Zinc bioavailability in maize grains in response of phosphorous–zinc interaction
Abstract
Phosphorous (P) and zinc (Zn) are plant nutrients that interact with each other in soil–plant systems. Such interactions may cause deficiency of one of the nutrients interacting with each other if interactions are antagonistic. In the present trial, a field experiment was conducted to investigate the interactive effect of Zn (0 and 16 kg ha−1) and P (0 and 60 kg ha−1) on growth, yield and grain Zn concentration of two maize (Zea mays L.) genotypes, i.e., Neelam (local) and DK-6142 (hybrid). Growth and yield of both maize genotypes were increased by the application of Zn and P treatments compared with control, but Zn+P was more effective than their sole application. When compared to control, combined application of Zn+P increased grain Zn and P concentrations by 52% and 32%, respectively, averaged for the two genotypes. Single application of P decreased grain Zn concentration by 10% over control. Application of P and Zn particularly in combination decreased the grain [phytate] : [Zn] ratio and increased the estimated human Zn bioavailability in grains based on a trivariate model of Zn absorption in both maize genotypes. Conclusively, combined Zn+P application appeared more suitable for enhancing grain yield and agronomic Zn biofortification in maize grains. However, Zn fertilization aiming at increasing grain yield and grain Zn concentration should consider the genotypic variations and P rate.
1 Introduction
Phosphorus (P) and zinc (Zn) deficiencies are well-known dietary limitations for growth and yield in agriculture in many parts of the world and have been widely investigated (Marschner, 1995). Increasing P availability in the root rhizosphere can induce Zn deficiency in plants by altering Zn uptake and Zn translocation into the shoots (Robson and Pitman, 1983) but the mechanisms are still not clear. Many studies have revealed that high P fertilization with low Zn supply remarkably increased P absorption, which in turn caused P toxicity and symptoms resembling Zn deficiency (Cakmak and Marschner, 1986; Webb and Loneragan, 1990). However, little information is available on the effect of P availability on uptake and translocation of Zn in maize.
Maize is the most important cereal crop of the world after wheat and rice. It is used as a staple food in many parts of the world including Latin America, Africa, and Asia (Ortiz-Monasterio et al., 2007; Menkir, 2008), as well as feed for livestock (Harris et al., 2007). It provides an expected 15% of the protein and 20% of the calories for the world and is a nutritional staple crop for ≥ 200 million people around the globe. Unfortunately, even though maize grains supply many macro- as well as micronutrients for human metabolic needs, the Zn amounts are insufficient for persons who depend on maize as a major staple food (Chomba et al., 2015; Nuss and Tanumihardjo, 2010). Particularly, Zn may be limiting for the growth and development of young children (Krebs, 2013).
Genetic variation of Zn concentrations in maize grains has been reported (Maziya-Dixon et al., 2000; Bänziger and Long, 2000; Fahad et al., 2015). The average Zn concentration in maize is 20 mg Zn kg−1 grain (Ortiz-Monasterio et al., 2007). However, concentration and bioavailability are lower in food. Many national and international projects are focusing on Zn biofortification of many edible crops (White and Broadley, 2009). The target set by the HarvestPlus Program of Zn concentrations is 28 μg g−1 dry matter (DM) in polished rice, 38 mg kg−1DM in wheat, 38 mg kg−1 DM in maize, 66 mg kg−1 DM in pearl millet, 56 mg kg−1 DM in beans, 34 mg kg−1 DM in cassava roots, and 70 mg kg−1 DM in roots of sweet potatoes (Bouis and Welch, 2010). Plant species also differ in their Zn requirements as well as tolerance of high Zn concentration in their tissues (Broadley et al., 2007; Fageria, 2009). Most of the crop plants require leaf Zn concentrations > 15–30 mg kg−1 DM for maximal yield and their growth is inhibited at leaf Zn concentrations >100–700 mg kg−1 DM, which might be a more realistic threshold (Reeves and Baker, 2000; Broadley et al., 2007).
Maize consumption to meet energy and protein needs is not sufficient to avoid the risk of Zn deficiency, especially in young children (Krebs, 2013). The bioavailability of minerals is reduced by phytic acid (PA) in food crops (Lestienne et al., 2005). Phytic acid is considered an anti-nutrient because of its ability to form strong metal-ion complexes, mainly Zn2+, Ca2+, and Fe3+ (Harland and Oberkas, 1987). As a strong chelating agent, PA reduces the bioavailability of Zn+2 by the development of insoluble compounds (Weaver and Kannan, 2002). As a result, PA produces deficit absorption of some dietary minerals, e.g., Zn and Fe (Reddy and Pierson, 1994; Saharan et al., 2001). Grain products have high concentrations of PA (Alabaster et al., 1996) and about 1–2% seed weight is PA and it can reach up to 3–6%. 90% of PA are found in maize germ, while in rice and wheat it is found to a larger extent in the external covers of husk and pericarp layer (Cheryan and Rackis, 1980).
Among several other factors which cause a decline in maize yield, imbalanced use of fertilizers is also one of them. Maize is a strongly nutrient-demanding crop which also requires micronutrients, especially Zn (Amrani et al., 1999; Obrador et al., 2003), along with major elements. Moreover, with the advent of the Green Revolution, production of high-yielding genotypes (hybrids) has further aggravated the situation (Dar, 2004). Due to intensive agriculture and use of high-purity fertilizers, soils have been depleted for micronutrients. Moreover, the alkaline calcareous nature of soils further aggravates the problem. However, in Pakistan, farmers generally apply nitrogen (N) and to some extent P totally ignoring micronutrients, which have become deficient. One of the strategies to alleviate Zn deficiency in maize-consuming populations in developing countries is Zn biofortification of maize grains (Saltzman et al., 2013).
The present study was conducted to explore the effects of Zn and P fertilization on maize growth, yield, tissue concentrations of Zn and P, and estimated Zn bioavailability in two contrasting maize genotypes.
2 Material and methods
2.1 Growth conditions
A field trial was carried out in the research area of the Faculty of Agricultural Sciences and Technology (FAS&T), Bahauddin Zakariya University, Multan. Before sowing, randomized surface soil samples (0–15 cm depth) were collected for soil characterization. Collected soil was air-dried, passed through a 2-mm sieve and mixed thoroughly. Representative subsamples of the soil were analyzed for varies physiochemical characteristics (Table 1). The soil was alkaline and calcareous with a low phytoavailability of Zn (AB- DTPA: 0.77 mg kg−1) and P (Olsen-P: 7.49 mg kg−1).
| Character | Unit | Value | Method |
|---|---|---|---|
| Textural class | – | Loam | USDA classification |
| Sand | % | 47.5 | Hydrometer method (Gee and Bauder, 1986) |
| Silt | % | 34.7 | |
| Clay | % | 17.8 | |
| pHs | – | 7.85 | pH of saturated soil paste |
| ECe | dS m−1 | 0.643 | Electric conductivity of saturated soil paste extract |
| CaCO3 | % | 0.63 | Acid dissolution (Allison and Moodie, 1965) |
| OM | % | 0.59 | Walkley-Black method (Jackson, 1962) |
| AB-DTPA extractable Zn | mg kg−1 | 0.77 | Extracted with AB-DTPA (Soltanpour, 1985) |
| Olsen P | mg kg−1 | 7.49 | NaHCO3 method (Olsen and Sommers, 1982) |
One maize hybrid (Zea mays L. cv. Monsanto DK-6142) and one indigenous (cv. Neelam) genotype were used in this experiment. Two levels of Zn (0, 16 kg ha−1) and two levels of P (0, 60 kg ha−1) were used in this trial. Zinc was applied as hydrated zinc sulfate (ZnSO4 × 7 H2O) and P as di-ammonium phosphate. These treatments were arranged in randomized complete block design (RCBD). The experimental area was divided into three blocks with a total of 24 plots each of 3 m × 5 m size and 89 healthy maize seeds of uniform size were sown per plot by maintaining a row-to-row distance of 75 cm and a plant-to-plant distance of 22.5 cm. At sowing, plots were uniformly supplied with 140 N and 60 K (in kg ha−1) by applying urea and potassium sulfate, respectively. Zinc was applied 15 d after sowing. A second dose of 60 kg N ha−1 was applied 30 d after sowing. Canal water was used to maintain the soil moisture at field capacity in all the plots during the experimental period.
2.2 Plant analyses
Whole plots were harvested at maturity and manually threshed to separately measure stover and grain yields. Subsamples of cobs and stover were taken from each plot. Stover was washed with distilled water and rapidly dried with tissue papers before oven-drying at 65°C for 72 h. Then, these samples were finely ground in a mill (IKA Werke, MF 10 Basic, Staufen, Germany). Ground subsamples of known weights were wet-digested in a di-acid mixture (HNO3 : HClO4, ratio 2 : 1; Jones and Case, 1990). Zinc concentration was measured in the digest with atomic absorption spectrophotometry (PerkinElmer, Analyst 100, Waltham, USA).
Phosphorus concentration in grains and shoot digests was determined spectrophotometrically with the vanadate-molybdate yellow color method (Chapman and Pratt, 1961). Phytate from maize grains was extracted with 10 mL of 0.2 N HCl at room temperature after shaking the mixture continuously for 2 h. Phytate in the extract was determined with an indirect method (Haug and Lantzsch, 1983) using a spectrophotometer at a 519 nm wavelength (Shimadzu, UV-1201, Kyoto, Japan; Hussain et al., 2011; Imran et al., 2015).
2.3 Human zinc bioavailability in grains
$$ \eqalign{ & {\rm{TAZ}} = 0.5\ \times \left( \matrix{ {{\rm{A}}_{{\rm{MAX}}}} + {\rm{TDZ}} + {{\rm{K}}_{\rm{R}}} \times \left( {1 + {{{\rm{TDP}}} \over {{{\rm{K}}_{\rm{P}}}}}} \right) - \hfill \cr \sqrt {{{\left( \matrix{ {{\rm{A}}_{{\rm{MAX}}}} + {\rm{TDZ}} + {{\rm{K}}_{\rm{R}}} \times \hfill \cr \left( {1 + {{{\rm{TDP}}} \over {{{\rm{K}}_{\rm{P}}}}}} \right) \hfill \cr} \right)}^2} - 4 \times {{\rm{A}}_{{\rm{MAX}}}} + {\rm{TDZ}}} \hfill \cr} \right), \cr} $$
(1)where AMAX (maximum Zn absorption) = 0.091, KR (equilibrium dissociation constant of zinc-receptor binding reaction) = 0.680, and KP (equilibrium dissociation constant of Zn-phytate binding reaction) = 0.033 related to Zn homeostasis in human intestine (Hambidge et al., 2010). In the model, total daily absorbed Zn (TAZ) (mg Zn d−1) is a function of total daily dietary phytate (TDP; mmol phytate d−1) and total daily dietary Zn (TDZ; mmol Zn d−1).
2.4 Statistical analysis
Analysis of variance (ANOVA) was based on two factorial randomize complete block design and treatment were ranked by Least Significant Difference (LSD) test at P ≤ 0.05 (Steel et al., 1997). Various statistical computations were run on Statistix 9® for Windows (Analytical Software, Tallahassee, USA).
3 Results
3.1 Growth and yield attributes
A significant influence of various fertilization treatments and genotypes was observed for the cob length of maize (Fig. 1A). Cob length ranged from 12.8–23.5 cm in both maize genotypes at different P and Zn rates. Maximum cob length (23.5 cm) was found in Zn+P combination followed by sole Zn application (19.8 cm) in DK-6142. Averaged across different fertilization treatments, maize genotype DK-6142 recorded a higher cob length compared to Neelam.
Grain and straw yield (t ha−1) of maize varied significantly under the influence of various fertilization treatments and genotypes (Fig. 1B, C). Grain and straw yield ranged from 4.23 to 7.4 and from 7.8 to 13.3 t ha−1, respectively, under different fertilization treatments. The maximum grain and straw yields were found with combined of Zn+P. In Neelam, the grain yield was increased by 12, 28, and 42% by application of P, Zn, and Zn+P, respectively, as compared with control. The same trend was found for DK-6142, but the increase was more than for Neelam. However, increase in straw yield was more in Neelam as compared with DK-6142. The Zn+P application resulted in 57 and 53% increase in straw yield of Neelam and DK-6142, respectively (Fig. 1C).
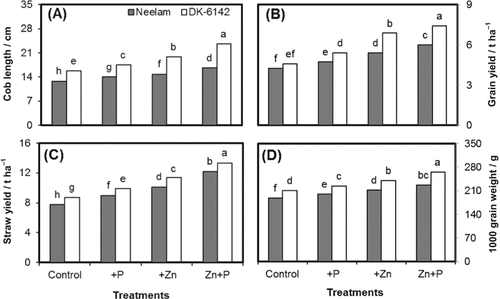
Cob length (A), grain yield (B), straw yield (C), and 1000-grain weight (D) of maize genotypes Neelam and DK-6142 after different Zn and P application. Different letters indicate significant differences by LSD at P ≤ 5%.
The 1000-grain weight showed highly significant effects of genotypes and different fertilization treatments (Fig. 1D). All the fertilization treatments increased the 1000-grain weight as compared to control. The 1000-grain weight was increased by 6.2, 12.76, 20.56% in Neelam and 6.67, 13.95, 26.52% in DK-6142 by application of P, Zn and Zn+P, respectively. Overall, maize genotype DK-6142 had a higher 1000-grain weight in all the treatments than Neelam.
3.2 Zn and P accumulation in plant parts
Significant main and interactive effects of genotypes and different fertilization treatments were observed for P concentration (μg g−1) in grains and stover (Fig. 2A, B). The P concentration in grains ranged between 1616 and 2231 μg g−1 for Neelam and 1876-2371μg g−1 for DK-6142. A stronger increase was found for combined application of Zn+P (2232 and 2371 μg g−1) followed by sole P application (1972 and 2140 μg g−1) in Neelam and DK-6142, respectively. A similar trend was found for P concentration (μg g−1) in stover of both maize genotypes.
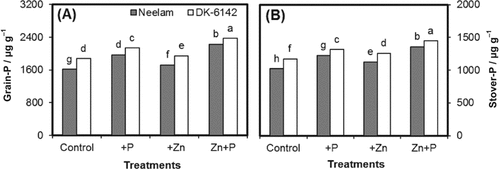
Phosphorus concentrations in grains (A) and stover (B) of maize genotypes Neelam and DK-6142 after different Zn and P application. Different letters indicate significant differences by LSD at P ≤ 5%.
Significant main and interactive effects of genotypes and different fertilization treatments were observed for Zn concentration (μg g−1) of grains and stover of maize. In grain, Zn concentration ranged from 22.48 to 34.56 μg g−1 in Neelam and 25.16–37.26 μg g−1 for DK-6142 (Fig. 3). It was observed that Zn concentrations of maize grains and stover were decreased (about 10%) with increasing level of P rate, but combined application of Zn+P increased the Zn concentration (about 49%) as compared with control (Fig. 3A, B). The same trend was found for the Zn concentration (μg g−1) of stover for both maize genotypes. Addition of single P fertilizer to soil dramatically decreased the tissue Zn contents (4–5%). However, addition of combined Zn+P fertilizer increased the Zn contents by 78 and 87% in Neelam and DK-6142 grains, respectively (Fig. 3C).
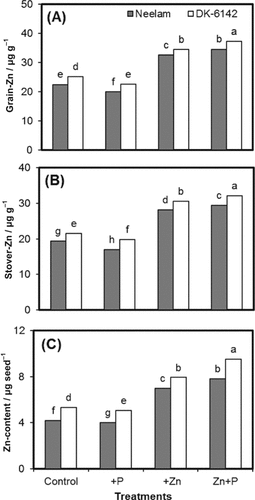
Zn concentrations of grain (A) and stover (B), and Zn content per seed (C) of maize genotypes (Neelam and DK-6142) at different Zn and P rates. Different letters indicate significant differences by LSD at P ≤ 5%.
3.3 Phytate in grains
Significant main and interactive effects of genotypes and different fertilization treatments were observed for phytate concentration (mg g−1) and content (mg seed−1) of maize (Fig. 4A, B). Phytate concentration ranged from 11.8 to 14.8 mg g−1 in Neelam and 13.2 to 16.2 mg g−1 in DK-6142, respectively, under different fertilization treatments. The maximum increase in phytate concentration was found by sole P application. In Neelam, the phytate concentration was increased by 22 and 2.5% for sole P and Zn+P, respectively. The same trend was found for DK-6142 but the increase was more than for Neelam. Reduction (2.5%) in phytate concentration was found by single Zn application in Neelam. Phytate content (mg seed−1) also followed a similar trend as phytate concentration in maize grains (Fig. 4B).
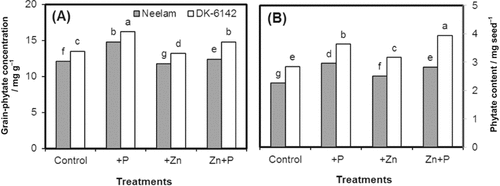
Phytate concentrations of grains (A) and phytate content per seed (B) of maize genotypes Neelam and DK-6142 after different Zn and P application. Different letters indicate significant differences by LSD at P ≤ 5%.
3.4 Estimated human Zn bioavailability in grains
Significant main and interactive effects of genotypes and different fertilization treatments were recorded for the [phytate] : [Zn] ratio and a non-significant interactive effect for the Zn bioavailability over control (Fig. 5A, B). The minimum [phytate] : [Zn] ratio of 35.6 and 39.4 (33 and 26% less than control) in maize grains was recorded with combined Zn+P followed by sole Zn application in the genotypes Neelam and DK-6142, respectively. However, in DK-6142 the maximum reduction in the [phytate] : [Zn] ratio was found by sole application of Zn (29%). The maximum increase in [phytate] : [Zn] ratio was found for single P application in Neelam (38%) and DK-6142 (34%), respectively. Contrary to the [phytate] : [Zn] ratio, the estimated Zn bioavailability was the minimum (1.08 and 1.18 mg Zn for 300 g maize grains) for single P application and maximum (1.66 and 1.77 mg Zn for 300 g maize grains) for combined Zn+P application over control in the genotypes Neelam and DK-6142, respectively. As compared to control, single Zn application also increased the estimated Zn bioavailability by 34 and 24% in Neelam and DK-6142, respectively.
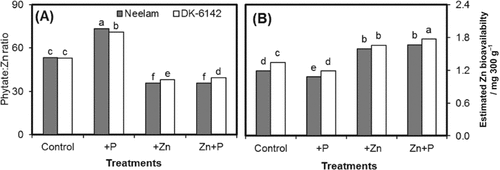
Grain [phytate] : [Zn] ratio (A) and estimated Zn bioavailability (B) in grains of maize genotypes Neelam and DK-6142 after different Zn and P application. Different letters indicate significant differences by LSD at P ≤ 5%.
4 Discussion
Low availability of Zn (< 1.0 mg of AB-DTPA extractable Zn kg−1soil) and P (< 10 mg of Olsen-P kg−1 soil) to plants occurs in calcareous soils because of their high fixation and low organic matter contents (Okalebo et al., 2002; Maqsood et al., 2011; Ahmad et al., 2012; Imran et al., 2015; Table 1). Therefore, application of Zn and P improved plant growth and yield parameters (Sattar et al., 2011; Hussain et al., 2012, 2013; Rehim et al., 2014), which is in agreement with the present study (Fig. 1). In most cases, application of Zn was much more effective in increasing straw and grain yield at higher levels of P application. Effects of nutrient application were much more obvious in grain yield as compared to straw yield. Application of either Zn or P considerably decreased the stress factor of the other nutrient, indicating higher requirement of a nutrient at a higher rates of the other nutrient. Farmers in Pakistan and other developing countries are only using macronutrient (e.g., N, P, and K) fertilizers while ignoring micronutrient application (Hussain et al., 2008; Aziz et al., 2011). The present study clearly indicates that Zn deficiency in maize may cause considerable reduction in grain yield.
Applications of Zn and P to the soil increased their concentrations of grains and stover (Figs. 2 and 3). At crop harvest, the grain concentrations of Zn as well as P were increased by combined application of Zn+P. Reduction in Zn and P concentration in maize grains and stover was observed after the single application of P and Zn to maize genotypes, respectively (Figs. 2 and 3). Relatively greater Zn accumulation in maize grains compared to stover (Fig. 3) is vital for human nutrition (Kanwal et al., 2010; Graham et al., 1992). Reduction in Zn concentration due to P supply may reduce the nutritional quality of foods, which is a major concern (Cakmak, 2002; Verma and Minhas, 1987) because of widespread Zn deficiency in human nutrition (Buerkert et al., 1998).
Single application of Zn caused a significant increase in Zn concentration of maize (Fig. 3). This is in general agreement with Kaya and Higgs (2001) and Montilla et al. (2003). The current results reveal that single P application markedly reduced shoot Zn concentration of both maize genotypes (Fig. 3). Large applications of P fertilizers to soils without Zn fertilization (low in available Zn) can depress tissue Zn concentration (Robson and Pitman, 1983; Gianquinto et al., 2000; Gill et al., 2004). The treatments receiving no P application had the highest Zn concentration in both genotypes, whilst single P application tended to reduce the Zn concentration. These results corroborate earlier findings of Farah and Solimon (1986), Olsen (1972), and Nair and Babu (1975). The decrease in Zn concentration due to P application at 60 kg ha−1 may result from the formation of soluble Zn-P compounds in the soil as reported by Krishnasamy (1993). Similarly, Harrel (2005) reported that P–Zn interactions in corn decreased P concentration and increased Zn bioavailability; they suggested that Zn and P fertilizers should be applied separately to overcome this problem.
A higher Zn content in maize was found after combined application of Zn+P followed by single Zn application in both genotypes (Fig. 3C). High Zn concentration of the grain was due to the genotypes and not tightly linked to agronomic Zn-efficiency traits, and varieties may have to be selected independently to increase the nutritional value of the grain for humans (Graham et al., 1992; Fageria, 2007). Phytate concentration was also decreased by single Zn application (Fig. 4). As most of the P in cereal grains is bound in phytate (an anti-nutritional factor), P concentration is strongly related with grain phytate concentration that reduces the Zn availability to human beings (Erdal et al., 2002; Stangoulis et al., 2007).
Single Zn application reduced the [phytate] : [Zn] ratio in both maize genotypes (Fig. 5A). A low [phytate] : [Zn] ratio might have caused an increase in Zn bioavailability (Fig. 5B). Hence, Zn fertilization together with P application would be considered for better nutritional quality of the products (Gill et al., 2004). The [phytate] : [Zn] ratio has recently been reported as a bioavailability trait in cereal grains and it should be in the range of 15–20 (Joy et al., 2014; Šimić et al., 2012). Single P application results in greater [phytate] : [Zn] ratio (73–71) indicating a Zn-deficiency hazard for plant growth and for human health. The [phytate] : [Zn] ratio in grains was decreased by 25.8–33.2% with single Zn and combined Zn+P application in both maize genotypes.
Similarly, combined Zn+P application followed by single Zn application increased the estimated Zn bioavailability from 1.19 to 1.66 and from 1.34 to 1.77 mg Zn for 300 g in grains of Neelam and DK-6142, respectively (Fig. 5B). The Zn-absorption requirement of an adult human is 3 mg d−1 (Institute of Medicine, 2001), while 300 g maize grains can only ensure about 55–59% of the daily Zn requirement (Fig. 5B).
5 Conclusions
The present study demonstrates that the combined application of Zn+P improved all growth and yield attributes in both maize genotypes compared with their single application. The concentrations of Zn and P were also increased with combined Zn+P application. Single Zn application decreased grain P concentration, phytate concentration, and [phytate] : [Zn] ratio in both genotypes. However, single P application significantly decreased grain Zn concentration and bioavailable Zn in maize grains. The Zn concentration, [phytate] : [Zn] ratio and estimated bioavailable Zn was much dependent on Zn application in both maize genotypes, especially when combined with P application.




