Response of white mustard (Sinapis alba) and the soil microbial biomass to P and Zn addition in a greenhouse pot experiment
Abstract
A 45-d pot experiment was carried out to investigate the response of white mustard and the soil microbial biomass after Zn and P addition to a P deficient silt loam. The underlying hypothesis was that P application reduces the Zn availability to crops and microbial biomass. White mustard was supplied with different levels of P (0, 50, and 100 µg g−1 soil) and Zn (0, 10, and 20 µg g−1 soil). Amendments of P generally reduced extractable Zn, shoot Zn and soil microbial biomass Zn. Amendments of P generally decreased the microbial biomass C/P ratio. At 20 µg Zn g−1 soil, a negative effect on the microbial biomass C/P ratio was observed, suggesting that high contents of extractable Zn have a negative impact on the microbial P uptake. However, the minimum Zn requirements of soil microorganisms and the consequences of microbial Zn deficiency for soil microbiological processes are completely unknown.
1 Introduction
Crop nutrition relies very much on soil microorganisms as they decompose organic matter (OM), release nutrients to plants, and store macronutrients such as P (Brookes et al., 1982) but also micronutrients such as Zn in their biomass (Wallander et al., 2003; Khan et al., 2009), protecting them against fixation by inorganic soil components (Khan et al., 2012). Phosphorus deficiency not only restricts crop growth but has also been found to have a negative impact on the microbial energy metabolism leading to low ATP to microbial biomass C ratios (Salamanca et al., 2006). Zinc deficiency is also very common in many parts of the world, affecting, e.g., nearly 50% of the cereal production areas in the world (Cakmak, 2008). Zinc is an essential micronutrient for numerous physiological processes as it serves as a cofactor in many enzymes and in the maintenance of protein structure in plants, animals, fungi, and bacteria (Blencowe and Morby, 2003).
In many soils, Zn deficiency is combined with P deficiency (Cakmak, 2008; Khan and Joergensen, 2010) causing problems for crop fertilization as there are negative interactions between these two nutrients (Loneragan et al., 1982; Barrow, 1987). High contents of extractable P reduce Zn availability to plants (Cakmak and Marschner, 1986; Zhang et al., 2012), whereas high contents of extractable Zn may reduce P availability to soil microorganisms as indicated by high microbial biomass C/P ratios in Zn contaminated soils (Khan et al., 2007).
Despite the importance for physiological processes, excessive contents of Zn have significant toxicity and act as a potent disrupter of biological systems (Blencowe and Morby, 2003), e.g., in soils contaminated by sewage sludge, mining dumps, and industrial exhaust gases (Chander et al., 2001a, b). A lot of research has been carried out on Zn toxicity to soil microorganisms (Chander and Brookes, 1991; He et al., 2005), but not much is known about microbial micronutrient deficiency, although soil bacteria and soil fungi certainly also have a demand for Zn (White and Gadd, 1987; Wallander et al., 2003; Khan and Joergensen, 2010).
A pot experiment was designed to investigate the response of white mustard (Sinapis alba L.) and the soil microbial biomass after Zn and P addition to a silt loam with low total P content. White mustard has a strong capacity to take up large amounts of P and Zn, increasing any existing limitations of soil microorganisms (Föhse et al., 1988; Khan et al., 2012). The underlying hypothesis was that P amendment reduces the Zn availability to both crops and microbial biomass. However, this reduced Zn availability might be counteracted by moderate application rates of Zn.
2 Material and methods
2.1 Soil
The soil was taken in September 2012 at 0–15 cm depth from the site “Saurasen” (Quintern et al., 2006) in N Hessia, Germany, sieved (< 2 mm), homogenized, and stored in polyethylene buckets at room temperature for about 1 week before the experiment started. The soil (6% sand, 77% silt, 17% clay) has been developed from eroded loess overlying clayey New Red Sandstone and was classified as Stagnic Luvisol according to the WRB-FAO classification system (FAO, 2014). The soil had a pH of 6.5 in water and contained 9.8 mg soil organic C (SOC), 1.0 mg total N, 370 µg total P, 0.66 mg oxalate extractable Al, 0.77 dithionite extractable Al, 2.2 mg oxalate extractable Fe, 7.5 mg dithionite extractable Fe, 9.1 µg 0.5 M NaHCO3 extractable P (Khan and Joergensen, 2012), and 0.43 µg NH4NO3 extractable Zn g−1 soil.
2.2 Pot experiment
The experiment consisted of two factors (P and Zn addition) distributed over 9 experimental treatments, each with three replicates (in µg g−1 soil): (1) +P-0 + Zn-0, (2) +P-0 + Zn-10, (3) +P-0 + Zn-20, (4) +P-50 + Zn-0, (5) +P-50 + Zn-10, (6) + P-50 + Zn-20, (7) +P-100 + Zn-0, (8) +P-100 + Zn-10, and (9) + P-100 + Zn-20. To reach the respective contents in soil, 50 µg P and 100 µg P g−1 soil were added as KH2PO4 solution, whereas 10 µg Zn and 20 µg Zn g−1 soil were adjusted with ZnSO4 · 7 H2O solution. Additionally, 200 µg N as NH4NO3 and 100 µg K as K2SO4 g−1 soil were applied to each pot, containing 2.0 kg (on an oven-dry basis) of soil. Three seeds of white mustard (Sinapis alba L. variety SITO) were sown at a depth of 0.5 cm. After emergence, plants were screened out so that each pot maintained two healthy plants. The pots were arranged in a completely randomized design and placed in a temperature-controlled greenhouse chamber with a 16–8 h light-dark cycle and a mean temperature of 25°C (day) and 12°C (night). The moisture was kept at 50% of the soil water holding capacity by weighing the pots twice a week and adding the water lost regularly. The experiment started on September 24, 2012, and was carried out until November 8, 2012. After the final harvest at day 45, the soil was sieved (< 2 mm) after removing the roots. Soil adhering to roots on top of the sieve was carefully removed from the roots and passed through the sieve. Separated roots were removed, rinsed with distilled water, dried and weighed.
2.3 Analytical procedures
Microbial biomass C was estimated by the fumigation-extraction method using 0.5 M K2SO4 as extractant (Vance et al., 1987). Microbial biomass C was calculated as EC / kEC, where EC = (organic C extracted from fumigated soils) – (organic C extracted from non-fumigated soils) and kEC = 0.45 (Wu et al., 1990). Soil microbial biomass P was also measured by the fumigation-extraction method using 0.5 M NaHCO3 (pH 8.5) as extractant (Brookes et al., 1982) as described by Joergensen et al. (1995). Microbial biomass P was calculated as EP / kEP / recovery, where EP = (PO4-P extracted from fumigated soil) – (PO4-P extracted from non-fumigated soil) and kEP = 0.40 (Brookes et al., 1982). Microbial biomass Zn was determined by extracting 10 g moist soil (on an oven-dry basis) with 50 mL 1 M NH4NO3 as described by Khan et al. (2009). Zinc in the extracts was determined by ICP-AES (Spectro Analytical Instruments, Kleve, Germany). Then, microbial biomass Zn was calculated as EZn = (Zn extracted from fumigated soil) – (Zn extracted from non-fumigated soil) without applying a conversion values.
Contents of 0.5 M NaHCO3-extractable PO4-P and 1 M NH4NO3 extractable Zn obtained from the non-fumigated soil by the fumigation-extraction method were taken as indices for the mobile (= plant available) fraction of total P (Joergensen et al., 1995) and total Zn (Chander et al., 2008; Krpata et al., 2009), respectively. In soil and plant material, total P and total Zn concentrations were determined by HNO3-pressure digestion as described by Chander et al. (2008) and measured by ICP-AES.
2.4 Statistical Analysis
The results presented in the tables are arithmetic means and expressed on an oven-dry basis (about 24 h at 105°C). Normal distribution of data and residues was tested by the Shapiro–Wilk test and equal variance by the Brown–Forsythe test. The significance of treatment effects was analysed by a two-way ANOVA, followed by the Holm–Sidak method as a pair-wise multiple comparison test. All statistical calculations were carried out using SigmaPlot 13.0 (Systat Inc., San José, USA).
3 Results
In the P-50 and P-100 treatments, shoot biomass pot−1 increased by 130 and 190%, respectively, in comparison with the P-0 treatment (Fig. 1a) and root biomass by 60 and 110% (Fig. 1b). Shoot P concentration additionally increased by 140 and 190% (Fig. 1c), whereas shoot Zn decreased by 52 and 66%, respectively (Fig. 1d). In the Zn-10 treatment, shoot biomass increased on average by 17% and root biomass by 30% in comparison with the Zn-0 treatment (Fig. 1a, b). The Zn-20 treatment had generally less positive effects on shoot and root biomass than the Zn-10 treatment. In the P-100/Zn-20 treatment, shoot and root biomass were even significantly lower than in the P-100/Zn-0 treatment, leading to significant P × Zn second order interactions. In the Zn-10 and Zn-20 treatments, the concentrations of shoot P decreased on average by roughly 7% in comparison with the Zn-0 treatment (Fig. 1c). The concentration of shoot Zn showed 140% and 210% increases in the Zn-10 and Zn-20 treatments, respectively, in comparison with the Zn-0 treatment (Fig. 1d).
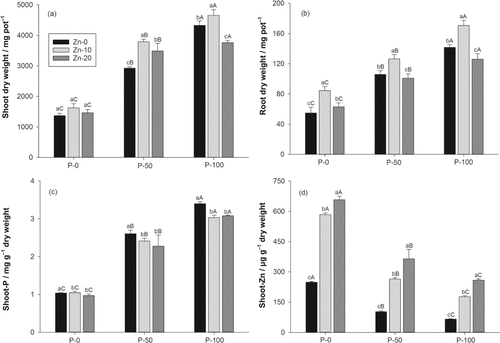
Parameters measured after 45-d pot experiment: (a) shoot dry weight of white mustard in the different P and Zn addition treatments, probability values: P < 0.01, Zn < 0.01, P × Zn < 0.01; (b) root dry weight, probability values: P < 0.01, Zn < 0.01, P × Zn = 0.01; (c) shoot P concentration, probability values: P < 0.01, Zn < 0.01, P × Zn = 0.07; (d) shoot Zn concentration, probability values: P < 0.01, Zn < 0.01, P × Zn < 0.01. Bars represent + 1 standard deviation; different small letters above the bar indicate a significant difference within a P level (Holm–Sidak test, P < 0.05); different capital letters above the bar indicate a significant difference within a Zn level (Holm–Sidak test, P < 0.05).
In the P-50 and P-100 treatments, NaHCO3 extractable P increased by 20 and 43 µg P g−1 soil, respectively, in comparison with the P-0 treatment (Fig. 2a). In the P-0/Zn-10 and P0-Zn-20 treatments, NH4NO3 extractable Zn increased by 1.7 and 4.4 µg Zn g−1 soil, respectively, in comparison with the Zn-0 treatment (Fig. 2b). In the P-50 and P-100 treatments, NH4NO3 extractable Zn decreased by 28 and 36%, respectively. The Zn-10 treatment had variable effects on NaHCO3 extractable P, a negative effect in the P0-treatment, no effect in the P-50, and a positive effect in the P-100 treatment (Fig. 2a). In contrast, the Zn-20 treatment generally led to an average significant 8% decrease.
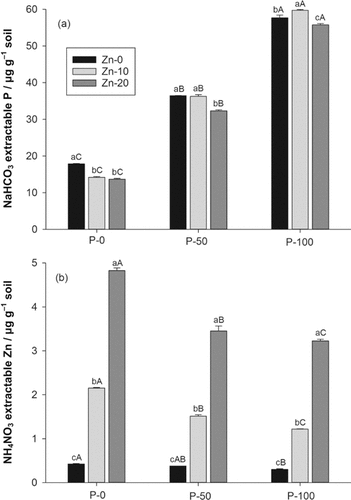
Parameters measured after 45-d pot experiment: (a) soil contents of NaHCO3 extractable P in the different P and Zn addition treatments, probability values: P < 0.01, Zn < 0.01, P × Zn = 0.01; (b) soil contents of NH4NO3 extractable Zn, probability values: P < 0.01, Zn < 0.01, P × Zn = 0.01. Bars represent +1 standard deviation; different small letters above the bar indicate a significant difference within a P level (Holm–Sidak test, P < 0.05); different capital letters above the bar indicate a significant difference within a Zn level (Holm–Sidak test, P < 0.05).
In the P-50 and P-100 treatments, microbial biomass C increased by 13 and 18%, respectively, in comparison with the P-0 treatment (Fig. 3a), and microbial biomass P by 44 and 80% (Fig. 3b), whereas microbial biomass Zn decreased by 11 and 15% (Fig. 3c). The stronger increase in microbial biomass P led to significantly decreased microbial biomass C/P ratios in the P-50 and P-100 treatments (Fig. 3d). Microbial biomass P showed a significant positive linear relationship with the root biomass (r = 0.95), which was not affected by the Zn treatments (Fig. 4). In the Zn-10 treatment, microbial biomass C significantly increased on average by 11% and microbial biomass P by 14% in comparison with the Zn-0 treatment (Fig. 3a, b). In the Zn-20 treatment, microbial biomass C significantly increased on average by only 6%, whereas microbial biomass P significantly declined by 5% in comparison with the respective Zn-0 treatments. Microbial biomass Zn showed nearly 5- and 9-fold increases in the Zn-10 and Zn-20 treatments, respectively, in comparison with the Zn-0 treatment (Fig. 3c). The additional increase in microbial biomass Zn from the Zn-10 to the Zn-20 treatments was stronger in the P-50 and P-100 treatments than in the P-0 treatment, leading to a significant P × Zn second order interaction.
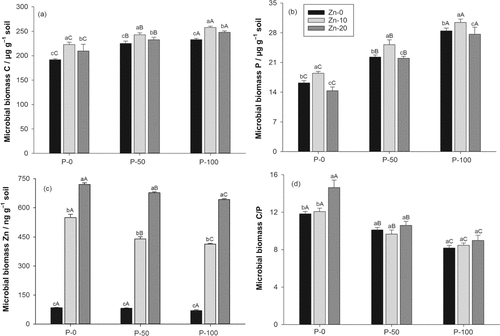
Parameters measured after 45-d pot experiment: (a) soil contents of microbial biomass C in the different P and Zn addition treatments, probability values: P < 0.01, Zn < 0.01, P × Zn = 0.36; (b) soil contents of microbial biomass P, probability values: P < 0.01, Zn < 0.01, P × Zn = 0.49; (c) soil contents of microbial biomass Zn, probability values: P < 0.01, Zn < 0.01, P × Zn < 0.01; (d) soil microbial biomass C/P ratios; probability values: P < 0.01, Zn < 0.01, P × Zn < 0.01. Bars represent +1 standard deviation; different small letters above the bar indicate a significant difference within a P level (Holm–Sidak test, P < 0.05); different capital letters above the bar indicate a significant difference within a Zn level (Holm–Sidak test, P < 0.05).
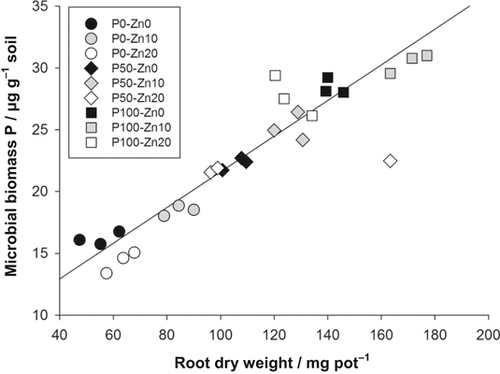
Linear relationship between root dry weight and microbial biomass P in the different P and Zn addition treatments in pots after 45 d; r = 0.96, P < 0.0001, n = 27.
4 Discussion
Phosphate amendment generally reduced the Zn concentration in white mustard shoots, in soil extracts, and also in the soil microbial biomass. This negative phosphate effect has already been observed for crops (Cakmak and Marschner, 1986; Watts-Williams et al., 2014) and soil extracts (Barrow, 1987; Norvell et al., 1987; Wang and Harrell, 2005; Zhao and Selim, 2010), but not for the soil microbial biomass. However, phosphate amendment had only minor effects on Zn uptake in comparison with the P-0 treatment. On average, 3.0% of the Zn applied were taken up by the white mustard shoots in the Zn-10 and 1.9% in the Zn-20 treatments, respectively.
Phosphate amendment not only increased P concentrations, but also resulted in significantly higher plant yields and microbial biomass contents. The relative P-induced biomass increases were much stronger for plant than for microbial biomass. This suggests that the increase in microbial biomass was mainly an indirect effect of P fertilization due to the higher C input by rhizodeposits. However, plant induced increases in microbial biomass have not always been observed in pot experiments (Chen et al., 2003; Muhammad et al., 2007), whereas P application resulted in some cases in rapid increases in microbial biomass C and especially microbial biomass P (Khan and Joergensen, 2009).
Soil fungi and bacteria are able to store excess available P as polyphosphates in the cytoplasm (Gächter and Meyer, 1993; Nielsen et al., 2002), in acidocalcisomes (Docampo et al., 2005, 2010; Docampo, 2006), in fungal vacuoles (Klionsky et al., 1990), but presumably also in the teichoic acids of Gram positive bacterial cell walls (Grant, 1979; Oberson and Joner, 2005). The microbial biomass C/P ratio in the present experiment varied between 8 and 15, i.e., in the range obtained by Joergensen and Emmerling (2006) or Brookes et al. (1982) for arable soils in a temperate climate of Europe. The significant increase of the microbial biomass C/P ratio in the Zn-20 treatments suggests negative effects of Zn on the P uptake of soil microorganisms as already proposed by Khan et al. (2007), investigating the effects of heavy metal pollution on the elemental stoichiometry of the soil microbial biomass. However, in their survey the microbial biomass C/P ratio ranged from 19 to 32 in highly Zn polluted soils, i.e., similar to arable soils in the tropics and sub-tropics, very low in P availability to soil microorganisms (Ghoshal and Singh, 1995; Muhammad et al., 2008; Joergensen, 2010). This suggests that phosphate application must lower Zn toxicity to plants and soil microorganisms in highly Zn contaminated soils.
Effects of Zn on phosphate turnover were dependent on the application rate. At 10 µg Zn g−1 soil, no effects on the microbial biomass C/P ratios and NaHCO3 extractable P were measured but positive effects on plant and microbial biomass. At 20 µg Zn g−1 soil, the content of extractable P decreased, suggesting counteracting Zn effects on soil P retention (Tu et al., 2002). The possibility of negative interactions between Zn and P availability has already been proposed by Khan and Joergensen (2010), investigating the microbial response to the application of 50 µg Zn g−1 soil, incubating a Zn and P deficient alkaline subsoil.
Application of Zn generally increased the Zn concentration in white mustard shoots, in NH4NO3 extracts, and in the soil microbial biomass. On average, 3.1% of the Zn applied remained NH4NO3 extractable in the Zn-10 and 4.3% in the Zn-20 treatment, whereas 3.9% of the Zn applied were taken up as the CHCl3-labile fraction by the soil microbial biomass in the Zn-10 and 3.0% in the Zn-20 treatments, respectively. Even for the Zn-0 treatment, the Zn concentration in the white mustard plants was in the middle of the range presented in literature, in which Zn concentrations between 30 and 1,200 µg g−1 dry weight have been reported, partly for highly Zn contaminated soils (Płociniczak et al., 2013; Jankowski et al., 2014; Zalewska and Nogalska, 2014). This indicates that the Zn availability in the current low P soil seems to be sufficient for crop growth (Sadeghzadeh and Rengel, 2011). It also indicates that the current white mustard variety has a strong capacity for Zn uptake (Płociniczak et al., 2013), probably combined with a low Zn use efficiency (Sadeghzadeh and Rengel, 2011; Kabir et al., 2014). Although none of the white mustard plants showed any visible symptoms of Zn toxicity, the Zn-20 treatment already seems to have toxic effects not only on plant yield but also on soil microorganisms. This suggests that there is only a small range for optimum fertilization rates for highly soluble Zn salts.
NH4NO3-extractable and microbial biomass Zn were closely correlated, indicating that the microbial uptake is controlled by Zn availability and not by microbial Zn demand. The microbial biomass Zn contents in the current Zn-0 treatments were in the range from 13 to 105 µg Zn g−1 soil as observed by Khan et al. (2009) for low metal soils. The average microbial biomass Zn content of 78 g Zn g−1 soil in the Zn-0 treatments corresponds to a Zn-concentration of 170 µg g−1 dry weight, assuming a C content of 45% for the soil microbial biomass. This value is nearly identical to the mean of 150 µg Zn g−1 dry weight measured by Wallander et al. (2003) in fungal mycelia, using PIXE (particle induced x-ray emission) analysis of fungal mycelia growing in sand mesh-bags buried in forest soils. In fungal fruit bodies and sporophores, sampled on the soil surface, concentrations between 17 and 300 µg Zn g−1 dry weight. have been repeatedly measured (Tyler, 1980; Gadd, 2007; Kalač, 2010), which are in line with the current results. A close relationship between the Zn concentrations of fungal mycelium and sporophores has been observed in Zn polluted litter layers (Krpata et al., 2009).
In soils with Zn deficiency, which is often combined with P deficiency, sole application of P fertilizers will further increase Zn deficiency to plants but also to soil microorganisms. If Zn deficiency in soil microorganisms reduces their mineralization activity, this will put additional pressure on nutrient supply to crops growing on P and Zn deficient soils. This hypothesis may be evaluated.
5 Conclusions
The application of 50 and 100 µg P g−1 soil reduced the contents of extractable Zn, shoot biomass Zn, and also microbial biomass Zn, which should be considered when applying P fertilizers to P and Zn deficient soils. Application of P also decreased the microbial biomass C/P. At 20 µg Zn g−1 soil a negative effect on the microbial biomass C/P ratio was observed, indicating that high contents of extractable Zn may have a negative impact on soil microbial P uptake. The minimum Zn requirements of soil microorganisms and the consequences of microbial Zn deficiency for soil microbiological processes are completely unknown.
Acknowledgements
Khalid Saifullah Khan is grateful to the Alexander von Humboldt Foundation for granting a Georg Forster fellowship. We thank Gabriele Dormann for providing skillful technical assistance.




