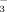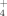Additional nitrogen in arctic-alpine soils and plants—a pilot study with 15NO
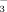 and 15NH
and 15NH
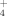 fertilization along an elevation gradient
fertilization along an elevation gradient
Abstract
Nitrogen (N) deposition has been increasing in alpine ecosystems, but its fate in soils and plants remains unclear. We assumed that the increased N load will be efficiently retained in alpine ecosystems but that the degree of N use efficiency changes with elevation. Thus, we performed a 3-year 15N tracer experiment, in which we added 1 g m−2 of either NH415NO3 or 15NH4NO3 fertilizer to a plot of 1 m2 in size at three elevations. Composite soil samples and aboveground plant material from lichens, dwarf shrubs, and graminoids were collected annually for three years and analyzed for their 15N accrual. We found a cumulative and plateauing rise in 15N concentration in soils and plants at all sites. However, overall recovery of the tracer decreased with time, amounting to 71% of fertilizer recovered in the soils in the first year, 69% recovered in soils and plants in the second year, and 37% in soils and plants in the third year. Moreover, the fertilizer use efficiency varied among fertilizer types and plant functional types. This utilization pattern appears to be modulated by elevation.
1 Introduction
Since 1960, atmospheric deposition of reactive N compounds has been increasing worldwide due to rising agricultural and industrial activities (Galloway et al., 2003). However, our knowledge about the fate of the added N in soils and their effects on plants is still incomplete (Lamarque et al., 2005; Gruber and Galloway, 2008; Peri et al., 2012). This is especially true for arctic-alpine regions (Beem et al., 2009; Futter et al., 2009; Bobbink et al., 1998, 2010a) which are highly sensitive to both increasing N load (Helliwell et al., 2008; Nowinski et al., 2008) and global climatic change (Klanderud, 2005; Huber et al., 2007; Sjögersten and Wookey, 2009).
In European alpine ecosystems the deposition rates of reactive N have already reached a critical load (Armitage et al., 2011; Department for Environment, Food and Rural Affairs, 2012); the deposition ranges between 0.5 and 1.5 g N m−2 y−1 (Bobbink and Roelofs, 1995; Nagel and Gregor, 1999; Pearce and Van der Wal, 2002, 2008; Fremstad et al., 2005; Bowman et al., 2006; Britton and Fisher, 2008; Bobbink et al., 2010b). A multi-model approach that refers to the IPCC SRES A2 scenario (Ehhalt et al., 2001) showed that recent reactive N deposition rates in W Europe are approx. 1 g N m−2 y−1, and the approach estimated doubled N deposition rates for the year 2100 (Lamarque et al., 2005). There is a huge potential of increased atmospheric N deposition to impact on alpine regions, because the vegetation cover there comprises slow growing and nutrient-poor species, and because of orographic rainfall and occult deposition (Helliwell et al., 2008; Armitage et al., 2011; Stevens et al., 2011). Exceeding the response threshold for reactive N deposition endangers these ecosystems by altering the nutrient cycle with associated C loss from deeper soil layers (Pearce and Van der Wal, 2008) and by leading to changes in alpine plant species composition due to vascular plants' tendency to outcompete low-growing cryptogams, herbs, and lichens (Heer and Körner, 2002; Nowinski et al., 2008; Britton and Fisher, 2008, 2010; Armitage et al., 2011). Thus, even low additional loads of N deposition might alter these alpine ecosystems (Nagel and Gregor, 1999; Pearce and Van der Wal, 2002; Fremstad et al., 2005; Bobbink et al., 2010b), underlining our need to understand the fate of added N in soils and plants.
Several studies have examined the effects of N deposition on alpine plant growth, cover, and diversity (Shaver and Chapin, 1995; Fremstad et al., 2005; Baron, 2006; Britton and Fisher, 2008, 2010; Armitage et al., 2011). These studies generally identified critical loads and found that plants react with a significant increase of growth and flowering in response to N fertilization, with lichen communities being most sensitive. Due to elevation, alpine ecosystems show high variability in a small area (Körner, 2003). Thus, we presumed that the fate of NH
 and NO
and NO
 in soils and plants might also vary across elevation. It seems reasonable that vegetation changes with elevation can significantly alter site-specific rates of the N mass balance (Nagel and Gregor, 1999; Sundqvist et al., 2011).
in soils and plants might also vary across elevation. It seems reasonable that vegetation changes with elevation can significantly alter site-specific rates of the N mass balance (Nagel and Gregor, 1999; Sundqvist et al., 2011).
The fate of N in soils and plants can be monitored by the use of the stable isotopes 14N and 15N. The average ratio of 15N/14N in non-reactive atmospheric N2 is 1:273; the molar proportion is thus 0.003663, amounting to 3.663‰, which is used to specify the natural 15N abundance in air as a reliable international standard due to its constant behavior (Mariotti, 1983; Hauck et al., 1994). Hence, we performed a fertilization pioneer experiment, in which we repeatedly added 15N-labeled material to track its fate in alpine soils and plants. In detail we aimed at identifying to what degree NO
 and NH
and NH
 are kept in soils and used by plants, and we wanted to know how these effects accumulate when the fertilizer is repeatedly deposited each year at different elevations.
are kept in soils and used by plants, and we wanted to know how these effects accumulate when the fertilizer is repeatedly deposited each year at different elevations.
2 Material and methods
The study was conducted in the Jetta mountain range in Vågå / Oppland, Norway (61°53'N, 9°15'E) (Löffler et al., 2006). Here, the most continental of Scandinavia's climates is characterized by the lowest mean annual precipitation (300–400 mm y−1 in the valleys) (Moen, 1999). Three alpine study sites along an elevation gradient were established on wind-exposed hilltops at 1050 m, 1175 m (both in the low alpine zone), and 1400 m asl (in the middle alpine zone). Throughout the sites, weathered phylitic bedrock, locally covered by thin glacial till layers, forms the parent material for skeleton-rich Cambic Cryosols. On the hilltops, lichen-heath communities are dominated by Bryocaulon divergens, Alectoria ochroleuca, and Cetraria nivalis, chionophobous terricolous lichens that are an indication of the rough climatic conditions due to the thin snow cover. These lichens form an almost continuous bottom layer from which dwarf shrubs and (at higher elevations) graminoids protrude.
From 2007 to 2010, 1 g m−2 y−1 of 15N-labeled NH4NO3 (98% 15N enrichment supplied by Cambridge Isotope Laboratories, Inc.) was experimentally added as wet deposition to the naturally existing N deposition at the end of the annual vegetation period. The fertilizer stayed in frozen soil during the winter and was available at the beginning of the next vegetation period, when plant N uptake is greatest. Together, these amounts approximated the estimated N deposition rates for the year 2100 (Lamarque et al., 2005). Each study site consisted of three separate plots with areas of 1 m2. To ascertain the impacts of different N compounds on the ecosystem, plots at each site were fertilized with 15NH4NO3 and NH415NO3, respectively. The third plot served as control. To ensure a homogenous tracer application across a plot, we divided the plot into 25 squares, dissolved 1 g of fertilizer in 1.25 L of distilled water, and sprinkled each square with 50 mL of the resulting solution. We used averaged soil in-situ density data from previous core cutter measurements as well as aboveground plant dry biomass, both obtained from measurements directly outside the plots (Table 1).
Soil sampling took place once a year before the fertilizer was added. The samples from 2007 showed the natural background of 15N and served as additional controls. The soil samples from 2008 showed the results after one year of fertilization (i.e., 1 g m−2), those from 2009 received two years of the fertilizer treatments (i.e., 2 × 1 g m−2), and the samples from 2010 received three treatments (i.e., 3 × 1 g m−2). In order to improve spatial coverage without increasing the sample number, as well as to achieve a sampling volume of about 5 cm3 (ca. 5 g), which was deemed sufficient for subsequent analyses, we divided each plot into five rows and took five evenly spaced samples from each row to form 5 composite samples per plot. The sampling depth of 7 cm (diameter 1 cm) covered the least available soil depth at all sites, which varied from 7 to 35 cm across the study area. The sampling holes in the ground were closed after each year due to cryoturbation.
In 2009 and 2010, plant leaf and lichen thallic tissues were sampled in the same manner as soils. Due to the expected differences in nutrient uptake, the plants were divided into three groups: terricolous lichens (Alectoria ochroleuca, Bryocaulon divergens, Cetraria nivalis, and Cladina stellaris), dwarf shrubs (Arctostaphylos alpinus, Betula nana, Vaccinium vitis-idea, and Empetrum nigrum ssp. hermaphroditum), and graminoids (Carex bigelowii, Juncus trifidus, and Luzula confusa). Both soil and plant samples were air-dried and stored until lab preparation.
To account for the extreme site heterogeneity at the fine scale in alpine soils and vegetation (Bednorz et al., 2000; Körner, 2003), the single samples were taken at a distance of 20 cm.
Z(s,p)= / mass% 15N(s,p) / 100 * mass(s,p) – mass 15N(ctr),
()where Z(s) stands for 15N g / 100 dm3 (i.e., 1 m2 with a depth of 7 cm) and Z(p) stands for 15N g m−2 aboveground biomass of lichens, dwarf shrubs, and graminoids (Hauck et al., 1994). Finally, the tracer recovery (15N excess) was calculated by subtracting the natural abundance of 15N(ctr), as measured in the control samples from 2007 (Providoli et al., 2005).
The experimental design of this pilot study did not allow us to apply inferential statistics. In the following, we therefore report only descriptive statistics. They, however, require that sampling points are spatially independent (Hakenes, 2015, pers. communication). For plant individuals, having a size of a few cm at maximum, the sampling distance of 20 cm may be sufficient for this purpose. When performing a Moran's I test for spatial autocorrelation with the δ15N values in each vegetation type and in the soils for every plot the results confirmed that except in two marginal cases (p = 0.025 and p = 0.05), rows within a plot were not spatially auto-correlated (at α = 0.05).
3 Results
3.1 Tracer recovery in soils and plants
The average total N content of the soils (with a soil depth of 7 cm) was 342 g m−2 (± 172). Lichens contained 3.78 g N m−2 on average, dwarf shrubs contained 4.18 g N m−2, and graminoids contained 1.67 g N m−2. Different N stocks in the aboveground biomass reflected the different dry masses of the plants at the respective sites (Table 1). Average natural abundance of 15N in soils in 2007 was 0.87‰ (± 1.14). Lichens showed a δ15N value of –3.21‰, and dwarf shrubs showed –4.22‰. Only graminoids showed a value of 7.03‰, which reflects the different isotope fractionation by the graminoids.
| Elevation / m asl | 1050 | 1175 | 1400 | |
|---|---|---|---|---|
| MAP / mm | 412 | 348 | 508 | |
| MAT of soil –15 cm / °C | 0.1 | –0.1 | –0.6 | |
| Mean soil density / kg dm−3 | 0.85 (0.1) | 1.46 (0.3) | 1.15 (0.1) | |
| Mean soil moisture / %* | 19.6 | 20.5 | 13.3 | |
| pH (H2O)* | 4.7 | 4.7 | 4.8 | |
| pH (CaCl2)* | 4.0 | 4.1 | 4.0 | |
| Corg / g kg−1* | 65 | 30 | 19 | |
| Lichens | 504 (12.5) | 733 (12.0) | 842 (14.4) | |
| Biomass / g m−2 | Dwarf shrubs | 372 (11.3) | 557 (12.8) | 408 (8.0) |
| Graminoids | 0 | 21 (8.7) | 107 (5.4) | |
| Soils | 4.2 (0.9) | 5.1 (2.3) | 4.6 (1.3) | |
| Ntot / g kg−1 | Lichens | 5.0 (0.6) | 4.9 (1.2) | 6.3 (2.1) |
| Dwarf shrubs | 8.9 (1.1) | 11.7 (1.3) | 8.1 (3.4) | |
| Graminoids | – | 12.9 (1.3) | 12.5 (5.0) | |
Amongst soil samples, the δ15N values for a given fertilization ranged between the different rows of a plot from 622‰ and 1811‰ at maximum (± 454). In plant samples, δ15N abundance was higher as was its variability (Fig. 1).
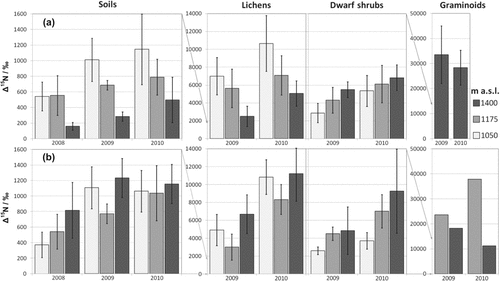
Mean δ15N (with standard deviations) in soils, lichens, dwarf shrubs, and graminoids from 1 m2 plots at three elevations fertilized with (a) NH415NO3 and (b) 15NH4NO3. No standard deviation bars are shown where the data are less abundant. Please note differences in the scales on the y-axis.
When calculating the total tracer recovery across both added N forms according to Eq. 1, we found that, after the first year of fertilization, the plots often recovered nearly 100% of the tracer addition on average (Fig. 2), despite high variability remaining between the different rows of a plot.
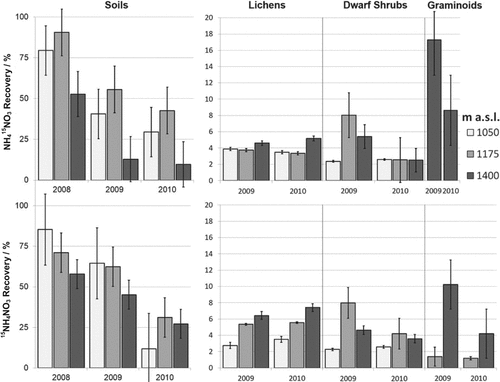
Average 15N fertilizer recoveries (in % of the added N) with standard errors in soils, lichens, dwarf shrubs, and graminoids fertilized with NH415NO3 (row above) and 15NH4NO3 (row below) at the three elevations. Please note the difference in the scales on the y-axis.
Most of the applied tracer ended up in the soils (cf Schleppi et al., 1999). When averaging across the three years, in the low-alpine zone at the sites at 1050 and 1175 m asl, 55% and 60% of the applied tracer was maintained in the soils, respectively. However, in the middle-alpine zone, at 1400 m asl, only 38% of the tracer remained. The plants responded differently to the additional N supply as well. In lichens, we found approx. 5%, in dwarf shrubs 4%, and in graminoids we found 6% of the applied tracer (Table 1). The total fertilizer uptake by plants varied with elevation in both years such that total fertilizer concentrations in dwarf shrubs increased with increasing elevation, while fertilizer uptake behavior in lichens differed along an elevation gradient related to the respective N form (Fig. 1).
3.2 Cumulative effects
Due to annual application of the tracer, the δ15N values increased with time (Fig. 1). In soils (with a natural background value of 0.87‰), the values approached a plateau: a doubling and tripling of the fertilizer amount with time did not double and triple the δ15N values in almost all samples, and the rise in the δ15N value in the soils from the second to the third year was only half of the rise from the first to the second year at sites fertilized with 15NO
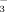 (Fig. 1). When calculating the tracer recovery, plant biomasses and soil densities at the respective sites were reflected in the recovery percentages (Fig. 2). Additionally, the phenomenon of approaching a plateau could be observed in both soils and plants: the overall recovery of the tracer in soils at all sites decreased with time, amounting to 71% of fertilizer recovered in the first, 49% in the second, and 26% in the third year after the start of the experiment. Dwarf shrubs contained 5% in 2009, and 3% in 2010, and graminoids (where they occurred) had 11% in 2009, and 4% of the applied tracer amount in 2010. Lichens showed 4% of fertilizer recovery in 2009, but 5% in 2010. The accumulation of the tracer in soil was usually lower at 1400 m asl than at the other sites (Fig. 2). The tracer recovery in the plants did not follow that in soils, which was related to variations in plant aboveground biomass (Table 1). Lichens and graminoids were more abundant with elevation, thus, storing more N in their biomass.
(Fig. 1). When calculating the tracer recovery, plant biomasses and soil densities at the respective sites were reflected in the recovery percentages (Fig. 2). Additionally, the phenomenon of approaching a plateau could be observed in both soils and plants: the overall recovery of the tracer in soils at all sites decreased with time, amounting to 71% of fertilizer recovered in the first, 49% in the second, and 26% in the third year after the start of the experiment. Dwarf shrubs contained 5% in 2009, and 3% in 2010, and graminoids (where they occurred) had 11% in 2009, and 4% of the applied tracer amount in 2010. Lichens showed 4% of fertilizer recovery in 2009, but 5% in 2010. The accumulation of the tracer in soil was usually lower at 1400 m asl than at the other sites (Fig. 2). The tracer recovery in the plants did not follow that in soils, which was related to variations in plant aboveground biomass (Table 1). Lichens and graminoids were more abundant with elevation, thus, storing more N in their biomass.
3.3 Uptake of 15NO
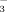 and 15NH
and 15NH
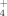
Along the elevation gradient, soils and plant functional types pointed at differences in the uptake patterns of NH
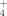 and NO
and NO
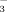 (Fig. 1): the 15NO
(Fig. 1): the 15NO
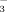 concentrations of soils consistently decreased with elevation, whereas the opposite pattern was indicated for NH
concentrations of soils consistently decreased with elevation, whereas the opposite pattern was indicated for NH
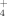 . Apparently, the soils were able to store additional ammonium more easily than 15NO
. Apparently, the soils were able to store additional ammonium more easily than 15NO
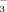 . Lichens showed the same pattern with elevation as the soils, whereas dwarf shrubs behaved differently, because foliar concentration of both 15NH
. Lichens showed the same pattern with elevation as the soils, whereas dwarf shrubs behaved differently, because foliar concentration of both 15NH
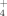 and 15NO
and 15NO
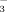 increased coincidently with elevation in 2009 and 2010 (Fig. 1). Being highly N-demanding, graminoids showed both the highest δ15N concentrations and the highest uptake of both N compounds among plants, slightly favoring 15NO
increased coincidently with elevation in 2009 and 2010 (Fig. 1). Being highly N-demanding, graminoids showed both the highest δ15N concentrations and the highest uptake of both N compounds among plants, slightly favoring 15NO
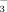 . This indicates that an input of different forms of reactive N leaves a characteristic fingerprint in this arctic-alpine ecosystem.
. This indicates that an input of different forms of reactive N leaves a characteristic fingerprint in this arctic-alpine ecosystem.
4 Discussion
4.1 Tracer recovery in soils and plants
Historically, decomposition of organic matter and nutrient cycling in arctic-alpine ecosystems is slow, and a large amount of N, which cannot be easily absorbed by plants, is stored in the soil with only minimal leaching (Bobbink et al., 1998). Our data show that we did not account for significant N losses during the first season. Gerzabek et al. (2004) has pointed to high recoveries of fertilizer N in high mountain soils, mainly because of the permafrost layer, which prevented the leaching of the fertilizer to deeper soil layers. At our sites, soils are frozen throughout the winter but thaw entirely during the vegetation period (Wundram et al., 2010).
The uptake pattern of the soils indicate that there was an even uptake of fertilizer N (in terms of NH
 or NO
or NO
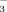 ) at all elevations in the first year after fertilization, but with time, soils at higher elevations had less capacity and ability to store the same amount of fertilizer than at lower elevations (Fig. 2). Consistent with the previous observation, a considerably large amount of the tracer in the soils was lost at 1400 m asl in our study, presumably due to enhanced precipitation and snowfall, lower temperatures, and less Corg with elevation (Table 1). These parameters also play an important role in determining the natural background of 15N (Amundson et al., 2003). In addition, daily maxima of soil temperatures in the middle-alpine zone frequently exceed temperatures in the low-alpine zone due to less vegetation coverage and corresponding exposed rock surface, which can lead to intermediate high increases in soil microbial activity in the vegetation period (Löffler et al., 2008).
) at all elevations in the first year after fertilization, but with time, soils at higher elevations had less capacity and ability to store the same amount of fertilizer than at lower elevations (Fig. 2). Consistent with the previous observation, a considerably large amount of the tracer in the soils was lost at 1400 m asl in our study, presumably due to enhanced precipitation and snowfall, lower temperatures, and less Corg with elevation (Table 1). These parameters also play an important role in determining the natural background of 15N (Amundson et al., 2003). In addition, daily maxima of soil temperatures in the middle-alpine zone frequently exceed temperatures in the low-alpine zone due to less vegetation coverage and corresponding exposed rock surface, which can lead to intermediate high increases in soil microbial activity in the vegetation period (Löffler et al., 2008).
The total fertilizer uptake concentration pattern in plants (Fig. 1) indicates a relationship with elevation, too, but the direction of the relationship seemed to vary by plant functional type. While both dwarf shrubs and lichens are considered to grow slowly in alpine regions compared to graminoids, their high levels of fertilizer uptake concentration suggests fast growth from pronounced nutrient demand. Additionally, the higher natural δ15N background of the graminoids is presumably due to higher overall N uptake. This indicates that with ongoing N deposition in the future, graminoid-dominated communities might supersede lichen-dominated communities in arctic-alpine ecosystems.
4.2 Cumulative effects
The accumulation of 15N in soils with time shows either a carry-over from the former year into the subsequent year, or, due to the N fertilization in the first year, more of the tracer N in the second year was available for plant uptake. Nevertheless, the capability of plants and especially soils to store additional fertilizer N clearly decreased in subsequent years (Fig. 2). This process has been assigned to saturation effects, i.e., a limited uptake of additional by a system that has already received excess N (Aber et al., 1998; Galloway et al., 2003; Fremstad et al., 2005). As a result, less of the additional fertilizer N was recycled in the subsequent years.
Above a critical N deposition of 0.75 g m−2 y−1, lichens may react with changes in thallic chemistry, cellular structure, species abundance, or, in cases of ongoing high deposition, even with death (Britton and Fisher, 2010; Nash, 1996). Death or color change was not yet observed after three years of adding 1 g N m−2 y−1, possibly because the soils still absorbed the majority of the added reactive N. Instead, we show that even if the lichens' fertilizer uptake started to approach a certain plateau after three years, lichens continued to utilize relatively high amounts of added 15N (e.g., approx. 0.2 g m−2 at 1400 m asl in 2009). Therefore, we suggest that the capacity of the system to store added 15N was though limited as outlined above but still larger than assumed from above cited critical loads.
4.3 Uptake of 15NO
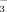 and 15NH4
and 15NH4
Our results support the assumption that the ratio of NH
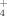 to NO
to NO
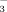 in both soils and plants changes along an elevation gradient. As NO
in both soils and plants changes along an elevation gradient. As NO
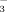 is usually less adsorbed by soils than the cationic NH
is usually less adsorbed by soils than the cationic NH
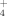 , it seems reasonable to assume that the enhanced loss of nitrates from the middle-alpine zone was due to leaching and runoff.
, it seems reasonable to assume that the enhanced loss of nitrates from the middle-alpine zone was due to leaching and runoff.
Lichens take up nutrients over their entire thallus body. As they are in contact with N sources of the soil, and nutrient transfer throughout the lichen thallus is possible (Stewart and Rowell, 1977; Rai et al., 2002). Under natural conditions, NH
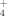 and NO
and NO
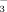 are both efficiently and equally taken up. However, when lichens encounter harsher environments or higher N depositions, the incorporation of 15NH
are both efficiently and equally taken up. However, when lichens encounter harsher environments or higher N depositions, the incorporation of 15NH
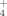 dominates (Dahlman et al., 2004). 15NH
dominates (Dahlman et al., 2004). 15NH
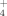 can be more easily converted into amino acids and DNA (both of which contain NH2 groups), thereby requiring less energy for cellular metabolism in harsher environments (Madigan et al., 2008). These findings and the elevational patterns in our data (Fig. 1) suggest that in our system lichens prefer NH
can be more easily converted into amino acids and DNA (both of which contain NH2 groups), thereby requiring less energy for cellular metabolism in harsher environments (Madigan et al., 2008). These findings and the elevational patterns in our data (Fig. 1) suggest that in our system lichens prefer NH
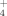 when growing under harsher living conditions (i.e., at higher elevations). The fertilizer uptake for the analyzed dwarf shrub species was most effective at the middle-alpine site (1400 m asl), where they approached the upper limits of existence (Bär et al., 2006). The accumulation of tracer N in the dwarf shrubs at higher elevations was most visible in the δ15N values, hence, it was a concentration effect (Fig. 1). We support the assertion that dwarf shrubs contain higher N concentrations and show higher N use efficiencies at higher elevations (Körner, 1989) because of less shoot growth but at the same time higher nutrient demands due to lower temperatures, later snow melt, and a shorter growing season at the middle-alpine sites. In the low-alpine zone, in contrast, increased shoot growth in response to added N and better growing conditions likely diluted the tissue 15N concentrations.
when growing under harsher living conditions (i.e., at higher elevations). The fertilizer uptake for the analyzed dwarf shrub species was most effective at the middle-alpine site (1400 m asl), where they approached the upper limits of existence (Bär et al., 2006). The accumulation of tracer N in the dwarf shrubs at higher elevations was most visible in the δ15N values, hence, it was a concentration effect (Fig. 1). We support the assertion that dwarf shrubs contain higher N concentrations and show higher N use efficiencies at higher elevations (Körner, 1989) because of less shoot growth but at the same time higher nutrient demands due to lower temperatures, later snow melt, and a shorter growing season at the middle-alpine sites. In the low-alpine zone, in contrast, increased shoot growth in response to added N and better growing conditions likely diluted the tissue 15N concentrations.
Graminoids produce aboveground plant material every year anew and therefore have a high demand for N. The usual faster and higher uptake of NO
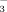 than of NH
than of NH
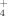 (Körner, 2003) was also reflected in our patterns.
(Körner, 2003) was also reflected in our patterns.
5 Conclusions
This study shows that (1) there is an efficient uptake of reactive N deposition in the arctic-alpine soils and plants. The majority of the added 15N was stored in the soils, followed by graminoids, lichens, and dwarf shrubs. However, our study also shows that (2) 15N uptake by plants declined in subsequent years, indicating saturation. Reduced storage of 15N after one year of fertilization shows how severely arctic-alpine ecosystems might be affected by expected elevated N deposition in the future. Excess N reaching the ecosystems via atmospheric deposition might not be completely retained in the system but might partially leave the system as nitrate leaching. Furthermore, (3) the uptake of 15N seems to depend on the provided N compound with elevation. We encourage additional studies in other arctic-alpine ecosystems, preferably by relating such studies on different slopes, in order to elucidate the role of elevation as key driver for the cumulative uptake and differential sequestration of NH
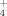 and NO
and NO
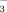 in both soils and plants.
in both soils and plants.
Acknowledgements
We thank Kirsten Unger for help with the lab analyses. We also thank Prof. Dr. Hendrik Hakenes, Dr. Kerstin Anschlag, and Dr. Anne Bjorkman for constructive discussions on this manuscript, as well as the family of Anders Svare and Ingrid Lunde Svare for their overwhelming hospitality and logistical support in Vågå, Norway. This study was supported by the University of Bonn and the German Academic Exchange Service (DAAD).