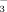Effect of HCO 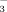 on rice growth and iron uptake under flood irrigation and drip irrigation with plastic film mulch
on rice growth and iron uptake under flood irrigation and drip irrigation with plastic film mulch
Abstract
There has been a partial shift away from conventional flood irrigation (FI) practices for rice (Oryza stativa L.) production in water-scarce northern China. Drip irrigation with plastic film mulch (DI-PFM) can maintain high rice yields with significant water savings. However, rice seedlings often develop chlorosis when grown with DI-PFM on calcareous soil. Bicarbonate is a concern with regard to chlorosis in calcareous soil. The objective of this simulation experiment was to determine the effect of irrigation method and irrigation water HCO
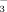 concentration on (1) soil pH and DTPA-Fe concentration, (2) chlorophyll, total Fe, and active Fe concentrations of rice leaves, and (3) rice root and shoot biomass. The experiment consisted of four treatments: FI with water containing either 2 or 10 mM HCO
concentration on (1) soil pH and DTPA-Fe concentration, (2) chlorophyll, total Fe, and active Fe concentrations of rice leaves, and (3) rice root and shoot biomass. The experiment consisted of four treatments: FI with water containing either 2 or 10 mM HCO
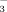 (referred to as FI-2 and FI-10, respectively) and DI-PFM with water containing 2 or 10 mM HCO
(referred to as FI-2 and FI-10, respectively) and DI-PFM with water containing 2 or 10 mM HCO
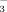 (referred to as DI-2 and DI-10, respectively). The results show that the HCO
(referred to as DI-2 and DI-10, respectively). The results show that the HCO
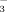 concentrations of the soil solution were greater under FI than under DI-PFM, because more irrigation water was applied in the FI system. Soil pH increased as the HCO
concentrations of the soil solution were greater under FI than under DI-PFM, because more irrigation water was applied in the FI system. Soil pH increased as the HCO
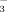 concentration of the irrigation water increased. The increase in soil pH was greater in DI-PFM than in FI. Soil DTPA-Fe concentration, leaf SPAD values, leaf total Fe concentration, leaf active Fe concentration, shoot biomass, and root biomass decreased as the HCO
concentration of the irrigation water increased. The increase in soil pH was greater in DI-PFM than in FI. Soil DTPA-Fe concentration, leaf SPAD values, leaf total Fe concentration, leaf active Fe concentration, shoot biomass, and root biomass decreased as the HCO
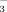 concentration of the irrigation water increased. The decreases were less under DI-PFM than under FI. Overall, the results indicate that rice plants are more sensitive to the HCO
concentration of the irrigation water increased. The decreases were less under DI-PFM than under FI. Overall, the results indicate that rice plants are more sensitive to the HCO
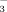 concentration of irrigation water under FI than under DI-PFM.
concentration of irrigation water under FI than under DI-PFM.
1 Introduction
Rice, the staple food for many people in Asia, is generally grown under flooded conditions (Maclean et al., 2002). As much as 43% of the world's irrigation water is used for rice production (Bouman et al., 2007). However, irrigation water is becoming increasingly scarce. Estimates indicate that 15–20 million ha of irrigated rice will suffer some degree of water scarcity by 2025 (Kijne et al., 2003; Tuong and Bouman, 2003). Water shortage has promoted the development of water-saving technologies for rice production, including alternate wetting and drying, non-flooded mulching cultivation, and aerobic cultivation (Belder et al., 2005; Liu et al., 2005; Tao et al., 2006; Belder et al., 2007).
Farmers in the Xinjiang Uyghur Autonomous Region of northwest China recently tried to grow rice using drip irrigation with plastic film mulch (DI-PFM). Rice yields under DI-PFM were as high as 12.0 t ha−2 (Guo and Chen, 2012). Furthermore, DI-PFM reduced the amount of irrigation water by 60% when compared with conventional flood irrigation (FI) (He et al., 2013). Rice grown on calcareous soil often suffers chlorosis. This phenomenon is usually attributed to Fe deficiency (Rutland and Bukovac, 1971; Falade, 1972; Fleming et al., 1984). One explanation is that the redox potential in calcareous soils leads to oxidation of Fe2+ to Fe3+, which is less available to plants. Alternatively, high pH in calcareous soils can cause Fe2+ to precipitate either as FeO or as more complex Fe oxide compounds (Loeppert et al., 1984). A third possibility is that HCO
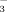 in calcareous soil interferes with plant Fe uptake (Fageria et al., 2011).
in calcareous soil interferes with plant Fe uptake (Fageria et al., 2011).
Bicarbonate in calcareous soils is a key factor causing Fe chlorosis in vine grape (Mengel et al., 1984; Gruber and Kosegarten, 2002), rose and pear (Boxma, 1972), soybean (Coulombe et al., 1984), and peach (Katkat, 2007). Bicarbonate neutralizes H+ released by proton pumps in root plasma membranes (Romera et al., 1991; Toulon et al., 1992), thus, inhibiting Fe translocation to shoots and affecting Fe distribution in leaves (Rutland, 1971; Rutland and Bukovac, 1971; Kosegarten and Koyro, 2001). Many papers have been published about the relationship between HCO
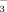 and chlorosis in rice grown under either hydroponic or flooded conditions (Xu et al., 2001; Yang et al., 2003; Kobayashi et al., 2010). However, less is known about the relationship between HCO
and chlorosis in rice grown under either hydroponic or flooded conditions (Xu et al., 2001; Yang et al., 2003; Kobayashi et al., 2010). However, less is known about the relationship between HCO
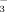 and chlorosis in upland rice, especially drip-irrigated rice. In Xinjiang, farmers observed that under DI-PFM chlorosis was more common when rice was irrigated with well water than with surface water. The HCO
and chlorosis in upland rice, especially drip-irrigated rice. In Xinjiang, farmers observed that under DI-PFM chlorosis was more common when rice was irrigated with well water than with surface water. The HCO
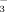 concentration of well water is normally greater than that of surface water. Therefore, we speculated that chlorosis in rice is related to the HCO
concentration of well water is normally greater than that of surface water. Therefore, we speculated that chlorosis in rice is related to the HCO
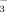 concentration of the irrigation water.
concentration of the irrigation water.
The objective of this simulation experiment was to determine the effect of irrigation method and water HCO
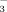 concentration on rice-seedling chlorosis. Specifically, we measured the effects of two irrigation methods (i.e., FI and DI-PFM) and two HCO
concentration on rice-seedling chlorosis. Specifically, we measured the effects of two irrigation methods (i.e., FI and DI-PFM) and two HCO
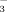 concentrations of the irrigation water (i.e., 2 and 10 mM) on (1) soil pH and DTPA-Fe content, (2) leaf SPAD value, total Fe, and active Fe concentration, and (3) rice root and shoot biomass.
concentrations of the irrigation water (i.e., 2 and 10 mM) on (1) soil pH and DTPA-Fe content, (2) leaf SPAD value, total Fe, and active Fe concentration, and (3) rice root and shoot biomass.
2 Material and methods
2.1 Soil properties
This study was conducted at the Shihezi University Agricultural Experiment Station, Shihezi City, Xinjiang Uyghur Autonomous Region, China (44°18′ N, 86°02′ E) in 2014. Soil was collected from the 0–20 cm depth at the experiment site. The soil, which has a sandy loam texture, is a Calcareous Fluvisol according the FAO-UNESCO classification system (FAO, 1998). Some properties of the soil are as follows: pH 8.0, 19.3 g kg−1 organic matter (Walkley–Black), 63.0 mg kg−1 alkaline hydrolyzable N, 41.9 mg kg−1 available P (Olsen-P), 203 mg kg−1 rapidly available K, 0.26 dS m−1 electrical conductivity, 52.0 mg kg−1 DTPA-Fe, 34.4 mg kg−1 DTPA-Mn, and 84.7 g kg−1 CaCO3. Soil organic matter, alkaline-hydrolyzable N, available P, and rapidly available K were measured using methods described by Page (1982). Soil DTPA-Fe and DTPA-Mn were measured using the methods of Lindsay and Norvell (1978). Soil CaCO3 was measured using the method of Horton and Newsom (1953).
2.2 Experimental design
The experiment consisted of four treatments: FI with water containing either 2 or 10 mM HCO
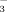 (referred to as FI-2 and FI-10, respectively) and DI-PFM with water containing 2 or 10 mM HCO
(referred to as FI-2 and FI-10, respectively) and DI-PFM with water containing 2 or 10 mM HCO
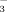 (referred to as DI-2 and DI-10, respectively). The HCO
(referred to as DI-2 and DI-10, respectively). The HCO
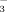 concentration in the irrigation water was adjusted by adding NaHCO3 to well water which had a natural HCO
concentration in the irrigation water was adjusted by adding NaHCO3 to well water which had a natural HCO
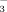 concentration of 2 mM. Each treatment was replicated four times and arranged in a completely randomized block design.
concentration of 2 mM. Each treatment was replicated four times and arranged in a completely randomized block design.
The soil was air-dried, passed through a 4-mm sieve, mixed with N, P, and K fertilizer, and then packed into plastic boxes (50 cm length × 40 cm width × 30 cm height). Each box contained 60 kg soil. Nitrogen fertilizer (urea, 0.20 g N kg−1 soil) was applied in four split applications: 15% at planting, 30% at tillering, 40% at jointing-booting, and the remaining 15% at the panicle stage. The P (triple superphosphate, 0.34 g P kg−1 soil) and K (potassium sulfate, 0.24 g K kg−1 soil, 0.07 g S kg−1 soil) fertilizers were both applied in one application at planting. The fertilizer rates were the same in all treatments and 50% greater than conventional practice because the soil volume in the boxes was limited. After preparing the boxes, enough water was applied to saturate the soil. The soil was allowed to dry naturally for 8 d and then the rice was sown.
Rice (Oryza sativa L. cv. T-43) seeds were pre-germinated by soaking in water for 24 h and then sown in small holes in the soil (12 seeds per hole). Each box contained twenty holes arranged in four rows (i.e., five holes of rice per row). The row spacing was 10 cm and with 10 cm between each hole within a row. The stand was thinned to six plants per hole after emergence.
The soil surface in the FI treatments was covered with 3–5 cm of water during the entire growth season. The bottom of each box had one or two drainage holes to simulate natural seepage at the rate of 3–6 mm d−1. The soil surface in the DI-PFM treatments was covered with transparent plastic film (7 μm thick). Water was added to the soil using an intravenous drip apparatus. The water content in the DI-PFM treatments was maintained at > 90% of the soil water holding capacity, but never flooded. The soil water content in the DI-PFM treatments was monitored by weighing the boxes. Irrigation was applied as needed, usually once every 3 d.
2.3 Soil and plant sampling
Plant and soil samples were collected at the seedling, shooting, and booting stages (32, 76, and 106 d after sowing, respectively). Six hills of rice were excavated from each replication on each sample date. The root ball of three of these hills was washed gently with water to remove the soil. The shoots and roots were then dried in an oven at 80°C and weighed. Rhizosphere soil (0–5 mm from the rice roots) was collected from the other three rice hills, placed in coolers, and returned to the laboratory to determine soil solution HCO
 and soil DTPA-extractable Fe. Leaf total Fe concentrations were determined using dry shoots from three rice hills as described in the following paragraph. Leaf active Fe concentrations were determined using fresh shoots from three rice hills. A SPAD-502 chlorophyll meter (Konica Minolta SPAD-502, Japan) was used to measure the greenness of the 30 uppermost leaves in each box on each sampling date. Plant height was measured at flowering.
and soil DTPA-extractable Fe. Leaf total Fe concentrations were determined using dry shoots from three rice hills as described in the following paragraph. Leaf active Fe concentrations were determined using fresh shoots from three rice hills. A SPAD-502 chlorophyll meter (Konica Minolta SPAD-502, Japan) was used to measure the greenness of the 30 uppermost leaves in each box on each sampling date. Plant height was measured at flowering.
2.4 Chemical analyses
The fresh plant and soil samples were analyzed immediately after collection in order to prevent changes because of drying (Wang et al., 2002). The soil solution was obtained by centrifuging fresh soil samples at 4100 × g using modified centrifuge tubes. Each tube was divided into an upper and a lower chamber by a piece of plastic containing small (1 mm diameter) holes. The upper surface of the plastic divider was covered with two pieces of moist filter paper. Fresh soil was placed on top of the filter paper. As the samples were centrifuged, the soil solution passed through the filter paper into the lower chamber. The filter paper prevented soil particles from moving into the lower chamber during centrifugation. The HCO
 concentration in the irrigation water and the soil solution were determined with 0.02 M HCl using bromophenol blue as an indicator (Page, 1982). Soil pH was measured with a combined electrode pH meter after shaking a 1.0 : 2.5 soil : water suspension for 3 min. Soil DTPA-extractable Fe was extracted with DTPA-TEA (0.005 M diethylene triamine pentaacetic acid + 0.1 M triethanolamine + 0.01 M CaCl2, adjusted to pH 7.3) after shaking a 2 : 1 extractant : soil suspension at 25°C for 2 h (Lindsay and Norvell, 1978).
concentration in the irrigation water and the soil solution were determined with 0.02 M HCl using bromophenol blue as an indicator (Page, 1982). Soil pH was measured with a combined electrode pH meter after shaking a 1.0 : 2.5 soil : water suspension for 3 min. Soil DTPA-extractable Fe was extracted with DTPA-TEA (0.005 M diethylene triamine pentaacetic acid + 0.1 M triethanolamine + 0.01 M CaCl2, adjusted to pH 7.3) after shaking a 2 : 1 extractant : soil suspension at 25°C for 2 h (Lindsay and Norvell, 1978).
To determine leaf total Fe concentrations, oven-dry leaf samples were ground to pass a 0.5-mm sieve. The samples were dry-ashed at 550°C for 12 h. The residues were digested twice with 1 : 3 HNO3 : H2O solution ((v/v)) and then dissolved in 1 : 30 HCl : H2O solution ((v/v)) (Yang et al., 1993). The filtrate solution was analyzed with an atomic absorption spectrophotometer (Hitachi Z-2000, Japan). To determine leaf active Fe concentrations, fresh uppermost leaves were collected from each plant and analyzed using methods described by Takkar and Kaur (1984). Briefly, the leaf samples were washed with running tap water, followed by acidified (0.1 M HCl) detergent solution to remove the contaminants. The samples were rinsed with distilled water and then placed between two sheets of clean filter paper to remove the water. The leaf samples were cut into small (1–2 mm wide) pieces with stainless steel scissors. Subsamples (2 g fresh weight) were transferred into bottles containing 20 mL 1 M HCl. The bottles were shaken for 5 h and then the filtrate solution was analyzed using atomic absorption spectrophotometry.
2.5 Statistical analysis
Statistical analysis was performed with SPSS analytical software (v. 11.0, SPSS Inc., 1996). Multiple comparisons were conducted with SAS 8.0 software, followed by Duncan's test for multiple comparisons.
3 Results
3.1 Effect of bicarbonate on soil pH
Soil solution HCO
 concentrations were significantly affected by irrigation method, HCO
concentrations were significantly affected by irrigation method, HCO
 concentration of the irrigation water and their interaction at all growth stages (Table 1, Fig. 1). Averaging the 2 and 10 mM treatments, HCO
concentration of the irrigation water and their interaction at all growth stages (Table 1, Fig. 1). Averaging the 2 and 10 mM treatments, HCO
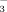 concentrations in the soil solution were significantly greater in FI than in DI-PFM (Table 2). Averaging FI and DI-PFM, HCO
concentrations in the soil solution were significantly greater in FI than in DI-PFM (Table 2). Averaging FI and DI-PFM, HCO
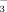 concentrations of the irrigation water were significantly greater in the 10 mM treatments than in the 2 mM treatments (Table 2). Comparing the individual treatments, HCO
concentrations of the irrigation water were significantly greater in the 10 mM treatments than in the 2 mM treatments (Table 2). Comparing the individual treatments, HCO
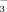 concentrations were significantly greater in FI-10 than in FI-2 at all three growth stages. However, HCO
concentrations were significantly greater in FI-10 than in FI-2 at all three growth stages. However, HCO
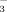 concentrations were significantly greater in DI-10 than DI-2 only at booting (Fig. 1).
concentrations were significantly greater in DI-10 than DI-2 only at booting (Fig. 1).
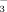 concentration of the irrigation water on soil HCO
concentration of the irrigation water on soil HCO
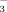 concentration, soil pH, soil DTPA-Fe concentration, leaf SPAD value, leaf active Fe concentration, leaf total Fe concentration, shoot dry weight, and root dry weight at three growth stages.a
concentration, soil pH, soil DTPA-Fe concentration, leaf SPAD value, leaf active Fe concentration, leaf total Fe concentration, shoot dry weight, and root dry weight at three growth stages.a| Growth stage | Source | DF | Soil HCO
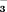 concentration concentration
|
Soil pH | DTPA-Fe | SPAD | Plant leaf active Fe | Plant leaf total Fe | Shoot dry weight | Root dry weight |
|---|---|---|---|---|---|---|---|---|---|---|
| Seedling | Irrigation (I) | 1 | 0.000** | 0.001** | 0.000** | 0.467ns | 0.000** | 0.000** | 0.006** | 0.733ns |
| Bicarbonate (B) | 1 | 0.005** | 0.019* | 0.000** | 0.000** | 0.000** | 0.005** | 0.511ns | 0.762ns | |
| I × B | 1 | 0.014* | 0.100ns | 0.004** | 0.001** | 0.000** | 0.678ns | 0.638ns | 0.520ns | |
| Shooting | Irrigation (I) | 1 | 0.000** | 0.000** | 0.000** | 0.000** | 0.000** | 0.000** | 0.000** | 0.000** |
| Bicarbonate (B) | 1 | 0.000** | 0.000** | 0.001** | 0.480ns | 0.000** | 0.126ns | 0.001** | 0.012* | |
| I × B | 1 | 0.000** | 0.572ns | 0.028* | 0.346ns | 0.004** | 0.283ns | 0.001** | 0.038* | |
| Booting | Irrigation (I) | 1 | 0.000** | 0.000** | 0.000** | 0.006** | 0.000** | 0.631ns | 0.000** | 0.000** |
| Bicarbonate (B) | 1 | 0.000** | 0.000** | 0.000** | 0.001** | 0.000** | 0.000** | 0.000** | 0.000** | |
| I × B | 1 | 0.001** | 0.032* | 0.000** | 0.000** | 0.016* | 0.137ns | 0.001** | 0.000* |
- a*and ** indicate significant differences at P < 5% and P < 1%; “ns” means no significant difference at P > 5%.
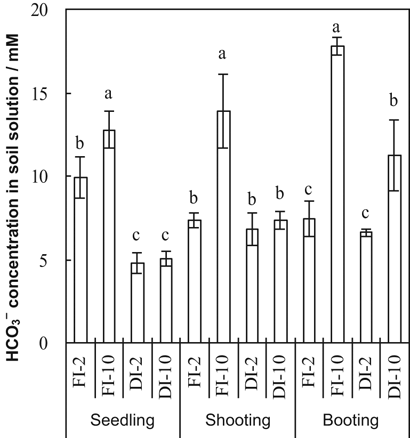
Bicarbonate concentrations of the irrigation water at three rice growth stages as affected by irrigation method and irrigation water HCO
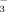 concentration. Error bars represent SE (n = 4). Columns within a growth stage with the same letter are not significantly different at 5% according to Duncan's multiple range test. Abbreviations: FI-2 and FI-10, flood irrigation with water containing 2 or 10 mM HCO
concentration. Error bars represent SE (n = 4). Columns within a growth stage with the same letter are not significantly different at 5% according to Duncan's multiple range test. Abbreviations: FI-2 and FI-10, flood irrigation with water containing 2 or 10 mM HCO
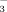 , respectively. DI-2 and DI-10, drip irrigation with water containing 2 or 10 mM HCO
, respectively. DI-2 and DI-10, drip irrigation with water containing 2 or 10 mM HCO
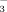 , respectively.
, respectively.
 concentration of the irrigation water on soil HCO
concentration of the irrigation water on soil HCO
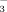 concentration, soil pH, soil DTPA-Fe concentration, leaf SPAD value, leaf active Fe concentration, leaf total Fe concentration, shoot dry weight, and root dry weight of rice at three growth stages.a
concentration, soil pH, soil DTPA-Fe concentration, leaf SPAD value, leaf active Fe concentration, leaf total Fe concentration, shoot dry weight, and root dry weight of rice at three growth stages.a| Growth stage | Source | Soil HCO
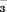 concentration / mM concentration / mM
|
Soil pH | Soil DTPA-Fe concentration / mg kg−1 | SPAD value | Plant leaf active Fe / µg g−1 FW | Plant leaf total Fe / µg g−1 DW | Shoot dry weight / g pot−1 | Root dry weight / g pot−1 |
| Seedling | DI-PFM | 4.90 (B) | 8.28 (A) | 20.7 (B) | 39.2 (A) | 41.6 (B) | 295 (B) | 0.82 (A) | 0.14 (A) |
| FI | 11.34 (A) | 8.04 (B) | 26.8 (A) | 39.0 (A) | 57.1 (A) | 460 (A) | 0.62 (B) | 0.14 (A) | |
| B2 | 7.34 (B) | 8.09 (B) | 25.2 (A) | 40.0 (A) | 57.2 (A) | 400 (A) | 0.74 (A) | 0.14 (A) | |
| B10 | 8.91 (A) | 8.24 (A) | 22.3 (B) | 38.2 (B) | 41.5 (B) | 354 (B) | 0.70 (A) | 0.14 (A) | |
| Critical Range at P = 5% | 1.23 | 0.12 | 1.3 | 1.2 | 8.5 | 28 | 0.12 | 0.03 | |
| Shooting | DI-PFM | 7.10 (B) | 8.43 (A) | 31.0 (B) | 40.6 (B) | 39.0 (B) | 92 (B) | 10.33 (B) | 2.06 (B) |
| FI | 10.64 (A) | 8.12 (B) | 100.0 (A) | 43.5 (A) | 47.5 (A) | 124 (A) | 19.01 (A) | 4.93 (A) | |
| B2 | 7.09 (B) | 8.06 (B) | 69.6 (A) | 42.2 (A) | 46.6 (A) | 113 (A) | 16.09 (A) | 3.82 (A) | |
| B10 | 10.61 (A) | 8.49 (A) | 61.3 (B) | 42.0 (A) | 39.9 (B) | 104 (A) | 13.25 (B) | 3.17 (B) | |
| Critical Range at P = 5% | 2.23 | 0.11 | 4.6 | 0.6 | 3.9 | 11 | 2.04 | 0.55 | |
| Booting | DI-PFM | 8.93 (B) | 8.24 (A) | 31.8 (B) | 38.6 (A) | 31.6 (B) | 100 (A) | 27.13 (B) | 9.49 (B) |
| FI | 12.63 (A) | 8.06 (B) | 96.1 (A) | 37.7 (B) | 40.2 (A) | 98 (A) | 40.17 (A) | 12.49 (A) | |
| B2 | 7.03 (B) | 7.99 (B) | 69.9 (A) | 39.0 (A) | 39.1 (A) | 109 (A) | 38.70 (A) | 12.78 (A) | |
| B10 | 14.53 (A) | 8.28 (A) | 58.0 (B) | 37.2 (B) | 32.7 (B) | 89 (B) | 28.61 (B) | 9.20 (B) | |
| Critical Range at P = 5% | 2.13 | 0.11 | 3.0 | 0.8 | 3.7 | 8 | 4.90 | 2.59 |
-
aWithin a column and within a growth stage, values followed by the same letter are not significantly different according to Duncan's Multiple Range Test. Critical range was calculated with SAS 8.0 software. Abbreviations: DI-PFM, drip irrigation with plastic film mulch; FI, flood irrigation; B2, irrigation water containing 2 mM HCO
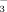 ; B10, irrigation water containing 10 mM HCO
; B10, irrigation water containing 10 mM HCO
 .
.
Soil pH was significantly affected by irrigation method and water HCO
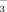 concentration at all growth stages (Table 1, Fig. 2). Averaging the 2 and 10 mM treatments, soil pH was significantly higher in DI-PFM than in FI (Table 2). Averaging DI-PFM and FI, soil pH was significantly higher in the 10 mM treatments than in the 2 mM treatments. Comparing the individual treatments, soil pH was 0.19 to 0.41 units higher in the FI-10 treatments than in the FI-2 treatments (Fig. 2). In comparison, soil pH was 0.1 to 0.5 units higher in the DI-10 treatments than in the DI-2 treatments. Overall, HCO
concentration at all growth stages (Table 1, Fig. 2). Averaging the 2 and 10 mM treatments, soil pH was significantly higher in DI-PFM than in FI (Table 2). Averaging DI-PFM and FI, soil pH was significantly higher in the 10 mM treatments than in the 2 mM treatments. Comparing the individual treatments, soil pH was 0.19 to 0.41 units higher in the FI-10 treatments than in the FI-2 treatments (Fig. 2). In comparison, soil pH was 0.1 to 0.5 units higher in the DI-10 treatments than in the DI-2 treatments. Overall, HCO
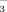 in irrigation water increased soil pH more in DI-PFM than in FI.
in irrigation water increased soil pH more in DI-PFM than in FI.
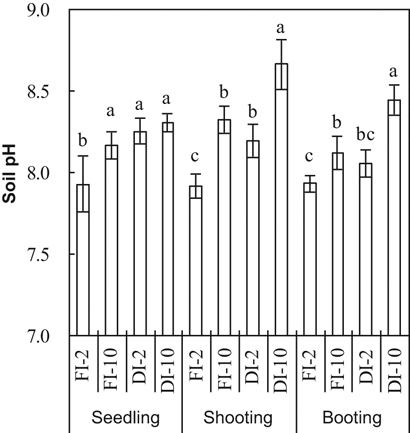
Soil pH at three rice growth stages as affected by irrigation method and HCO
 concentration of the irrigation water. Error bars represent SE (n = 4). Columns within a growth stage with the same letter are not significantly different at 5% according to Duncan's multiple range test. Abbreviations: FI-2 and FI-10, flood irrigation with water containing 2 or 10 mM HCO
concentration of the irrigation water. Error bars represent SE (n = 4). Columns within a growth stage with the same letter are not significantly different at 5% according to Duncan's multiple range test. Abbreviations: FI-2 and FI-10, flood irrigation with water containing 2 or 10 mM HCO
 , respectively. DI-2 and DI-10, drip irrigation with water containing 2 or 10 mM HCO
, respectively. DI-2 and DI-10, drip irrigation with water containing 2 or 10 mM HCO
 , respectively.
, respectively.
3.2 Effect of bicarbonate on soil DTPA-extractable Fe
Soil DTPA-Fe concentrations were significantly affected by irrigation method, HCO
 concentration of the irrigation water, and their interaction at all three growth stages (Table 1, Fig. 3). Soil DTPA-Fe concentrations were greater at the shooting and booting stages than at the seedling stages, especially in the FI treatments. Averaging the 2 and 10 mM treatments, soil DTPA-Fe concentrations were significantly lower in DI-PFM than in FI (Table 2). Averaging DI-PFM and FI, soil DTPA-Fe concentrations were significantly lower in the 10 mM treatments than in the 2 mM treatments. Comparing the individual treatments, soil DTPA-Fe concentrations were significantly lower in FI-10 than in FI-2 at all growth stages (Fig. 3). Soil DTPA-Fe concentrations were significantly less in DI-10 than in DI-2 at both the shooting and the booting stages, but not at the seedling stage. The DI-10 treatment generally had the lowest DTPA-Fe concentration among all the treatments. Although the 10 mM HCO
concentration of the irrigation water, and their interaction at all three growth stages (Table 1, Fig. 3). Soil DTPA-Fe concentrations were greater at the shooting and booting stages than at the seedling stages, especially in the FI treatments. Averaging the 2 and 10 mM treatments, soil DTPA-Fe concentrations were significantly lower in DI-PFM than in FI (Table 2). Averaging DI-PFM and FI, soil DTPA-Fe concentrations were significantly lower in the 10 mM treatments than in the 2 mM treatments. Comparing the individual treatments, soil DTPA-Fe concentrations were significantly lower in FI-10 than in FI-2 at all growth stages (Fig. 3). Soil DTPA-Fe concentrations were significantly less in DI-10 than in DI-2 at both the shooting and the booting stages, but not at the seedling stage. The DI-10 treatment generally had the lowest DTPA-Fe concentration among all the treatments. Although the 10 mM HCO
 treatments reduced DTPA-Fe in both DI-PFM and FI, the decrease was greater in DI-PFM than in FI (e.g., at booting the decrease was 20.7% in DI-PFM vs. 15.8% in FI).
treatments reduced DTPA-Fe in both DI-PFM and FI, the decrease was greater in DI-PFM than in FI (e.g., at booting the decrease was 20.7% in DI-PFM vs. 15.8% in FI).
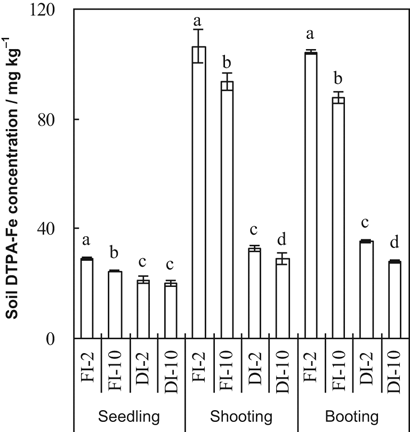
Soil DTPA-Fe concentrations at three rice growth stages as affected by irrigation method and HCO
 concentration of the irrigation water. Error bars represent SE (n = 4). Columns within a growth stage with the same letter are not significantly different at 5% according to Duncan's multiple range test. Abbreviations: FI-2 and FI-10, flood irrigation with water containing 2 or 10 mM HCO
concentration of the irrigation water. Error bars represent SE (n = 4). Columns within a growth stage with the same letter are not significantly different at 5% according to Duncan's multiple range test. Abbreviations: FI-2 and FI-10, flood irrigation with water containing 2 or 10 mM HCO
 , respectively. DI-2 and DI-10, drip irrigation with water containing 2 or 10 mM HCO
, respectively. DI-2 and DI-10, drip irrigation with water containing 2 or 10 mM HCO
 , respectively.
, respectively.
3.3 Effect of bicarbonate on rice leaf SPAD value, available Fe and total Fe
Leaf SPAD values were significantly affected by irrigation method, HCO
 concentration of the irrigation water, and interaction between irrigation method and HCO
concentration of the irrigation water, and interaction between irrigation method and HCO
 concentration of the irrigation water at booting (Table 1, Fig. 4). The effects of these factors were not always significant at the seedling and shooting stages. Averaging the 2 and 10 mM treatments, leaf SPAD values were significantly lower in DI-PFM than in FI at shooting (Table 2). Averaging DI-PFM and FI, leaf SPAD values were significantly lower in the 10 mM treatments than in the 2 mM treatments at the seedling and booting stages (Table 2). Comparing the individual treatments, leaf SPAD values were significantly less in FI-10 than in FI-2 (Fig. 4). In contrast, there was no significant difference in leaf SPAD value between DI-10 and DI-2 (Fig. 4).
concentration of the irrigation water at booting (Table 1, Fig. 4). The effects of these factors were not always significant at the seedling and shooting stages. Averaging the 2 and 10 mM treatments, leaf SPAD values were significantly lower in DI-PFM than in FI at shooting (Table 2). Averaging DI-PFM and FI, leaf SPAD values were significantly lower in the 10 mM treatments than in the 2 mM treatments at the seedling and booting stages (Table 2). Comparing the individual treatments, leaf SPAD values were significantly less in FI-10 than in FI-2 (Fig. 4). In contrast, there was no significant difference in leaf SPAD value between DI-10 and DI-2 (Fig. 4).
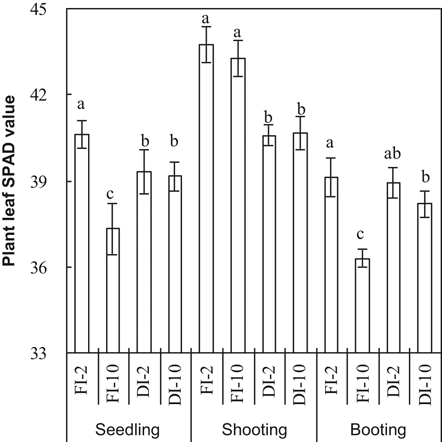
Leaf SPAD values at three growth stages as affected by irrigation method and HCO
 concentration of the irrigation water. Error bars represent SE (n = 4). Columns within a growth stage with the same letter are not significantly different at 5% according to Duncan's multiple range test. Abbreviations: FI-2 and FI-10, flood irrigation with water containing 2 or 10 mM HCO
concentration of the irrigation water. Error bars represent SE (n = 4). Columns within a growth stage with the same letter are not significantly different at 5% according to Duncan's multiple range test. Abbreviations: FI-2 and FI-10, flood irrigation with water containing 2 or 10 mM HCO
 , respectively. DI-2 and DI-10, drip irrigation with water containing 2 or 10 mM HCO
, respectively. DI-2 and DI-10, drip irrigation with water containing 2 or 10 mM HCO
 , respectively.
, respectively.
Active Fe concentrations in rice leaves were significantly affected by the irrigation method, HCO
 concentration of the irrigation water and their interaction at all growth stages (Table 1, Fig. 5a). Averaging the 2 mM and 10 mM treatments, leaf active Fe concentrations were significantly lower in DI-PFM than in FI at all three growth stages (Table 2). Averaging DI-PFM and FI, leaf active Fe concentrations were significantly lower in the 10 mM treatments than in the 2 mM treatments (Table 2). Comparing the individual treatments, active Fe concentrations were significantly lower in FI-10 than in FI-2 at all growth stages. In contrast, there was no significant difference in active Fe between DI-10 and DI-2 at any growth stage.
concentration of the irrigation water and their interaction at all growth stages (Table 1, Fig. 5a). Averaging the 2 mM and 10 mM treatments, leaf active Fe concentrations were significantly lower in DI-PFM than in FI at all three growth stages (Table 2). Averaging DI-PFM and FI, leaf active Fe concentrations were significantly lower in the 10 mM treatments than in the 2 mM treatments (Table 2). Comparing the individual treatments, active Fe concentrations were significantly lower in FI-10 than in FI-2 at all growth stages. In contrast, there was no significant difference in active Fe between DI-10 and DI-2 at any growth stage.
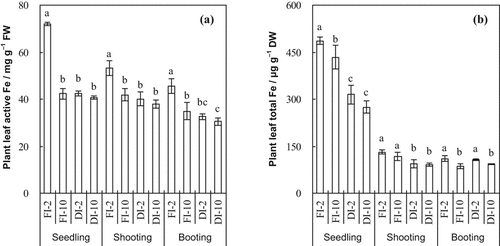
Leaf active Fe and leaf total Fe concentrations at three rice growth stages as affected by irrigation method and HCO
 concentration of the irrigation water. Error bars represent SE (n = 4). Columns within a growth stage with the same letter are not significantly different at 5% according to Duncan's multiple range test. Abbreviations: FI-2 and FI-10, flood irrigation with water containing 2 or 10 mM HCO
concentration of the irrigation water. Error bars represent SE (n = 4). Columns within a growth stage with the same letter are not significantly different at 5% according to Duncan's multiple range test. Abbreviations: FI-2 and FI-10, flood irrigation with water containing 2 or 10 mM HCO
 , respectively. DI-2 and DI-10, drip irrigation with water containing 2 or 10 mM HCO
, respectively. DI-2 and DI-10, drip irrigation with water containing 2 or 10 mM HCO
 , respectively.
, respectively.
Total Fe concentrations in rice leaves were significantly affected by irrigation method at the seedling and shooting stages and by irrigation water HCO
 concentration at the seedling and booting stages (Table 1, Fig. 5b). Averaging the 2 mM and 10 mM treatments, total Fe concentrations were significantly lower in DI-PFM than in FI at the seedling and shooting stages (Table 2). Averaging DI-PFM and FI, leaf total Fe concentrations were significantly lower in the 10 mM treatments than in the 2 mM treatments (Table 2). Comparing the individual treatments, total Fe was significantly lower in FI-10 than in FI-2 at the seedling and booting stages but not at the shooting stage (Fig. 5b). There was no significant difference in total Fe concentrations between DI-10 and DI-2 except at booting. Comparing the individual treatments, total Fe concentrations were 14.48–51.41 units higher in the FI-2 treatments than in the FI-10 treatments (Fig. 2). In comparison, total Fe concentrations were 2.72–40.03 units higher in the DI-2 treatments than in the DI-10 treatments (Fig. 5b). Overall, the HCO
concentration at the seedling and booting stages (Table 1, Fig. 5b). Averaging the 2 mM and 10 mM treatments, total Fe concentrations were significantly lower in DI-PFM than in FI at the seedling and shooting stages (Table 2). Averaging DI-PFM and FI, leaf total Fe concentrations were significantly lower in the 10 mM treatments than in the 2 mM treatments (Table 2). Comparing the individual treatments, total Fe was significantly lower in FI-10 than in FI-2 at the seedling and booting stages but not at the shooting stage (Fig. 5b). There was no significant difference in total Fe concentrations between DI-10 and DI-2 except at booting. Comparing the individual treatments, total Fe concentrations were 14.48–51.41 units higher in the FI-2 treatments than in the FI-10 treatments (Fig. 2). In comparison, total Fe concentrations were 2.72–40.03 units higher in the DI-2 treatments than in the DI-10 treatments (Fig. 5b). Overall, the HCO
 affected total Fe concentrations decreased sharply in FI but not in DI-PFM at all growth stages.
affected total Fe concentrations decreased sharply in FI but not in DI-PFM at all growth stages.
3.4 Effect of bicarbonate on rice biomass and plant height
Shoot and root dry weights were significantly affected by irrigation method, HCO
 concentration of the irrigation water, and their interaction at the shooting and booting stages (Table 1, Fig. 6). At the seedling stage, shoot biomass was significantly greater in DI-PFM than in FI (Table 2). There was no significant difference in root biomass between DI-PFM and FI. At the shooting and booting stages, shoot and root biomass were both significantly lower in DI-PFM than in FI. Shoot and root dry weights were significantly lower in the 10 mM treatments than in the 2 mM treatments at shooting and booting but not at the seedling stage (Table 2). Plant height at flowering decreased in the order FI-2 > FI-10 > DI-2 > DI-10 (Fig. 6).
concentration of the irrigation water, and their interaction at the shooting and booting stages (Table 1, Fig. 6). At the seedling stage, shoot biomass was significantly greater in DI-PFM than in FI (Table 2). There was no significant difference in root biomass between DI-PFM and FI. At the shooting and booting stages, shoot and root biomass were both significantly lower in DI-PFM than in FI. Shoot and root dry weights were significantly lower in the 10 mM treatments than in the 2 mM treatments at shooting and booting but not at the seedling stage (Table 2). Plant height at flowering decreased in the order FI-2 > FI-10 > DI-2 > DI-10 (Fig. 6).
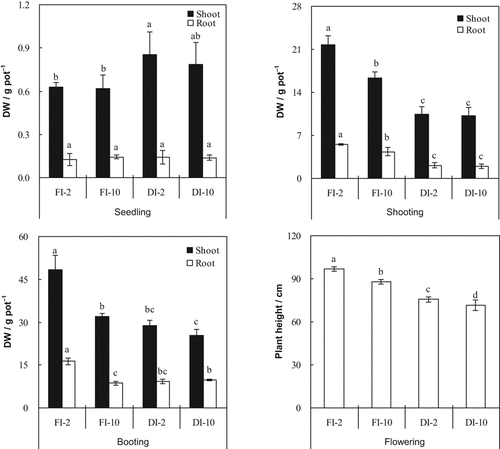
Effects of irrigation method and HCO
 concentration of the irrigation water on (1) rice shoot and root dry weights at three growth stages and (2) plant height at flowering. Error bars represent SE (n = 4). Columns within a growth stage with the same letter are not significantly different at 5% by Duncan's multiple range test. Abbreviations: FI-2 and FI-10, flood irrigation with water containing 2 or 10 mM HCO
concentration of the irrigation water on (1) rice shoot and root dry weights at three growth stages and (2) plant height at flowering. Error bars represent SE (n = 4). Columns within a growth stage with the same letter are not significantly different at 5% by Duncan's multiple range test. Abbreviations: FI-2 and FI-10, flood irrigation with water containing 2 or 10 mM HCO
 , respectively. DI-2 and DI-10, drip irrigation with water containing 2 or 10 mM HCO
, respectively. DI-2 and DI-10, drip irrigation with water containing 2 or 10 mM HCO
 , respectively.
, respectively.
4 Discussion
4.1 Bicarbonate increases soil pH and reduces Fe availability more under DI-PFM than under FI
Our experiment demonstrates that soil HCO
 concentration was greater under FI than under DI-PFM even though the HCO
concentration was greater under FI than under DI-PFM even though the HCO
 concentration of the irrigation water was the same (Fig. 1). More irrigation water was applied in FI than in DI-PFM. This resulted in the accumulation of HCO
concentration of the irrigation water was the same (Fig. 1). More irrigation water was applied in FI than in DI-PFM. This resulted in the accumulation of HCO
 in the soil under FI. Although FI had a greater soil HCO
in the soil under FI. Although FI had a greater soil HCO
 concentration, soil pH was lower in FI than in the corresponding DI-PFM treatment. This contradiction can be explained by differences in soil redox potential. Redox potentials are low under flooded conditions, causing soil pH to shift towards neutral (Larson et al., 1991).
concentration, soil pH was lower in FI than in the corresponding DI-PFM treatment. This contradiction can be explained by differences in soil redox potential. Redox potentials are low under flooded conditions, causing soil pH to shift towards neutral (Larson et al., 1991).
Iron availability is highly affected by soil pH (Lindsay, 1995). Soil DTPA-Fe concentration decreased as the HCO
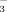 concentration in the irrigation water increased from 2 mM to 10 mM. The decrease was greater in DI-PFM than in FI (e.g., at booting the decrease was 20.7% in DI-PFM vs. 15.8% in FI). Soil DTPA-Fe content was much greater in FI than in DI-PFM, especially at the shooting and booting stages (Table 2). This is probably because anaerobic conditions in FI can promote Fe hydrolysis, resulting in greater Fe availability. The DTPA-Fe concentrations in DI-2 were 32.85 g kg−1 at shooting and 35.42 g kg−1 at booting. These values were enough for the needs of the rice plants. Therefore, the rice did not show any visual (naked-eye) chlorosis.
concentration in the irrigation water increased from 2 mM to 10 mM. The decrease was greater in DI-PFM than in FI (e.g., at booting the decrease was 20.7% in DI-PFM vs. 15.8% in FI). Soil DTPA-Fe content was much greater in FI than in DI-PFM, especially at the shooting and booting stages (Table 2). This is probably because anaerobic conditions in FI can promote Fe hydrolysis, resulting in greater Fe availability. The DTPA-Fe concentrations in DI-2 were 32.85 g kg−1 at shooting and 35.42 g kg−1 at booting. These values were enough for the needs of the rice plants. Therefore, the rice did not show any visual (naked-eye) chlorosis.
4.2 Cool soil temperatures may play a major role in rice Fe deficiency
Soil DTPA-Fe concentrations were much lower at the seedling stage than at either the shooting or booting stage both in DI-PFM and in FI (Fig. 3, Table 2). The lower availability of Fe at the seedling stage may be caused by cool temperatures (Cho and Ponnamperuma, 1971). Yoshida et al. (1996) reported that temperatures of 15 or 17°C significantly reduced rice growth and chlorophyll content. In this study, average air temperatures were lower at the seedling stage (20.5°C) than at the shooting (24.8°C) and booting (26.4°C) stages (Fig. 7). Rice chlorosis often occurs at the seedling stage. The seedling stage was the time when soil DTPA-Fe concentrations were at their lowest during the experiment (Fig. 3), whereas leaf active Fe and total Fe concentrations were at their highest (Fig. 5). The low soil DTPA-Fe concentration is the reason why rice chlorosis occurs during the seedling stage. It also explains why chlorosis is more common in rice irrigated with well water than with surface water. It is because well water is much cooler than surface water (the temperature of well water in our study was about 17°C, compared with > 25°C for surface water). High HCO
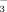 concentrations of the irrigation water reduced soil Fe availability during the seedling stage, especially in FI (Fig. 3). However, the effect of HCO
concentrations of the irrigation water reduced soil Fe availability during the seedling stage, especially in FI (Fig. 3). However, the effect of HCO
 concentration was less than the effect of soil temperature.
concentration was less than the effect of soil temperature.
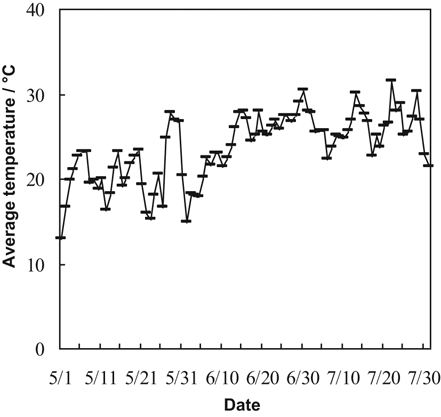
Daily average temperature at the experiment site between May 1 (sowing) and August 1 (harvest).
4.3 Bicarbonate reduced rice Fe uptake and SPAD value more severely under FI
Rice chlorosis is often due to Fe deficiency. In this study, the N, P, and K rates were sufficient for rice nutrition. The soil in this study contains high SO
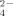 concentrations due to the arid climate. Furthermore, the potassium sulfate fertilizer used in this study contained enough S to meet the needs of the rice plants. Overall, rice chlorosis in this study is most likely due to Fe deficiency.
concentrations due to the arid climate. Furthermore, the potassium sulfate fertilizer used in this study contained enough S to meet the needs of the rice plants. Overall, rice chlorosis in this study is most likely due to Fe deficiency.
Some researchers reported that chlorotic leaves can contain as much or more Fe than green leaves (Römheld, 2000; Gruber and Kosegarten, 2002). This has been referred to as the “chlorosis paradox” (Römheld, 1987). For this reason, it has been suggested that active Fe or available Fe concentrations are more important than total Fe concentrations in plant tissues. Active Fe in plants is considered to be Fe2+ (Clarkson and Hanson, 1980; Katyal and Sharma, 1980; DeKock, 1981; Olsen et al., 1982). In this study, the active Fe concentrations in leaves were significantly greater in FI than in DI-PFM at all three growth stages (Fig. 5). This indicates that aerobic conditions under DI-PFM significantly restricted rice Fe uptake. Aerobic conditions under DI-PFM not only restricted rice Fe2+ uptake but also reduced total Fe concentrations. The HCO
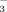 reduced leaf active Fe and total Fe concentrations in both DI-PFM and in FI (Table 2). Thus, no “chlorosis paradox” was observed in this study. Active Fe concentrations were significantly greater in FI-2 than in FI-10 at all three growth stages (Fig. 5a), whereas the difference between DI-2 and DI-10 was relatively small. This indicates that HCO
reduced leaf active Fe and total Fe concentrations in both DI-PFM and in FI (Table 2). Thus, no “chlorosis paradox” was observed in this study. Active Fe concentrations were significantly greater in FI-2 than in FI-10 at all three growth stages (Fig. 5a), whereas the difference between DI-2 and DI-10 was relatively small. This indicates that HCO
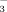 restrained Fe uptake more under FI than under DI-PFM. The reason could be (1) the higher HCO
restrained Fe uptake more under FI than under DI-PFM. The reason could be (1) the higher HCO
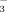 concentration in the soil solution under FI or (2) that higher concentrations of HCO
concentration in the soil solution under FI or (2) that higher concentrations of HCO
 restrained Fe transport from roots to shoots (Fleming et al., 1984; De la Guardia and Alcántara, 2002; Zribi and Gharsalli, 2002).
restrained Fe transport from roots to shoots (Fleming et al., 1984; De la Guardia and Alcántara, 2002; Zribi and Gharsalli, 2002).
Under FI, leaf SPAD values at the seedling and booting stages significantly decreased as the HCO
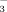 concentration increased. In contrast, irrigation water HCO
concentration increased. In contrast, irrigation water HCO
 concentration had no significant effect on leaf SPAD values under DI-PFM. Zuo et al. (2007) reported that Fe chlorosis was more severe when peanuts were grown on soil with high water content than low water content. Leaf SPAD values were generally less in DI-PFM than in FI (Fig. 4). This may be because the high soil redox potential and soil moisture conditions under DI-PFM restrained rice growth and Fe uptake. The HCO
concentration had no significant effect on leaf SPAD values under DI-PFM. Zuo et al. (2007) reported that Fe chlorosis was more severe when peanuts were grown on soil with high water content than low water content. Leaf SPAD values were generally less in DI-PFM than in FI (Fig. 4). This may be because the high soil redox potential and soil moisture conditions under DI-PFM restrained rice growth and Fe uptake. The HCO
 restrained Fe uptake in DI-PFM and in FI. When HCO
restrained Fe uptake in DI-PFM and in FI. When HCO
 concentrations of the irrigation water increased from 2 to 10 mM, leaf SPAD values decreased more in FI than in DI-PFM. The response of rice to HCO
concentrations of the irrigation water increased from 2 to 10 mM, leaf SPAD values decreased more in FI than in DI-PFM. The response of rice to HCO
 stress may have been less in DI-PFM than in FI, because rice in the DI-PFM treatment was also under stress due to soil redox potential and soil moisture.
stress may have been less in DI-PFM than in FI, because rice in the DI-PFM treatment was also under stress due to soil redox potential and soil moisture.
In general, high HCO
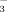 concentration restricted rice growth more under FI than under DI-PFM. Therefore, chlorosis in rice seedlings is primarily due to changes in soil redox potential rather than HCO
concentration restricted rice growth more under FI than under DI-PFM. Therefore, chlorosis in rice seedlings is primarily due to changes in soil redox potential rather than HCO
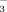 concentration.
concentration.
4.4 Bicarbonate of the irrigation water inhibited rice shoot and root growth
Bicarbonate in the irrigation water inhibited both rice shoot and root growth (Fig. 6, Table 2). This is similar to a report by Gruber and Kosegarten (2002) who observed that calcareous soil reduced the growth of grape vine and induced chlorotic symptoms in young leaves. Rice biomass at the shooting and booting stages was lower in DI-2 than in FI-2, showing that aerobic conditions restricted rice growth and biomass accumulation. In contrast, shoot dry weights at the seedling stage were significantly greater under DI-PFM than under FI. The likely reason is that soil temperatures were higher under DI-PFM, both because of the plastic film and because the soil was drier. Tao et al. (2006) and Zhang et al. (2008) reported similar results. Cool root zone temperatures are known to reduce the dry weights of both rice shoots and roots (Yang et al., 1993). Bicarbonate concentrations of the irrigation water had no significant effect on rice growth at the seedling stage regardless of the irrigation method (Table 2). At shooting and booting, high HCO
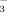 concentrations significantly reduced rice shoot and root biomass in FI but not in DI-PFM (Fig. 6). These data imply that HCO
concentrations significantly reduced rice shoot and root biomass in FI but not in DI-PFM (Fig. 6). These data imply that HCO
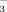 in irrigation water causes greater harm to rice in FI than in DI.
in irrigation water causes greater harm to rice in FI than in DI.
5 Conclusion
Bicarbonate in irrigation water had a negative effect on rice Fe uptake and growth. This was more severe when rice was flood-irrigated rather than drip-irrigated. Drip irrigation with plastic film mulch is an irrigation method/cultivation system helpful to alleviate HCO
 stress. The results indicate that chlorosis in rice seedlings under DI-PFM on calcareous soils maybe due to low temperature or low availability of soil Fe. To prevent chlorosis in rice grown on calcareous soil, it is suggested to irrigate rice seedlings with warm (surface) water.
stress. The results indicate that chlorosis in rice seedlings under DI-PFM on calcareous soils maybe due to low temperature or low availability of soil Fe. To prevent chlorosis in rice grown on calcareous soil, it is suggested to irrigate rice seedlings with warm (surface) water.
Acknowledgements
This study was supported by the National High Technology Research and Development Program of China (2011AA100508), and by the National Science Funds of China (31471947). The authors would like to thank Dr. William Gale for help with the English in this manuscript.