 20 kg of FN soil substrate, which was fertilized to 0.1 mg and 16 mg B kg−1 soil in a clean cement mixer as described above, was prepared to fill one rhizobox (900 mm × 600 mm × 50 mm). Rhizoboxes were mounted in the Rhizotron system for automated plant root and shoot phenotyping located in a large-scale fully climate-controlled plant cultivation facility (IPK Plant Cultivation Hall – IPK PhenoSphere) (Langstroff et al., 2022; https://www.ipk-gatersleben.de/en/infrastructure/phenotyping/ipk-phenosphere). Rhizoboxes were stored at 45° angle. Two seeds per rhizobox were initially planted, and the most homogenously germinated seedlings were kept for root phenotyping. Plants were grown at 20/18°C day/night cycle with a 16-h light-period at 60% humidity up to growth stage BBCH19 (Meier, 2001). Every 24 h, images of the roots and the shoots were automatically captured by three high-resolution cameras (shoot top and side view and root side view through a plexiglass pane) in imaging cabinets moving along the rhizoboxes and pulling each rhizobox into the imaging cabinet. Root parameters were detected and calculated using the image analysis software ‘semi-automated Root Image Analysis – saRIA v0.1’ (Narisetti et al., 2019). The accuracy of the root and shoot imaging platform was previously demonstrated by comparing image data to established software and with physically measured biomass data (Shi et al., 2023). Twenty-nine days after sowing (DAS), the biomass of roots and shoots was determined as described before.
2.7 Gene expression
Plant tissue was collected and immediately frozen in liquid N. Total RNA was extracted using the RNeasy Plant Mini Kit (Macherey-Nagel GmbH & Co KG). cDNA was synthesized out of 500 ng total RNA using MuLV-Reverse Transcriptase (Fermentas) in a total volume of 20 µL and diluted to 1:20 with nuclease-free water. RT-qPCR was performed in a 384-well thermocycler (CFX384 Touch TM Real-Time PCR Detection System, Bio-Rad) using the GoTaq qPCR Master Mix (Promega) and 2 µL diluted cDNA (1:20) per reaction. Biological replicates were run in two technical replicates. The thermocycler protocol was as follows: 3 min at 95°C, followed by 45 cycles of 10 s at 95°C, and 40 s at 58°C. Primer specificity (Table S1) was validated by sequencing of obtained qPCR products. A standard curve was generated using a homogenously mixed pool of all samples which was serially diluted (1–1, 1–2, 1–4, 1–8, 1–16, 1–32 and 1–64) with nuclease-free water. PCR efficiencies were determined for each primer pair. Assays having PCR efficiencies >90% and < 115% were used for expression data analyses. Relative expression of all genes was normalized to the geometric mean of ZmAct1 and ZmGapDH as internal references.
3 RESULTS
3.1 Distribution patterns of mineral elements in young maize seedlings under nutrient-sufficient growth conditions
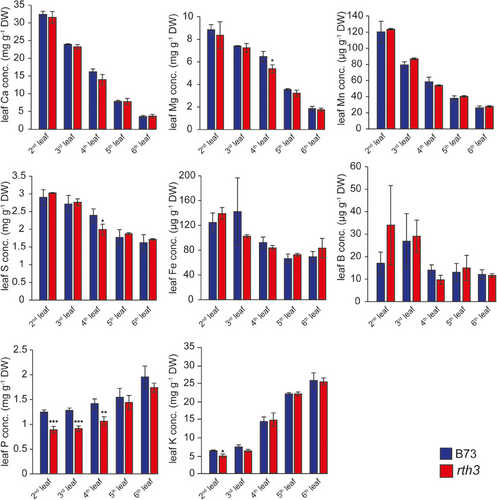
To investigate whether root hairs impact on the uptake of nutritionally relevant elements in maize, we grew B73 and rth3 plants in a loamy soil substrate that was sufficiently supplemented with nutrients (Lippold et al., 2021). Typically, Ca, Mg, Mn, S, Fe and B concentrations increased with leaf age (Figure 1) and the concentration of K and P decreased with leaf age in both, the B73 and the rth3 mutant (Figure 1). These observed nutrient patterns reflect nutrient allocation patterns, which are typical and mirror nutrient-specific mobilities within plants (Marschner, 2012). However, we observed that old leaves of rth3 plants possessed significantly lower P levels than the WT, whereas this difference disappeared in younger leaves (Figure 1). Leaf B concentrations did not significantly differ between both genotypes and were above 10 µg g−1 dry weight, a leaf B concentration indicating an adequate B nutritional status. Thus, in this loamy substrate, the presence of root hairs did not contribute to B uptake, whereas they significantly contributed to P acquisition.
3.2 The soil substrate has significant impact on the extractability and uptake of soil B in maize
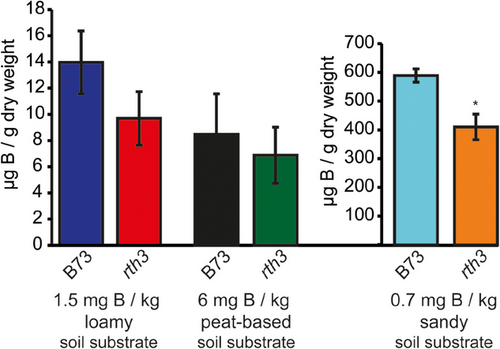
Next, we elucidated the ability of WT and rth3 maize plants to acquire B from different growth substrates in order to identify substrates, in which B-deficient growth conditions prevail and the contribution of root hairs to B uptake can be assessed. Boron adsorption in soils and therewith plant bio-availability is influenced by soil pH and texture, and the clay-, oxide- and organic matter content. High B availability for plants is observed in coarse-textured sandy soils, fixation maxima are found in soils with a high organic carbon content such as peat and clay-rich soils seem to possess an intermediate B availability (Chaudhary et al., 2005; Goldberg, 1997). To this aim, a sand, loam and peat-based substrate were supplemented with B to achieve 0.7 mg, 1.5 mg and 6 mg B kg−1 substrate, respectively. WT and rth3 maize plants were grown until BBCH15. Leaf B concentrations differed substantially in the order of sandy soil > loamy soil > peat-based substrate grown plants (Figure 2). On the loamy soil and the peat-based substrate, both genotypes had leaf B concentrations between 7 and 14 µg B g–1 DW which is in the range of reported tissue B levels of maize plants being sufficiently supplied with B (Gupta, 1983; Lordkaew et al., 2011). B73 and rth3 plants grown on sandy soil had leaf B concentrations of 400 and 600 µg B g–1 DW, respectively, clearly being greater than 100 µg B g–1 DW which has been shown to induce B toxicity in maize shoots (Gupta, 1983). This high tissue B level is reasoned by the high B availability for plants when supplied in coarse-textured sandy soils (Goldberg, 1997). Accordingly, B toxicity symptoms in leaves were observed in the sandy substrate (Figure 3B), whereas B symptom-free growth was observed in the loamy and peat-based substrate (data not shown). These results showed that the B nutritional status of maize does highly depend on the growth substrate. Obtained results suggested that the peat and the sandy substrate are suitable to investigate the contribution of maize root hairs to the uptake of B, under B-deficient or high B available growth conditions, respectively.
3.3 Under excessive B supply B73 WT maize plants possess higher shoot B concentrations than its corresponding root hairless rth3 mutant
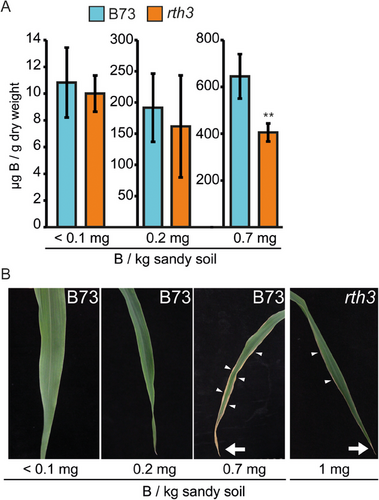
To investigate the impact of root hairs on shoot B levels in luxury consumption or excessive B availability situations, B73 and rth3 plants were grown on no additional (<0.1 mg B kg−1), 0.2 and 0.7 mg B kg−1 sandy soil substrate (Figure 3). On no additional and 0.2 mg B kg−1 sandy soil substrate, a trend towards lower B concentrations in the fourth leaf of the rth3 genotype was detected though not significantly different (Figure 3A). When grown on 0.7 mg B kg−1 sandy soil substrate, the rth3 mutant had significantly lower fourth leaf B concentrations than B73 (Figure 3A). Moreover, B73 displayed typical B toxicity symptoms such as leaf tip necrosis and chlorotic/necrotic leaf blade margins to a greater extent than rth3 (Figure 3B). Therefore, the presence of root hairs seemed to promote B uptake in the sandy soil substrate (Figure 3) in which the B bio-availability is suggested to be very high (Figure 2; Goldberg, 1997).
3.4 Boron-deprived B73 WT maize plants possess higher shoot B concentrations than its corresponding root hairless rth3 mutant
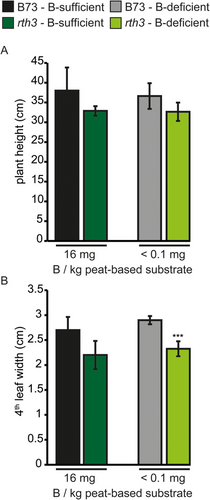
To investigate the impact of maize root hairs on B uptake under B-limiting conditions, we optimized a previously established peat substrate-based cultivation system for rapeseed, which allows establishing B-deficient or B-sufficient growth conditions, for Z. mays (Pommerrenig et al., 2018). Boron uptake efficiency of maize plants from this substrate was very low; hence, the soil B content was set to 16 mg B kg−1 substrate to obtain a sufficiently high maize plant B content (Figure 4A). Phenotypic B-deficiency symptoms in shoots and roots of B73 became visible during the first 20 DAS, when no additional B was added to the peat-based soil substrate. In these conditions, B73, but not rth3, roots had formed more root hairs compared to B-sufficient growth conditions (Figure S2). Maize seems to share this typical B-deficiency response with many other plant species. Symptoms of B deficiency in shoots of both maize genotypes grown under B-deficient but not B-sufficient conditions were visible approximately 3 weeks after gemination in form of white longitudinal expanding stripes on the blades of the youngest developing leaves (Figure 4B). Measuring the B concentration of the fourth leaf in both maize genotypes grown on 16 mg B kg−1 substrate showed sufficient B tissue levels above 10 µg B g−1 DW (Figure 4A). Under B-deficient growth conditions, B concentrations of B73 dropped far below 3–5 µg B g−1 DW (Figure 4A), a threshold value which was previously described to be critical and supposed to result in deficiency symptoms in maize (Lordkaew et al., 2011). Under the same growth conditions, the B concentration of the rth3 mutant was significantly lower than that of B73 (Figure 4A).
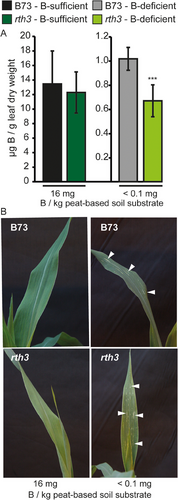
Though B-deficiency symptoms were visible on young leaf blades and B concentrations were in the deficiency range when plants grew under B-deficient growth conditions, plant height (Figure 5A), the number of leaves and shoot fresh weight (data not shown) did not differ substantially between the WT and the rth3 mutant neither on B-sufficient nor B-deficient growth conditions.
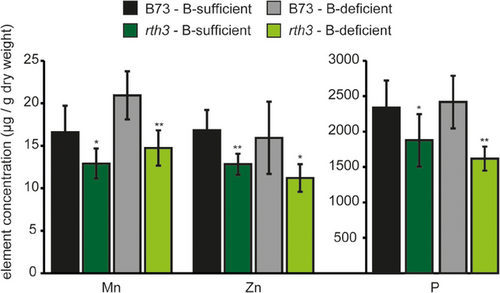
3.5 B73 plants possess higher shoot Mn, Zn and P concentrations than its corresponding root hairless rth3 mutant when the bio-availability of these nutrients is limited
Neutral to alkaline calcareous soils are known to impair the extractability and plant availability of manganese (Mn) and zinc (Zn), as well as P (Rengel, 2015). Accordingly, though the pH-neutral peat substrate was sufficiently fertilized with these minerals, their shoot concentrations were rather low in B73 plants (Figure 6). Strikingly, under B-sufficient as well as B-deficient growth conditions, the rth3 plants had significantly lower Mn, Zn and P concentrations compared to B73, whereas the other micro- and macronutrients, including B, did not differ when sufficiently supplied (Figures 4A and 6; data not shown). This result suggested that maize root hairs contribute to the uptake of Mn, Zn and P from soil substrates in which the bio-availability of these nutrients is limited.
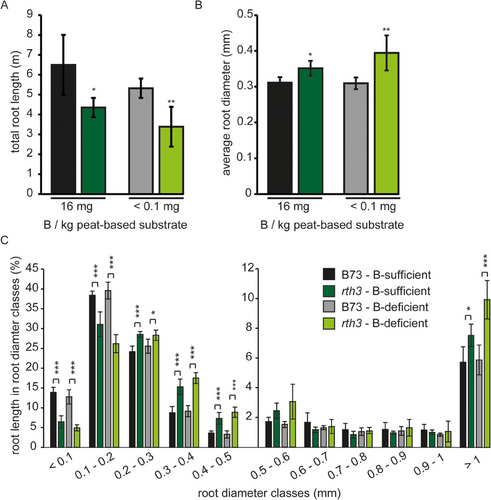
3.6 The root length of the rth3 mutant is significantly shorter than that of B73 plants independent of the B treatment
Root system architecture can be modulated by the plant as a response to exposed stresses or genetic prerequisites. Investigating the total root length of B73 and rth3 plants grown in soil columns intended to clarify whether the absence of root hairs in rth3 results in compensatory effects in other root traits to attenuate the loss of epidermal root surface. Interestingly, total root length of the rth3 mutant was significantly smaller than that of the WT, whereas the mean root diameter was significantly larger in rth3 (Figure 7). In this context, the absence of root hairs did not provoke the formation of more very fine roots (diameter <0.2 mm) but rather increased the percentage of roots having a diameter between 0.2 and 0.5 mm compared to WT plants (Figure 7B,C).
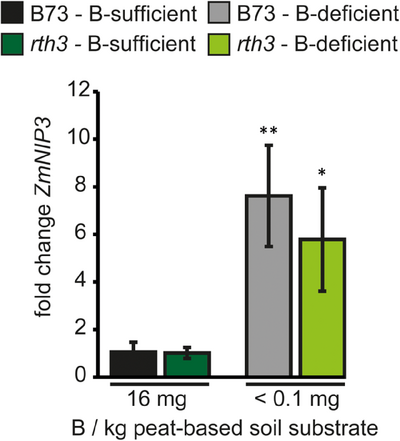
3.7 Transcript abundance of the crucial B transport regulator ZmNIP3;1 does not differ between the WT and the rth3 mutant
ZmNIP3;1 is a B channelling transporter which is important for B homeostasis in maize (Durbak et al., 2014; Leonard et al., 2014). Although its orthologue from Arabidopsis, AtNIP5;1, is root-specific, ZmNIP3;1 is expressed in various plant organs and much less up-regulated under B deficiency than its Arabidopsis counterpart (Durbak et al., 2014; Leonard et al., 2014). Genes encoding the B-transporting NIP protein channels are typically strongly up-regulated at the transcriptional level under B-limiting conditions, and their expression positively correlates with the B nutritional status of plants. Accordingly, NIP genes represent the major reliable transcript markers for B deficiency and as an indicator to understand B uptake into plants (Takano & Tanaka, 2023). Therefore, ZmNIP3;1 transcript abundance was quantified in B73 and the rth3 mutant both under B-sufficient and B-deficient growth conditions. ZmNIP3;1 was very lowly expressed, both in B73 and the rth3 mutant under B-sufficient growth conditions. Upon B-deficient conditions, ZmNIP3;1 was up-regulated eight- or sixfold in B73 and the rth3 mutant, respectively. However, the up-regulation did not significantly differ between B73 and the rth3 mutant (Figure 8). This result suggested that neither a lack of root hairs nor the smaller root systems are compensated by a total higher up-regulation of this important B uptake channel protein in the rth3 mutant compared to the WT under B deficiency.
3.8 Dynamic assessment of root and shoot growth in an automated rhizotron system reveals that rth3 mutant and B73 root growth is neither inhibited nor stimulated under B deficiency
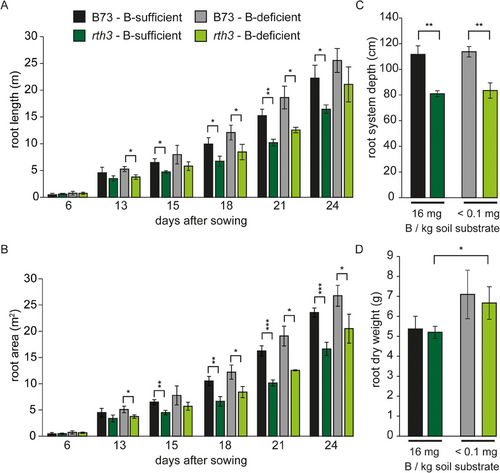
Having established B-deficient growth conditions for maize in soil columns and having obtained knowledge on B-deficiency response reactions on the shoot and root level therein, it was tested how root traits dynamically develop in more field-like conditions both with respect to growth conditions and rooting volume. Therefore, growth experiments of B73 and rth3 mutant plants under B-sufficient and B-deficient growth conditions were repeated in rhizoboxes mounted in the Rhizotron System for automated plant root and shoot phenotyping located in a large-scale fully climate-controlled plant cultivation facility (IPK PhenoSphere) at the IPK Gatersleben (Langstroff et al., 2022; https://www.ipk-gatersleben.de/en/infrastructure/phenotyping/ipk-phenosphere). This allowed us to automatically capture images of shoots (Figure 9A) and roots (Figure 9B) of individual plants every 24 h, for subsequent analyses of root and shoot growth parameters. When grown for 24 days in rhizotrons, the rth3 mutant plants developed a significantly smaller total root length (Figure 10A) and root area (Figure 10B) from 18 DAS onwards under both, B-deficient and B-sufficient growth conditions. However, these traits did not differ in the dependence of the growth conditions (Figure 10A,B). Strikingly, root system depth (Figure 10C) was consistent for each genotype independent of the B treatment but was significantly lower for the rth3 mutant compared to the WT (Figure 10C). This observation has not been reported before. The root dry weight tends to increase under B-deficient conditions though only significant for the rth3 mutant (Figure 10D). These results showed that the rth3 mutant has a decreased root system depth and length when the rooting volume is large enough to allow expanded growth at >18 DAS.
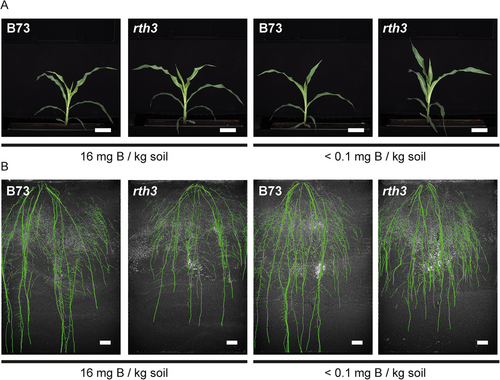
3.9 Maize shoot growth is marginally impaired under B-deficient rhizotron growth conditions
When looking at plant development under B-sufficient growth conditions, the plant height, shoot fresh weight and the number of leaves did not differ between the WT and the rth3 mutant (Figure 11A–C). However, under B-deficient growth conditions, the plant height and the shoot fresh weight, but not the number of leaves, are marginally but significantly lower in rth3 mutants compared to the WT. In accordance with data from the literature (Lordkaew et al., 2011), B deficiency had imperceptible effects on vegetative biomass parameters of WT maize plants.
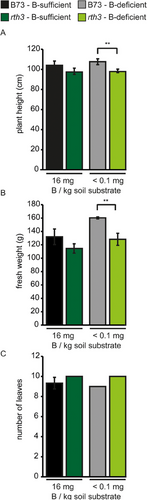
There was no corresponding significant effect of B on the root:shoot ratios neither for the WT nor the rth3 mutant both on B-deficient and on B-sufficient growth condition calculated using either the fresh weight or the dry weight parameters (Table S2). Calculating the amount of B in mg which was taken up of each genotype per root area (in cm2), per total root length (in cm), per specific root length (in cm) or per root dry- or root fresh weight (in g) (Table S2) suggested collectively and independent of the five root-unit parameters that B73 and rth3 roots are evenly effective in taking up B along the growth period both under B-deficient and B-sufficient growth conditions. Considering that the calculation underlying root system parameter values are based on the complete root system including in root zones without root hairs, these results support the notion that root hairs in B73 maize roots are not responsible for the significant higher shoot B concentration in WT plants under B-deficient growth conditions. Similarly, the amount of P in mg which was taken up of each genotype per four of the above-mentioned root system ‘size’ parameters (Table S2) did not differ between the genotypes and also not comparing B-deficient versus B-sufficient growth conditions, although in both B growth conditions, the P concentration was significantly lower (approx. 15% reduced) in the rth3 mutant compared to the WT (Figure 6). An exception represented the amount of P in mg which was taken up per root dry weight (in g) which was significantly higher in the WT compared to the rth3 mutant under B-sufficient growth conditions but not under B-deficient growth conditions.
4 DISCUSSIONS
Among cereals, Z. mays belongs to those with a rather high demand for B (Lordkaew et al., 2011; Shorrocks 1997). Although architectural and morphological responses to B-deficient conditions in maize have been described as imperceptible at the vegetative shoot level and detrimental at the flowering stage, specific root responses remained unknown. A few studies described in detail root responses to B deficiency of maize plants grown in a ‘paper towel roll’ or a ‘Hoagland's fortified GelRite gellan gum gel’ growth system (Housh et al., 2020; Wilder et al., 2022), but studies on roots of in terra grown plants are scarce. Moreover, the qualitative and quantitative contribution of root hairs to B nutrition in maize (and other plants) is unclear. The comparison of B73 WT maize and its root hairless rth3 mutant subjected to different B treatments therefore provides new insights into root responses to B deficiency and a putative role of root hairs in B uptake.
4.1 Soil type has a decisive impact on the bio-availability of B for maize plants
B availability for plants varies depending on physicochemical properties of the soil such as the pH, the soil B adsorption and retention capacity (low in sandy acidic soils and high in soils with a high pH, or which are rich in clay minerals, iron or aluminium oxides, or organic matter) and the dynamic of the mass flow by which B is transmitted to the root surface. Plants have evoked species and probably even cultivar-specific different plasticity to adapt their root system and root characteristics in dependence of the nutrient and water availability. Here, we demonstrate that B availability for Z. mays varies tremendously in dependence of the soil substrate the plants develop in. Boron uptake of maize plants from the peat-based substrate used in this study was very low; hence, the soil B content was increased up to 16 mg B kg−1 substrate to guarantee a sufficient high typical maize plant B content (Figure 4A). For comparison, 2.4 mg B kg−1 in this substrate is enough to sustain the growth of Brassica napus for the same time period, though being a plant species having a much higher B demand than maize (Pommerrenig et al., 2018). In contrast, less than 1 mg B kg−1 sandy substrate caused B toxicity symptoms in the assayed maize genotypes. This observation highlights that supplying B to a plant species in an adequate manner is a balancing act (Brdar-Jokanović, 2020) that demands knowledge on the demand of the genotype and its ability to take up B from a specific soil type in a specific environment. A low B uptake ability of maize in soils with a high organic matter content may explain the observation that despite their generally assumed low B-demand, maize plants with suboptimal B tissue levels are frequently found on fields which were supplied with assumingly sufficient amounts of B fertilizer (https://www.winfieldunited.com/news-and-insights/don-t-let-a-boron-deficiency-compromise-your-corn).
4.2 The B uptake channel ZmNIP3;1/TLS1 is not expressed to a higher degree in rth3 roots to compensate for the lack of root hairs
One explanation for the low ability of maize plants to acquire B from soils with a high adsorption and retention capacity might be the lack of root-specific B uptake transporters which are highly responsive to B-deficient conditions. Although in Arabidopsis or B. napus, root-specific B uptake channels (AtNIP5;1, BnaNIP5s) exist and are strongly up-regulated (>20 times) under B-deficient conditions (Diehn et al., 2019; Takano et al., 2006), the maize uptake channel encoding ZmNIP3;1/TLS1 gene is only up-regulated >5 times and not specifically in roots (Durbak et al., 2014; Figure 8). Surprisingly, the B uptake channel ZmNIP3;1/TLS1 was not expressed to a higher degree in rth3 compared to B73 roots (Figure 8) independent of the B supply level, which suggests that neither the shorter root system nor the lack of root hairs are compensated at the molecular level by a higher expression of this B uptake facilitator. Keeping in mind that the separation of roots from the substrate for subsequent RNA extraction most likely removed also a number of root hairs from B73 roots, it might even be possible that an induction of ZmNIP3;1/TLS1 expression in root hairs in the WT has to be additionally accounted as a positive B-uptake determining factor in comparison to the mutant.
4.3 Is a targeted B root tissue allocation more decisive for a proper root development than the overall root B nutrition?
Interestingly, although tls1 mutants grown in the greenhouse under sufficient B supply did not exhibit any visible shoot defects, their root system was shorter compared to the WT with thinner cell walls in the xylem and a reduced Casparian strip (Durbak et al., 2014). These results suggest that ZmNIP3;1/TLS1 plays an important role in root development by delivering available B both under B-deficient but also under B-sufficient conditions to targeted cells and tissues where it is physiologically needed, and that, if specifically allocated by ZmNIP3;1/TLS1, a ‘low’ B supply (<0.1 mg B kg−1 substrate; this study) is sufficient for proper maize root development. However, if quantitative and qualitative B patterns are disturbed by improper B distributions caused by, for example, the lack or mislocalization of a B transporter, normal root development will be impaired. Such a role of B transporters in a fine-tuned B allocation essential for root development is supported by the analysis of the rte-1;rte2-2 mutant which displays an altered B distribution pattern but is not modified in its primary root B uptake ability (Chatterjee et al., 2017). This suggests that a specific B allocation within tissues is crucial for a proper root development in addition to the overall B uptake ability of roots in maize. Similar conclusions derived from a study in Arabidopsis show that the overexpression of AtNIP5;1 only in its native cell types resulted in B-deficiency tolerant plants but not, when AtNIP5;1 was ubiquitously overexpressed in roots under the control of the CaMV 35S promoter (Kato et al., 2009).
4.4 Rth3 maize plants develop a larger average root diameter in a peat-based substrate independent of the B supply conditions
Recently, it was described that the average root diameter of B73 and rth3 varies depending on the soil substrate they develop in. The average root diameter in a sandy soil substrate was larger compared to that of roots growing in a loamy soil substrate (Lippold et al., 2021). Interestingly, the rth3 mutant showed a tendency towards having a larger average root diameter than B73 in both substrates. Here, we grew these genotypes in a peat-based soil substrate in which they developed average root diameters around 290 and 320 µm at BBCH15, which is in between the reported values for sand and loam. Strikingly, we detected a significant difference between the average root diameter of the genotypes being larger for the rth3 mutant which is in line with the tendency observed in sandy and loamy substrates (Lippold et al., 2021). Although root hairs and fine roots are defined to enable the root to explore niches in the soil substrate more efficiently, root hairs are also a radial factor bridging the gap between the roots and the soil. In that sense, an increased average diameter might ensure a tight contact of the root to the soil structure to compensate for the radial diminution caused by the lack of root hairs. An increased average root diameter was for instance observed in tomato and mungbean plants exposed to P-deficient growth conditions (Reddy et al., 2020; Tiziani et al., 2020). Moreover, a genome-wide association study assaying 13 morphological traits in 356 maize lines under P-sufficient and P-deficient conditions suggested that roots with a larger diameter mediated by an up-regulated H+-ATPase activity enhance the P absorption ability in maize seedlings (Wang et al., 2019). Interestingly, on average, a 20% increased root diameter was found in B-deficient tomato plants (Choi et al., 2015). Whether an increased root diameter is indeed of physiological relevance for the acquisition of certain nutrients under certain nutrient-limiting conditions remains to be demonstrated.
4.5 Estimating the contribution of root hairs to maize B acquisition under B-deficient and B-excess conditions using the root hairless rth3 mutant
Plants have evolved a genetically encoded phenotypic plasticity to adapt their root system and root characteristics in dependence of the nutrient and water availability. One of the root surface areas decisively determining root parameter is the presence of root hairs which can enlarge the root surface up to 70% of the total root and have the power to modulate the contact area between the plant and the rhizosphere (Raghothama & Karthikeyan, 2005). A symptom typical for B deficiency in many dicot plants is the rapid formation of large numbers of very long root hairs close to the root tip (i.e., the so-called hairy root phenotype) which is assumed to facilitate the uptake of B under such limiting B growth conditions (Bienert et al., 2021; Claeijs & Vissenberg, 2022; González-Fontes et al., 2016; Liu et al., 2021). More root hairs were also visible in B73 under B-deficient compared to B-sufficient growth conditions in the peat-based soil substrate suggesting that root hairs play also a role in B uptake in maize (Figure S2). To estimate the importance of Z. mays root hairs on nutrient acquisition, particular in B-deficient and B-excess conditions, we included the root hairless rth3 mutant in our study. Under B-deficient growth conditions, the rth3 mutant possessed and accumulated significantly lower shoot B concentration than B73. Moreover, under conditions in which B is highly bio-available, as in the sandy substrate experiment, the rth3 mutant also possessed lower levels of B in the shoot and developed less intense visible B toxicity symptoms than B73 (Figure 3). Independent of the B availability, the absence of root hairs entailed lower shoot Mn, Zn and P concentrations, nutrient whose uptake has been already interconnected with the quantity and length of root hairs in plants in the past (reviewed in Bienert et al., 2021). These results could hint towards the importance of root hairs for maize nutrient uptake and content. However, the total root length and depth were additionally significantly decreased over time in the rth3 mutant compared to B73, both under B-sufficient and B-deficient growth condition (Figure 10). Therefore, the performance of rth3 mutants may be biased by secondary effects, such as an altered overall root growth, in addition to the root hair phenotype. This study does therefore not allow to discriminate whether the smaller root surface area of rth3 or the missing root hairs are responsible for a modified B uptake and consequently for altered shoot B levels. The calculated amount of B in mg which was taken up of each genotype per root area, per total root length or per root dry- or root fresh weight rather suggests that root hairs have a minor role in B uptake in maize. Our results match those of a study assessing the role of rice root hairs in the uptake of another nutritionally important metalloid, namely that of silicon (Si) (Ma et al., 2001). Ma et al. (2001) demonstrated that root hairs do not play a role in Si uptake. Similar to our study, Klamer et al. (2019) reported in an attempt to estimate the importance of maize root hairs in low P conditions and under drought that the maize rth2 mutant has in addition to the root hair phenotype, also an altered root architecture. Future studies on the contribution of maize root hairs on nutrient or water uptake will depend on the identification of root hair mutants with an otherwise non-altered root system architecture.
Furthermore, more striking physiological effects of root hairs on the B nutritional status of maize seedlings grown under B-deficient growth condition may be likely masked by the fact that maize plants are able to sustain vegetative growth with only very imperceptible phenotypic effects despite possessing very low B tissue levels. The importance of root hairs may, however, drastically change when the reproductive development in maize plants starts, as tassels, pollen and the silk are highly sensitive to B deficiency.
4.6 B-deficient maize roots do not display foraging behaviour
In contrast to a progressive reduction of the total root length caused by an impaired root growth in response to certain nutrient deficiencies, plants are able to adapt to the shortage of other certain nutrients or localized nutrient availability by altering their root system architecture in a compensatory manner to efficiently exploit yet unexplored soil zones, still containing little amounts of the limited nutrient (Giehl & von Wirén, 2014). Such a so-called root nutrient foraging behaviour is generally observed for plant roots grown under N-deficient growth conditions (Giehl & von Wirén, 2014) or has been reported for the root hairless rth2 maize mutant exposed to P limitations, under which the mutant displayed a higher number of fine roots compared to the WT, most likely to enlarge the exploration capacity in an alternative way to the typical root hair elongation (Klamer et al., 2019). However, neither for the WT nor for the rth3 mutant an increased total root length indicative for a B foraging behaviour was observed under the presented B-deficient growth conditions.
4.7 Rooting depth and total root length of B73 and rth3 maize plants are not decreased in B-deficient growth conditions
To shed light into responses of maize roots facing B-deficient growth conditions, we developed a soil substrate-based cultivation system for Z. mays in which a naturally developing root system and the formation of a physiologically relevant rhizosphere are ensured, whereas the B content and availability can be fine-tuned to specific B levels. Unexpectedly, and in contrast to a striking strong and very rapidly occurring growth inhibition of dicot primary and lateral roots upon B-deficient growth conditions (Gruber et al., 2013; Liu et al., 2021), we did not observe any significant reduction in maize rooting depth or total root length in response to B-deficient growth conditions and that in the two experimentally independent rhizotube and rhizotron approaches (Figures 7 and 10). Boron deficiency-induced root growth inhibition was neither present in the rth3 mutant nor the WT, although in both genotypes, typical visual B-deficiency symptoms in form of white necrotic stripes between leaf veins were already visible at a growth stage when four leaves have been unfolded (Figures 4, 7 and 10). In dicot plants, such as Arabidopsis and Brassica visual B-deficiency symptoms usually first appear in roots (e.g., by readily responding to B deficiency by decreasing their primary root elongation) and then proceed to shoot parts (Liu et al., 2021; Matthes et al., 2020; Pommerrenig et al., 2018). However, in rice, another monocot species, plants do not show a significant growth defect on the root and shoot level when grown <4 weeks on B-deficient hydroponic medium (Uraguchi and Fujiwara, 2011). That suggests that in contrast to dicots but similar to other monocots, maize seems to possess the ability to sustain root growth for several weeks even under severe B-deficient growth conditions, in which B-deficiency symptoms are already visible in leaf blades. It will be of high interest to identify in future the mechanisms allowing maize root meristems and other root tissues to withstand this nutrient stress.
5 CONCLUSIONS
In summary, the root system growth of Z. mays behaves, in comparison to other plant species, atypically under B-deficient conditions: Neither a progressive reduction of root growth nor a root foraging behaviour was observed, though B-deficiency symptoms in shoots were visible and shoot B levels were clearly in a range of being regarded deficient. Because the rth3 genotype displayed secondary effects in root length and depth, future experiments on the relevance of root hairs are needed to assess their exact contribution to B nutritional status of maize.
ACKNOWLEDGEMENTS
We acknowledge Dr. Yudelsy Tandron Moya (IPK) for excellent technical assistance in the ICP-MS analysis, Jacqueline Fuge (IPK) for RT-qPCR analysis, and Ingo Mücke (IPK) for his support in management of the PhenoSphere operations. We also thank Anja Schmidt (TUM), Johanna Tebbing (TUM), Jacqueline Fuge (IPK) and Annett Bieber (IPK) for the excellent assistance in the plant growth and data processing. This project was carried out in the framework of the priority programme 2089 ‘Rhizosphere spatiotemporal organization – a key to rhizosphere functions’ funded by the German Research Foundation (DFG) (403625794). This work was supported by the DFG grant 1668 / 4-1 from the German Research Foundation (DFG) to G.P. Bienert. Seeds of B73 and the maize mutant rth3 were provided by Caroline Macron and Frank Hochholdinger (University Bonn).
Open access funding enabled and organized by Projekt DEAL.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




