Zinc deficiency alters soybean susceptibility to pathogens and pests†
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
Abstract
Inadequate plant nutrition and biotic stress are key threats to current and future crop yields. Zinc (Zn) deficiency and toxicity in major crop plants have been documented, but there is limited information on how pathogen and pest damage may be affected by differing plant Zn levels. In our study, we used soybean plants as a host, a soybean pest, and three soybean pathogens to determine whether plant Zn levels change pest and disease assessments. Two soybean cultivars were grown in sand culture with a soluble nutrient solution that ranged from Zn-deficient to toxic. Detached leaves from these plants were either inoculated with Aphis glycines, the soybean aphid, Xanthomonas axonopodis pv. glycines, a bacterium that causes bacterial pustule, Sclerotinia sclerotiorum, the necrotrophic fungus responsible for stem rot, or Phakopsora pachyrhizi, a biotrophic obligate pathogen that causes soybean rust. There were significant (P < 5%) effects on aphid colonization, positive counts for bacterial pustule, S. sclerotiorum leaf area affected, and numbers of rust lesions associated with the Zn treatments. Plants grown with the physiologically optimal levels of Zn (2 µM) had less (P < 5%) soybean aphids cm−2 leaflet than plants grown without Zn, at 0.1× Zn (0.2 µM), or at 100× Zn fertilization (200 µM). Plants grown with the normal fertilization of Zn or 100× Zn had fewer (P < 5%) positive counts for bacterial pustule and less lesion area affected by S. sclerotiorum than plants grown without Zn or fertilized with 0.1× Zn. For soybean rust, plants grown with the physiologically optimal fertilization of Zn or 100× Zn had higher (P < 5%) lesions cm−2 on leaflets from plants grown without Zn or fertilized with 0.1× Zn. These results indicate different Zn nutrition levels in soybean significantly affected aphid and disease development.
1 Introduction
The world area harvested in soybean was over 11.2 billion ha in 2013 just behind wheat, maize, and rice (FAO, 2014). Despite the considerable importance of soybean and a sizeable body of literature documenting the potential effects of Zn on biotic stress severity in other plants (Graham and Webb, 1991; Dordas, 2008; Huber et al., 2012), literature on Zn effects on altering soybean susceptibility to diseases and pests is limited. Zn plays an important part in the plant–pathogen–nutrient triangle, as both plants and their pathogens need certain concentrations of Zn for their metabolism (Marschner, 1995a). In plants, Zn serves as an integral component of thousands of plant enzymes as well as plant auxins and is involved in enzyme activation, membrane integrity, and binding of radicals (Broadley et al., 2007). Furthermore, the metal defense hypothesis postulates that hyper-accumulation of Zn and other metals serves as a self-defense mechanism against pathogens and pests (Poschenrieder et al., 2006). The metal defense hypothesis has not been tested for soybean.
There has been little controlled research evaluating the effect of Zn on diseases and pests in soybean. Soybean field experiments conducted in India showed that Zn fertilizers decreased disease severity of charcoal rot (Macrophomina phaseolina; Latha et al., 1997) and blight caused by Sclerotium rolfsii (Deb and Dutta, 1992). While these studies provided valuable practical knowledge for local farmers, field experiments allow only limited control of nutrients and results across environments and years are often inconsistent. A soil-pot study on aerial blight (caused by Rhizoctonia solani) concluded that higher leaf Zn concentrations increased disease severity (Silva et al., 2012). While Silva et al. (2012) measured Zn leaf contents, they did not study how different Zn treatments affected the concentrations of other nutrients such as N, P, and K. Zinc has interactions with other plant nutrients (Loneragan and Webb, 1993), and nutrient interactions are a key in understanding other plant–biotic stress–nutrient relationships (Walter and DiFonzo, 2007).
Increasing Zn nutrition has increased biotic stress severity in some crops, but decreased biotic stress severity in other crops (Huber et al., 2012). Due to the wide range in methods (field trials to hydroponics) and limited or incomplete characterization of plant nutritional status, it is difficult to be confident if differential results are due to different plant–pathogen–Zn interactions or if they are due to different experimental methodologies or environmental conditions. Beanland et al. (2003) grew soybeans under various amounts of B, Fe, and Zn, and found that the growth and development of three soybean arthropod herbivores (Ancarsia gemmatalis, Epilachna varivestis, and Pseudoplusia includens) increased in plants deficient in B, whereas the effect on Zn levels on the insects depended on the ratio between its level with iron and boron levels (Beanland et al., 2003). Toxic levels of Zn and other nutrients were not studied.
The main objective of this study was to determine the effects of different levels of Zn, applied in nutrient solutions to soybean host plants, on the development of plant damage caused by three important soybean pathogens and one pest. Soybean plants were grown without Zn (–Zn), 0.1× Zn, the full recommended Zn concentration (Zn), or 100× Zn levels, and these plants were assayed for changes in susceptibility to the soybean aphid (Aphis glycines Matsumura), a bacterium [Xanthomonas axonopodis pv. glycines (Nakano) Vauterun et al. 1995 that causes bacterial pustule, a necrotrophic fungus [Sclerotinia sclerotiorum (Lib.) de Bary] that causes stem rot, and a biotrophic obligate fungal pathogen (Phakopsora pachyrhizi Syd.) that causes soybean rust disease (Hartman et al., 2015). To our knowledge, none of these organisms or the diseases or plant damage they cause has been studied in relation to Zn and soybeans. These organisms were selected because of their importance to soybean production, their different modes of obtaining nutrition for growth, and because they are easy to manipulate, inoculate, and assess non-destructively on detached soybean leaves.
Additionally, we developed a biomass growth response curve (Marschner, 1995b) to characterize the nutrient status of the plants and to assess the whole-plant responses to the treatments. Leaf Zn contents and contents of the macro- and micronutrients N, P, K, Ca, Mg, S, Mn, Fe, Cu, and B were measured. The concentrations of each nutrient in each of the Zn treatments were analyzed.
2 Material and methods
2.1 Plant growth conditions
Plants were grown in sand culture in 16.5 cm diameter pots filled with 2.75 kg of coarse silica sand (size 0.60–2.38 mm: Best Sand Corporation, Chardon, USA) in a temperature-controlled greenhouse from September to December 2014. Cotton cheesecloth was placed on the bottom of each pot and replaced regularly to prevent sand from falling through. Day temperatures were held at 23–27°C, night temperatures at 18–22.5°C, and supplementary illumination, provided by a mixture of high pressure sodium vapor and metal halide lamps, was set to provide a 14 h photoperiod. Due to the low water-retaining capacity of the sand, the plants were irrigated two to three times daily until sand was saturated and excess solution dripped from the container. For the first week during seed germination, the plants were irrigated with distilled water and subsequently with treatment-specific Hoagland's solutions with formulations adapted for this study.
As a control, we used Hoagland's solution for soybeans consisting of 0.5 mM KH2PO4, 5 mM KNO3, 5 mM Ca(NO3)2, 2 mM MgSO4, 0.1 mM C10H12N2O8FeNa, 0.05 mM KCl, 0.025 mM H3BO3, 0.002 mM MnSO4, 0.002 mM ZnSO4, 0.0005 mM CuSO4, and 0.0005 mM Na2MoO4 (Pfahler et al., 2013). To minimize mineral contamination, only plastic utensils were used to prepare and store the solutions. All utensils were washed thoroughly, soaked in 10% HNO3, and rinsed twice with deionized water (Impa et al., 2013). Concentrations of zinc sulfate for the predetermined levels of Zn tested were 0 µM for the –Zn treatment, 0.2 µM for 0.1× Zn, 2 µM for Zn standard ZnSO4, and 200 µM 100× Zn treatment.
2.2 Experimental design and sampling of plant material
We used two soybean cultivars (Glycine max L. cvs. Alpha and Fairbault), of which neither has a known resistance to any of our test pathogens or pest or nutrient tolerances (Orf and MacDonald, 1994, 1995). The soybean host plants were arranged on a greenhouse bench in a randomized complete block design (RCBD) with four blocks. Each block had all eight factorial treatment combinations, with two cultivars for the cultivar factor, and the four different Zn concentrations as the Zn treatment factor. The experimental unit was one pot of two plants of a single cultivar with a single Zn treatment applied. Soybean seeds were surface-disinfected (Kremer et al., 2005), and five seeds were planted per pot, and the plants were thinned to the two most vigorous plants 14 d after planting (DAP). The whole experiment—plant growth measurements, detached leaf assays, and plant nutrient analyses—was repeated once using a different randomization of host plants and Zn treatments in each trial.
Following 34 DAP when the plants reached growth stage R1 (first observed flower), one plant per pot was selected at random and the leaflets from the youngest (topmost) fully expanded trifoliolates were detached with a fresh razor blade and immediately placed on agar media for the pathogen and pest assays. The rest of the aboveground biomass from the selected plant was dried at 70°C for 3 d (Yamagishi and Yamamoto, 1994), weighed, and the leaves and petioles were ground for analysis of nutrients using ICP-MS AOAC 985.01 (A&L Great Lakes Laboratories, Fort Wayne, IN).
2.3 Detached leaf experimental setup
The aphid, bacterial pustule, and Sclerotinia stem rot detached-leaf assays were setup in a RCBD. For all three tests, soybean leaflets, detached from each plant, were placed adaxial side up in a 9-cm petri dish filled with 20–25 mL 1% water agar (Bacto Agar; Becton, Dickinson and Co., Sparks, MD 21152) amended with 2 mL 6-benzylaminopurine (BAP) solution (1 mg mL−1 pre-dissolved; Sigma-Aldrich Co., St. Louis, MO), applied after cooling the medium to 45–50°C following autoclaving. Each assay was repeated once using a different randomization of treatment combinations. Soybean aphid population (Michel et al., 2010), area of S. sclerotiorum infection (Valdés-López et al., 2011), and rust lesions per cm2 (Twizeyimana and Hartman, 2010) were evaluated using pre-established methods. No assay for evaluating bacterial pustule infection on detached soybean leaves has been previously reported.
The soybean rust assay was set up as a split-plot design with four blocks. The main plots were the two cultivars and the sub-plots were the four Zn treatments. Susceptible and resistant check cultivars were added to each main plot. Four leaflets of either soybean Alpha or Fairbault, detached from plants with each of the four Zn treatments and from one block, and one leaflet of Williams 82 (rust-susceptible soybean) and UG5 (rust-resistant soybean), for a total of six leaflets, were placed abaxial side up on sterile paper towels moistened with sterile water inside plastic clamshell boxes (22 cm × 12 cm × 3.5 cm; Andex, Escamba, MI, USA).
2.4 Aphid assay
To ensure the validity of the test, the soybean breeding line LD10-30014 (acquired from Dr. Brian Diers, University of Illinois) with Rag1 and Rag2 aphid-resistance genes in a pyramid, and the susceptible cultivar Williams 82 were used as controls (Hill et al., 2009; Michel et al., 2010). Ten viviparous apterous adult soybean aphids were placed on each leaflet and the dishes were incubated at 22°C and a photoperiod of 16 h in a plant tissue-culture chamber (Percival, TC-2). The dependent variable was the population size at day 12 divided by the leaf area to account for different leaf sizes (Michel et al., 2010).
2.5 Bacterial pustule assay
Leaflets were placed on BAP-amended media as described above. The resistant and susceptible cultivars used in the bacterial pustule assay were Williams 82 and Spencer, respectively. For inoculation, each leaflet was wounded with a 22 mm diameter flower frog, which made 36 wounds in a circular pattern, just prior to dropping 1 mL of 108 bacterial cells mL−1 suspension (based on optical density) onto the leaf surface, fully covering the wounds (Goradia et al., 2009; Groth and Braun, 1986). After inoculation, the dishes were incubated at 25°C and a photoperiod of 16 h for 15 d in an incubator (Percival, I-35VL). The dependent variable was the number of wounds with visible pustule signs and symptoms that included water soaking and bacterial ooze around the wound site.
2.6 Sclerotinia stem rot assay
Only a susceptible control, Williams 82, was included in the Sclerotinia stem rot experiment, since no completely resistant soybean genotypes are known (Hartman et al., 2015). A 4 mm diameter mycelial plug was taken from the growing margin of a culture of S. sclerotiorum growing on potato dextrose agar (PDA) and placed mycelial side down in the center of each detached leaflet (Valdés-López et al., 2011). The petri dishes were sealed with parafilm and placed in a tissue chamber at 22°C with a photoperiod of 16 h for 4 d (Percival, TC-2). The affected area (cm2) was assessed by measuring the major and minor axes of each lesion, which served as the dependent variable for this assay.
2.7 Soybean rust assay
For the rust assay, susceptible (Williams 82) and resistant (UG5) cultivar checks were included to ensure the validity of the test as described above. The leaflets were inoculated by thoroughly spraying them with an un-quantified urediniospore water suspension amended with Tween 20 (0.01%). A blank PDA plate was also sprayed to monitor urediniospore viability. There were 8 and 16 germinating spores per cm2 in the first and second trials, respectively. The clamshell, boxes containing the inoculated leaflets, were stored in the dark for 24 h, then kept at 22°C and a photoperiod of 12 h in a plant tissue culture chamber (Conviron, TC 16). After 14 d following inoculation, the soybean rust lesions that developed were counted at 25× magnification under a compound microscope.
2.8 Statistical analyses
Statistical analyses were performed in R (R Core Team, 2014) and JMP 11 (SAS Institute, Cary, NC). The susceptible and resistant cultivar checks for the bioassays were included in statistical analysis, but since these leaves had a different age, growing environment, and were not treated with the Zn treatments, their means were only used for frame of reference with the Zn treatments and were not included in mean separation. Data for measurements were transformed by adding 1 before taking the log to base 10 to correct for heterogeneity of variance among the trials. Bartlett's test for variance homogeneity was performed using JMP Fit Y by X platform on the transformed data to test the homogeneity of variance between the trials of each biotic stress assay, to determine if the data sets from each trial could appropriately be combined for analysis. Then, a full-factorial analysis of variance model (ANOVA) was fitted using the JMP Fit Model platform to analyze the fixed effects of the cultivars, treatments, and blocks for aboveground dry biomass, aphid population cm−2, number of bacterial infections, area of S. sclerotiorum infection, lesions cm−2 for rust, and Zn concentration of the leaves. Mass and Zn concentration of leaves were also log10-transformed to correct for non-constant variance found among the treatments for the untransformed data prior to analysis.
A restricted maximum likelihood analysis (REML) was performed using the JMP Fit Model platform for number of soybean rust lesions using a split-plot model, with main plots the cultivars placed in separate boxes and sub-plots— Zn treatments—as fixed effects. Blocks were random effects. Cultivars × blocks were also used as a source of variation in the REML analysis. Since blocks were random effects, their effect and the cultivar × blocks effect were not reported in the results of the REML analysis. Post hoc, means of the Zn treatments for all tests were separated by Fisher's least significant difference test (P < 5%).
Asymptotic or polynomial regression was performed to create trend lines for nutrient interactions. Third-order polynomial regressions were conducted and reduced to second order if the cubic term did not prove significant.
3 Results
3.1 Aphid bioassay
Significant differences were found between the Zn treatments. The treatment without Zn (–Zn) had an aphid population of 11 aphids cm−2, which was significantly higher than the Zn control treatment that had 8 aphids cm−2 (Table 1, Fig. 1a). The populations on the 0.1× Zn and 100× Zn treatments did not significantly differ from the Zn control, each treatment having 9 aphids cm−2. Williams 82, the aphid-susceptible check, had an average aphid density of 8 aphids cm−2, whereas LD10-30014, the resistant check, had 6 aphids cm−2 (Fig. 1a). The cultivar and cultivar × Zn treatment effects were non-significant.
| Sourceb | DF | Plant biomassc | Aphid counts | Bacterial counts | Area of necrosis | Number of Lesionsd |
|---|---|---|---|---|---|---|
| Block | 7 | 0.81 | 1.6 | 0.85 | 14*** | 0.56 |
| Cultivar | 1 | 12.4*** | 0.4 | 0.33 | 3.4 | 2.39 |
| Zn treatment | 3 | 45.4*** | 3.7* | 13.6*** | 4.5** | 3.09* |
| Cv × Zn treatment | 3 | 0.15 | 0.6 | 1.47 | 0.7 | 2.51 |
| Error γ | 48 |
- a*P < 5%, **P < 1%, and ***P < 0.1%.
- bBlock = randomized complete block design with four replications in each of two trial; Cultivar = Alpha and Fairbault; Zn treatment = –Zn, 0.1× Zn, Zn (physiologically optimal concentration), and 100× Zn; Error γ = degrees of freedom was 48 for each analysis the last which was 37 due to missing data.
- cDried whole-plant biomass mass at first flower, which coincided with the detached leaf assays.
- dRestricted maximum likelihood analysis (REML) was performed using a split-plot model, with main plots the cultivars placed in separate boxes and sub-plots—Zn treatments—as fixed effects.
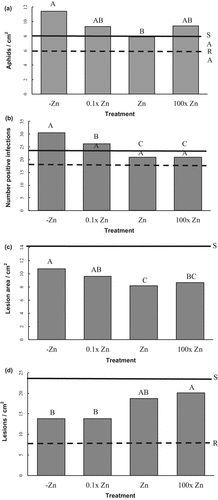
Effects of four Zn treatments on (a) soybean aphid density 12 d after inoculation; (b) number of wounds out of 36 with signs and symptoms of bacterial pustule 15 d after inoculation; (c) Lesion area caused by Sclerotinia sclerotiorum 4 d after inoculation; and (d) number of soybean rust pustules 14 d after inoculation. All bioassays were carried out on detached leaflets of soybean (growth stage R1, first flower); the figure combines results from two soybean cultivars. Means followed by different letters are significantly different according to Fischer's Least Significant Difference test (P = 5%). Horizontal lines indicate the mean values of the resistant (R) and susceptible (S) cultivar checks for frame of reference on the bioassay.
3.2 Bacterial pustule bioassay
Highly significant differences were found between Zn treatments (Table 1). The –Zn treatment had 30 out of 36 wounds which counted as positively infected and the 0.1× Zn treatment had 26 out of 36 infected wounds, which was significantly higher than the Zn control and 100× Zn treatments, both with 21 out of 36 infected wounds (Fig. 1b). The resistant check Williams 82 had 17 wounds that were positively infected, compared with 22 wounds on the susceptible Spencer (Fig. 1b). The cultivar and cultivar × Zn treatment effects were non-significant.
3.3 Sclerotinia stem rot bioassay
There were highly significant differences among the Zn treatments for the S. sclerotiorum infection assay (Table 1). The –Zn treatment had a significantly larger necrotic lesion (10.7 cm2) than the Zn control (8.2 cm2). The 0.1× Zn treatment (9.6 cm2) and 100× Zn (8.7 cm2) treatment did not significantly differ from the Zn control for area of necrosis (Fig. 1c). The necrotic leaf area produced by S. sclerotiorum infection on the susceptible cultivar Williams 82 averaged 14.0 cm2. The cultivar and cultivar × Zn treatment effects were non-significant.
3.4 Soybean rust bioassay
All rust lesions that developed on soybean Alpha and Fairbault were TAN-infection type (Twizeyimana and Hartman, 2010). There were significantly higher numbers of lesions produced on leaflets from plants treated with 100× Zn (20 lesions cm−2) than the –Zn (14 lesions cm−2) or 0.1× Zn (14 lesions cm−2) treatments, but not significantly higher than the Zn treatment (19 lesions cm−2). The mean number of lesions on resistant UG5 was 8 lesions cm−2, which were all RB infection type, while susceptible Williams 82 had 24 lesions, which were all TAN-infection type (Fig. 1d). The cultivar and cultivar × Zn treatment effects were non-significant.
3.5 Biomass and Zn concentration
Both cultivar and treatment main effects were highly significant for the plant dry biomass (Table 1). Treatment means fit a theoretical growth response curve with lower biomass production in the –Zn, 0.1× Zn, and 100× Zn treatments compared to the optimal Zn treatment (Fig. 2). The mass of plants in the 0.1× Zn (6 g) and 100× (6 g) Zn treatments were not significantly different. The biomass of the –Zn treatment (5 g) was significantly lower than the Zn control (9 g). Soybean Alpha (7 g) had a significantly higher biomass than Fairbault (6 g).
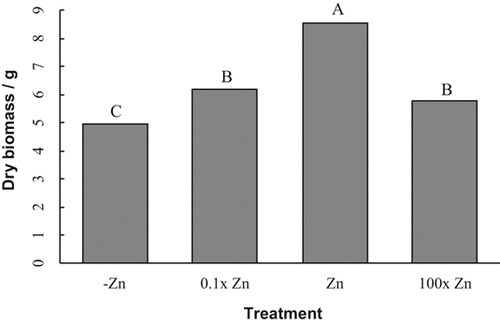
Dried aboveground biomass of soybean plants at the growth stage R1 (first flower) in response to four Zn treatments. Means followed by different letters are significantly different based on Fischer's Least Significant Difference test (P = 5%). The figure combines results from two cultivars.
There were highly significant differences for Zn concentrations among Zn treatments. Zinc concentration was 9, 11, 37, and 1050 mg kg−1 for the –Zn, 0.1× Zn, Zn, and 100× Zn treatments, respectively. Zn concentrations in all of the Zn treatments were significantly different from each other (Fig. 3).
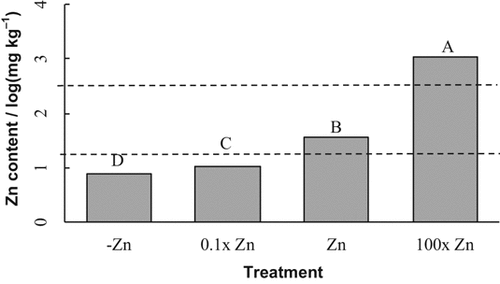
5%). The figure combines results from two soybean cultivars.
3.6 Nutrient interactions
Zinc treatments affected the leaf concentrations of other nutrients analyzed (Fig. 4). Increasing leaf Zn concentrations resulted in higher leaf N, K, S, and B concentrations and decreasing P, Mg, and Fe concentrations. A parabolic relationship between Zn and Ca, Mn, and Cu was found with the lowest concentrations of those nutrients in leaves with moderate Zn levels and higher concentrations in leaves with low or high Zn levels. Concentrations of other nutrients (Cl, Mo, and Ni) were below the detection limit for all of the treatments.

Interaction of Zn with other nutrients in R1 growth stage soybean leaves. Values are from two trials; each point is one measurement. The x-axis is the Zn concentration in log10 of mg kg−1; the y-axis is the nutrient concentration in percent dry weight for the macronutrients N, P, K, Ca, Mg, and S and in mg kg−1 for Mn, Fe, Cu and B. The R2 value is only provided for polynomial regressions. The figure combines results from two soybean cultivars.
4 Discussion
The metal-defense hypothesis postulates that certain plant species hyper-accumulate metals as a self-defense strategy to deter pathogen attack (Poschenrieder et al., 2006). Leaf Zn concentrations for the 100 × Zn treatment averaged 1063 mg kg−1 and were well above the accepted toxic threshold of 300 mg kg−1 (Marschner, 1995a). However, the 100 × Zn treatment did not increase resistance, relative to the physiologically optimal Zn level, to soybean aphids, bacterial pustule, or S. sclerotiorum. For soybean rust, the 100 × Zn treatment produced a higher level of susceptibility than the physiologically optimal Zn control, which was opposite to the expected trend of greater resistance predicted by the metal-defense hypothesis. This result is consistent with the results found in an earlier study of the effects of nutrients on sugar cane rust severity, which reported a similar relationship with rust severity increasing as Zn concentration increased (Anderson and Dean, 1986). Soybean rust and aerial blight, found to increase in severity with increasing Zn, belong to the same class of fungi—the Basidiomycetes (Silva et al., 2012). However, drawing general conclusions on the relationship between Zn nutrition and fungal pathogen classification will require further research.
In contrast to the results found with soybean rust, leaflets from plants in the –Zn treatment had higher populations of aphids, higher numbers of wounds infected with bacterial pustule, and larger areas of necrosis caused by S. sclerotiorum compared with the physiologically optimal Zn treatment. Similar contrasting results between pathogens in other host-pathogen systems from field and soil pot trials have been reported (Deb and Dutta, 1992; Latha et al., 1997; Silva et al., 2012).
A non-destructive assay was necessary to measure the effects of the Zn treatments on biotic stress organisms to allow the test plants to mature for measurement of whole-plant mineral composition. Use of detached leaf assays served this purpose in this study. Limitations on the use of detached leaves for measuring resistance to fungal pathogens have long been known (Hare, 1966) and should be considered before use. Detached-leaf assays have previously been used successfully to measure soybean rust resistance traits (Twizeyimana and Hartman, 2010) and infection by S. sclerotiorum (Kim et al., 2000). In contrast, resistance to soybean aphids is not maintained in detached leaves of some soybean genotypes (Michel et al., 2010). However, the soybean cultivars Alpha and Fairbault which were treated with Zn were susceptible to soybean aphids, and therefore loss of resistance was not an issue in this study. There are no reports on the use of detached leaves to measure resistance to bacterial pustule in soybean; however, there was no obvious influence of the use of this technique on the results in this study.
While our study does not reveal mechanisms behind the observed Zn biotic stress severity relationships, it underlines the importance of considering indirect effects of Zn nutrition, i.e., Zn treatment effects on concentrations of other nutrients. Various plant stress-response systems are influenced by Zn directly, and it is likely that these Zn functions explain pathogen and pest severity. For example, Zn is an important inhibitor of the reactive oxygen species (ROS) generating NADPH odixdase. Stress response includes increased ROS production, which leads to excessive cell damage, if regulatory mechanisms, foremost superoxide dismutases, are defective due to Zn deficiency (Alscher et al., 2002). This mechanism was shown to cause leaf chlorosis under abiotic stress exposure such as high light intensity (Cakmak, 2000). Also, Zn-finger proteins are involved in the production of small RNAs, crucial in plant biotic stress response (Ruiz-Ferrer and Voinnet, 2009).
Aside from direct effects of Zn in plant response mechanisms, it is also likely that interactions of Zn with other nutrients may explain disease responses. Interactions between Zn and other nutrients may arise through substitution effects, cation-inhibition effects, or Zn's role in membrane permeability (Graham et al., 1987; Marschner, 1995a). The Zn treatments in our experiments significantly affected N, P, K, Ca, Mg, S, Mn, Fe, Cu, and B leaf concentrations. Plants under low Zn treatments had lower leaf concentrations of N, K, S, and B, but higher concentrations of P, Mg, Mn, Fe, and Cu than the normal treatment. For example, N concentration was around 3% of leaf mass for –Zn treatment but around 4.3% for plants not deficient in Zn, suggesting a much higher protein concentration in healthy plants (Fig. 4). For herbivores, which are often N-limited, higher plant N concentration is often associated with more severe pest attacks (Veresoglou et al., 2013). However, in this study aphid population density showed a negative correlation with leaf N concentration.
In another study, increased aphid abundance on soybeans deficient in K was attributed to K's effect on phloem nitrogen (Walter and DiFonzo, 2007). It is thus conceivable that Zn's interactions with other nutrients play an important role in unlocking the mechanisms behind Zn's role in plant defense. Overall, direct effects of Zn on biotic stress resistance may be coupled with or intensified by the abnormal accumulation or deficiency of other nutrients. For example, ROS scavenging, impaired under low Zn conditions, is exacerbated by inherently higher Fe concentrations as Fe potentiates the production of free radicals (Cakmak, 2000).
While soybean displays considerable breadth in genotype responses to Zn treatments (White et al., 1979), we did not find any significant cultivar × treatment effect (Table 1). Thus, the size of cultivar biomass was not dependent on Zn treatment in this study. Over all the Zn treatments, cultivar Alpha had larger biomass than Fairbault and this was consistent across all treatments.
5 Conclusions
In this study, we provide the first evidence of Zn effects on a herbivore (soybean aphid) and three important soybean pathogens that cause bacterial pustule, Sclerotinia stem rot, and soybean rust. Soybean leaflets deficient in Zn supported significantly more soybean aphids and increases in disease severity of bacterial pustule and Sclerotinia stem rot without significantly affecting soybean rust. The effect of Zn on reduced growth of stress organisms may be explained in one of two ways: (1) Zn directly affects plant defense and/or pathogen virulence, or (2) the effect is caused indirectly through multiple nutrient interactions. Leaf Zn concentration had a positive interaction with N, K, S, and B concentrations, and negative interaction with P, Mg, and Fe concentrations, and a parabolic relationship with Ca, Mn, and Cu. In the search for Zn mechanisms in plant defense, it is thus important to consider interactions with other nutrients. Furthermore, to allow the comparison between different studies, we stress that future plant–pathogen–nutrient research provides a full characterization of the nutrient status of the plant material used. This includes a growth-response curve which allows insight on possible treatment contamination.
Acknowledgements
The authors would like to thank Drs. Susan Tandy, Anja Gramlich, Verena Pfahler, Julian Smith, and Jurin Vatren for suggestions on the experimental design, Dr. James Haudenshield and Theresa Herman for laboratory assistance, and Heather Lash and Nathan Deppe for greenhouse monitoring.




