The relationship between knee loading during gait and cartilage thickness in nontraumatic and posttraumatic knee osteoarthritis
Abstract
The relationship between knee moments and markers of knee osteoarthritis progression has not been examined in different knee osteoarthritis subtypes. The objective was to examine relationships between external knee moments during gait and tibiofemoral cartilage thickness in patients with nontraumatic and posttraumatic knee osteoarthritis. For this cross-sectional study, participants with knee osteoarthritis were classified into two groups: nontraumatic (n = 22; mean age 60 years) and posttraumatic (n = 19; mean age 56 years, history of anterior cruciate ligament rupture). Gait data were collected with a three-dimensional motion capture system sampled at 100 Hz and force plates sampled at 2000 Hz. External knee moments were calculated using inverse dynamics. Cartilage thickness was determined with magnetic resonance imaging (T1-weighted, 3D sagittal gradient-echo sequence). Linear regression analyses examined relationships between cartilage thickness with knee moments, group, and their interaction. A higher knee adduction moment impulse was negatively associated with medial to lateral cartilage thickness ratio (B = −1.97). This relationship differed between participants in the nontraumatic osteoarthritis group (r = −0.56) and posttraumatic osteoarthritis group (r = −0.30). A higher late stance knee extension moment was associated with greater medial femoral condyle cartilage thickness (B = −0.86) and medial to lateral cartilage thickness (B = −0.73). These relationships also differed between participants in the nontraumatic osteoarthritis group (r = −0.61 and r = −0.51, respectively) and posttraumatic osteoarthritis group (r = 0.10 and r = 0.25, respectively). Clinical Significance: The relationship between knee moments with tibiofemoral cartilage thickness differs between patients with nontraumatic and posttraumatic knee osteoarthritis. The potential influence of mechanical knee loading on articular cartilage may also differ between these subtypes.
1 INTRODUCTION
Osteoarthritis (OA) is the most common joint disorder worldwide and is often associated with significant disability1 with at least 19% of Americans aged 45 years and older being affected by knee OA.2 Medial knee joint loading has a role in knee OA progression.3 Specifically, the knee adduction moment (KAM) is a proxy for medial/lateral load distribution within the knee.4, 5 The KAM is associated with radiographic knee OA severity, where a higher KAM is associated with greater disease severity.6-8 Furthermore, a higher KAM is associated with longitudinal cartilage loss measured using magnetic resonance imaging (MRI)9, 10 and radiographic knee OA progression3 in patients with medial tibiofemoral knee OA.
The knee flexion moment (KFM) and extension moment (KEM) represent the net muscular contribution to the knee and have also been shown to play an important role in knee loading in individuals with knee OA. For instance, the KFM has been shown to influence medial knee joint contact forces,11, 12 and baseline KFM values were shown to predict 5-year tibiofemoral cartilage changes in individuals with medial compartment knee OA.10 However, another longitudinal study found no association between baseline peak KFM on any medial knee OA progression outcome measures after 2 years.13 Patients with severe knee OA also exhibit smaller knee extension angles and a reduced KEM in late stance when compared with asymptomatic individuals and older individuals with moderate knee OA.14 This has been hypothesized to be a compensatory strategy to increase knee joint stiffness and reduce the external load on the knee joint in individuals with knee OA.15 Consequently, further investigation is required to better understand how the KFM and KEM may impact knee OA-related cartilage changes.
Knee OA can be categorized as nontraumatic (patients with no history of a previous knee injury or trauma) or posttraumatic (OA diagnosed secondary to trauma, injury, surgery).16 Previous research suggests that there are differences in the structural changes that occur between nontraumatic and posttraumatic knee OA progression.16-19 Patients with knee OA and concomitant anterior cruciate ligament (ACL) tears have demonstrated more frequent OA-related findings in the lateral compartment, including increased denuded area and bone surface area, bone marrow lesions, and meniscal alterations, compared to patients with knee OA with an intact ACL.19 Radiographic findings such as joint space narrowing and osteophytes were found to be predominantly in the medial compartment of the knee joint in patients with nontraumatic knee OA, whereas it was found to be evenly distributed between medial and lateral compartments in patient with posttraumatic knee OA.16 Lastly, frontal plane knee kinetics during gait20 and the relationship between knee alignment and cartilage thickness differ between patients with and nontraumatic and posttraumatic medial compartment knee OA.21 Therefore, differences in structural OA-related changes and knee mechanics exist between patients with nontraumatic and posttraumatic knee OA.
Considering there are differences in gait metrics between nontraumatic and posttraumatic knee OA, the relationship between measures of disease progression (e.g., cartilage thickness) and knee moments might also differ between these knee OA subtypes. This has not been previously examined. A greater understanding of this relationship would provide valuable insight into the potential difference in mechanisms affecting disease progression between these OA subtypes. The objective of this study was to examine the relationship between external knee joint moments (KAM, KFM, and KEM) during gait and cartilage thickness, measured using MRI, in patients with nontraumatic and posttraumatic knee OA. We hypothesized that the relationship between knee joint moments during gait and cartilage thickness would differ between participants with nontraumatic and posttraumatic knee OA.
2 METHODS
2.1 Participants
This observational, cross-sectional study (level II evidence) recruited participants diagnosed with symptomatic knee OA, according to clinical criteria from the American College of Rheumatology.22 Participants were recruited from three tertiary hospitals in Montreal, Canada and the local community from January 2015 to March 2017. They were between the ages of 35 and 75. Exclusion criteria included knee trauma or surgery within the last 12 months, history of joint arthroplasty in the lower extremities, neurological conditions (e.g., previous stroke), severe cardiovascular conditions (e.g., angina pectoris), or any other conditions affecting gait. Participants were part of an ongoing longitudinal study,21, 23 and all available participants were analyzed for the current study. Participants provided written, informed consent before enrollment. Procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (Jewish General Hospital) and with the Helsinki Declaration of 1975, as revised in 2000.
Participants that reported no previous knee trauma resulting in an ACL rupture were classified as nontraumatic OA (n = 22). Participants in the posttraumatic knee OA group (n = 19) had a history of ACL injury. This posttraumatic OA group included participants with an ACL deficiency (n = 9) or reconstructed ACL (n = 10). Other traumatic injuries (e.g., posterior cruciate ligament tear, isolated meniscal tear) were excluded from the study to ensure homogeneity. ACL status for all participants (injured, normal, and/or reconstructed) was confirmed on MRI by a fellowship trained, musculoskeletal radiologist with 8 years of experience. Participants provided an estimate of when the ACL tear occurred. In patients with bilateral knee OA, the side with the most severe symptoms was selected based on participants' reporting of pain intensity.
Demographic variables were self-reported (i.e., age and sex) from participants (Table 1). Height was measured with a measuring tape and mass using a force plate. Pain was assessed using the Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP), which is a multidimensional, 11-item measure designed to comprehensively evaluate the pain experience in people with hip or knee OA. The ICOAP has demonstrated excellent test-retest reliability, internal consistency, and convergent validity with other instruments used to assess OA pain.24-26
| Measure | Nontraumatic OA (n = 22) | Posttraumatic OA (n = 19) | p-value |
|---|---|---|---|
| Age (year) | 60 (7) | 56 (9) | 0.191 |
| Sex, n (%) | |||
| Men | 6 (27) | 11 (58) | 0.047 |
| Women | 16 (73) | 8 (42) | |
| Body mass index (kg/m²) | 29.6 (7.5) | 26.0 (3.2) | 0.023 |
| Gait speed (m/s) | 1.14 (0.14) | 1.23 (0.15) | 0.265 |
| ICOAP | |||
| Constant Pain (/100) | 19 (24) | 16 (19) | 0.704 |
| Intermittent Pain (/100) | 31 (20) | 27 (21) | 0.512 |
| Total Score (/100) | 26 (21) | 22 (19) | 0.584 |
| Radiographic knee OA severity, n (%) | |||
| Grade 1 | 2 (10) | 1 (5) | |
| Grade 2 | 6 (28) | 11 (58) | |
| Grade 3 | 8 (38) | 5 (26) | |
| Grade 4 | 5 (24) | 2 (11) | 0.297 |
- Note: Mean (SD) and number of participants (n) are provided for group descriptors. Significant associations (p < 0.05) are in bold. The p-value for Radiographic Knee OA severity is for all Kellgren–Lawrence grades.
- Abbreviations: ICOAP, Intermittent and Constant Osteoarthritis Pain; OA , osteoarthritis; SD, standard deviation.
2.2 Radiographs
Participants underwent hip to ankle, anterior-posterior radiographs in standing. The participants stood barefoot, with feet and toes facing forward and the patella centered on the femoral condyles.27 Kellgren–Lawrence disease severity scores (0 = no OA to 4 = severe OA) provided a measure of disease severity.28
2.3 Gait data collection
Gait was measured using an eight camera, motion capture system (OQUS 300 + , Qualisys) sampled at 100 Hz and two synchronized force plates (BP400600, AMTI) sampled at 2000 Hz. Marker and force plate data were captured with commercial software (Qualisys Track Manager, Qualisys). Similar procedures have previously demonstrated acceptable test-retest reliability.29, 30 Thirty-four reflective markers (12.7 mm diameter) were placed on the participants according to guidelines.29 Eighteen individual markers were placed with adhesive tape over boney landmarks including: acromion, anterior and posterior superior iliac spines, femoral greater trochanters and lateral epicondyles, lateral malleoli, first and fifth metatarsal heads, and calcanei. Four reflective marker clusters of four markers were also placed on the mid-shank and mid-thigh bilaterally using a Velcro strap. Six additional markers were placed bilaterally during static standing trials only to determine joint center position: femoral medial epicondyles, medial malleoli, and second metatarsal heads.
Data collection began with participants completing a standing trial on a force plate to identify knee and ankle joint centers and to measure body mass. Afterward, they completed two trials (one trial per side) to identify functional hip joint centers. These trials required participants to flex/extend and abduct/adduct their hip three times in each direction.31, 32 Before collecting gait trials, participants were allowed at least four practice gait trials to adjust to the environment. Participants were then required to walk barefoot, at self-selected speeds, along an 8-m walkway for seven successful trials for each leg. Participants were instructed to walk at their normal walking speed. A trial was deemed successful if an adequate force plate strike was achieved with one foot. Only five trials were processed. Additional trials were collected to account for potential errors.
2.4 Gait data processing
Data processing was completed using Visual3D (v 5.02, C-motion). Marker and force plate data were filtered with dual pass, Butterworth filters (fourth order) with cut-off frequencies of 6 Hz and 20 Hz, respectively. Inverse dynamics using Newton–Euler equations with previously published segment inertial properties were used to calculate net external knee moments.33 Moments were calculated about the knee joint coordinate system, amplitude normalized to body mass, and time normalized to 100% gait cycle. Gait parameters included peak KAM during early stance, peak KFM during early stance, and peak KEM in late stance. The KAM impulse during stance was also determined from waveforms not normalized to 100% gait cycle. Gait speed was determined by tracking the speed of the posterior superior iliac spine markers. Discrete parameters were determined for each trial and averaged across five trials for each participant, and thus average values were used in subsequent statistical analyses.
2.5 Cartilage thickness measurement
Images of articular cartilage at the tibiofemoral joint were obtained with a 3T MRI system (GE Discovery MR750) with a knee coil. The sequence was a three-dimensional (3D) sagittal T1* spoiled gradient-echo sequence with fat suppression (TR = 13 ms, TE = 4 ms, NEX = 0.6, FoV = 160 mm, matrix = 512 × 512 pixels, flip angle = 14°, pixel bandwidth = 434 Hz/px). To limit patient movement during the MRI, a strict immobilization protocol was used.
Cartilage thickness was determined according to a previously described and validated method, which has demonstrated excellent test-retest reliability (Pearson correlation coefficients of 0.97 for the global knee, 0.96 for the femur, and 0.83 for the tibia) with low measurement error (−0.3 ± 1.6% for the global knee, 0.14 ± 1.7% for the femur).34 To briefly summarize, the bone was first segmented using a ray-casting approach,35 then the cartilage was quantified using a texture analysis process in a bone proximity resampled MRI image.34 For the bones (i.e., femur and tibia), the image was resampled in a polar coordinate system using a ray casting method following a coarse localization of the joint. The bone contours were localized along the radii using characteristics of a Gaussian of Laplacian filter. A spline surface representing the bone surface was built using the selected 3D positions. For the femur and tibia cartilage assessment, the MRI image was resampled perpendicularly to each surface independently. A texture analysis based on spatial gray level dependency (SGDL) properties allowed for the delineation of the cartilage-to-soft-tissues interface while helping with the tuning of the bone-to-cartilage interface. Some final processing allowed for the consideration of fluid exclusion, and image inhomogeneity. Cartilage thickness was quantified for the following regions: medial femoral condyle, lateral femoral condyle, medial tibial plateau, and lateral tibial plateau. The ratio of medial:lateral cartilage thickness from these compartments was also determined. These regional cartilage thickness measures were chosen to increase the sensitivity to detect regional differences in cartilage thickness associated to different joint moments.
2.6 Statistical analysis
Descriptive statistics were determined for each group for sample descriptors, discrete gait parameters, and cartilage thickness measures. An independent student t-test was used to compare the age, gait speed, and ICOAP between groups (nontraumatic and posttraumatic). A Welch test was used to compare body mass index (BMI) between groups given a statistically significant Levene test for homogeneity of variance. χ2 test compared Kellgren–Lawrence grades and sex proportions between groups.
Forced entry linear regression analyses examined relationships between cartilage thickness measures (dependent variable) with gait parameters, OA group, and their interaction. Four analyses were conducted for each of the cartilage thickness measures. Each analysis varied in the gait parameter examined (peak KAM, KAM impulse, peak KFM, and peak KEM). Age, sex, BMI, and radiographic disease severity (Kellgren–Lawrence grades) were entered first, as covariates. Gait parameter and OA group (0 = nontraumatic and 1 = posttraumatic) were entered in the second and third steps, respectively. Next, their interaction was entered and was only retained if it was significant. Unstandardized regression coefficients (B) with 95% confidence intervals and total explained variance (R2) were reported. Statistical significance was set at p < 0.05. Appropriateness of the analyses was evaluated by examining data normality, residuals, and multicollinearity using histogram of residuals, plots of residuals, collinearity statistics, or variance proportions. Analyses were performed with SPSS (v24, IBM Corp).
3 RESULTS
Participant demographics and group descriptors showed that there were no significant differences between groups for age, radiographic disease severity, gait speed, and pain (Table 1). However, significant differences were found between groups for BMI and sex in which the nontraumatic knee OA group had a greater mean BMI and higher proportion of women when compared with the posttraumatic OA group. Regression coefficients are provided in Table 2.
| Gait variable | Cartilage region | Gait variable B (95% CI) | OA group B (95% CI) | Interaction B (95% CI) | Model explained variance (R2) |
|---|---|---|---|---|---|
| Peak KAM | Med Femoral Condyle | −0.58 | −0.01 | – | 41.7% |
| (−1.02 to −0.14) | (−0.18 to 0.15) | ||||
| Lat Femoral Condyle | 0.58 | 0.06 | – | 34.1% | |
| (0.10 to 1.06) | (−0.12 to 0.24) | ||||
| Med Tibial Plateau | −0.50 | −0.06 | – | 27.5% | |
| (−1.02 to 0.02) | (−0.25 to 0.13) | ||||
| Lat Tibial Plateau | 1.05 | −0.01 | – | 36.9% | |
| (0.35–1.75) | (−0.27 to 0.26) | ||||
| Med:Lat Cartilage | −0.67 | −0.02 | – | 49.5% | |
| (−0.97 to −0.38) | (−0.13 to 0.09) | ||||
| KAM Impulse | Med Femoral Condyle | −1.13 | −0.03 | – | 37.6% |
| (−2.24 to −0.03) | (−0.20 to 0.14) | ||||
| Lat Femoral Condyle | 1.23 | 0.07 | – | 31.5% | |
| (0.05–2.41) | (−0.11 to 0.25) | ||||
| Med Tibial Plateau | −1.21 | −0.07 | – | 27.5% | |
| (−2.46 to 0.04) | (−0.26 to 0.13) | ||||
| Lat Tibial Plateau | 2.07 | 0.02 | – | 31.1% | |
| (0.30–3.84) | (−0.25 to 0.29) | ||||
| Med:Lat Cartilage | −1.97 | −0.27 | 1.47 | 47% | |
| (−2.92 to −1.01) | (−0.52 to −0.01) | (0.01, 2.93) | |||
| Peak KFM | Med Femoral Condyle | −0.18 | −0.05 | – | 30.6% |
| (−0.66 to 0.29) | (−0.23 to 0.13) | ||||
| Lat Femoral Condyle | 0.28 | 0.10 | – | 24.9% | |
| (−0.23 to 0.79) | (−0.09 to 0.29) | ||||
| Med Tibial Plateau | −0.20 | −0.09 | – | 20.3% | |
| (−0.74 to 0.34) | (−0.29 to 0.11) | ||||
| Lat Tibial Plateau | 0.72 | 0.07 | – | 27.7% | |
| (−0.02 to 1.47) | (−0.21 to 0.35) | ||||
| Med:Lat Cartilage | −0.34 | −0.07 | – | 25.5% | |
| (−0.70 to 0.02) | (−0.20 to 0.07) | ||||
| Peak KEM | Med Femoral Condyle | −0.86 | 0.42 | 1.42 | 45.9% |
| (−1.50 to −0.22) | (0.05–0.79) | (0.40–2.44) | |||
| Lat Femoral Condyle | 0.37 | 0.10 | – | 25.3% | |
| (−0.25 to 0.99) | (−0.09 to 0.29) | ||||
| Med Tibial Plateau | −0.37 | −0.09 | – | 22.2% | |
| (−1.03 to 0.28) | (−0.29 to 0.11) | ||||
| Lat Tibial Plateau | 0.22 | 0.05 | – | 19.7% | |
| (−0.74 to 1.18) | (−0.24 to 0.34) | ||||
| Med:Lat Cartilage | −0.73 | 0.25 | 0.94 | 35.9% | |
| (−1.23 to −0.22) | (−0.05 to 0.54) | (0.14–1.75) |
- Note: Age, sex, and radiographic knee OA severity were controlled for in the regression analyses. Unstandardized coefficients (B) with 95% confidence intervals are provided. Significant associations (p < 0.05) are in bold. Only significant interactions were retained in the model.
- Abbreviations: CI, confidence interval; KAM, knee adduction moment; KEM, knee extension moment; KFM, knee flexion moment; OA, osteoarthritis.
3.1 Peak KAM
Higher peak KAM was associated with lower medial femoral condyle cartilage thickness (p = 0.01), lower medial:lateral cartilage thickness ratio (p < 0.01), higher lateral femoral condyle cartilage thickness (p = 0.02), and higher lateral tibial plateau cartilage thickness (p < 0.01), after accounting for the covariates. For all five analyses, OA group and its interaction with peak KAM were not statistically significant. The explained variance (R2) in cartilage thickness for the regression models ranged from 28% to 50%, depending on the regions evaluated (Table 2).
3.2 KAM impulse
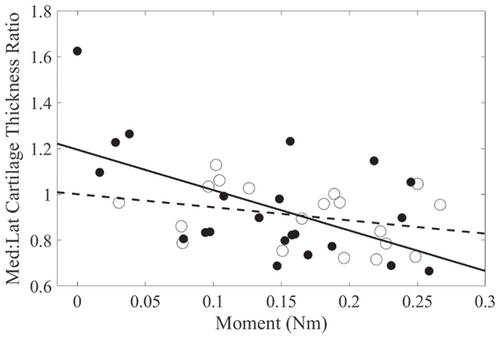
Higher KAM impulse was associated with lower medial femoral condyle cartilage thickness (p = 0.04), higher lateral femoral condyle cartilage thickness (p = 0.04), and higher lateral tibial plateau cartilage thickness (p = 0.02). For medial:lateral cartilage thickness, KAM impulse, OA group, and their interaction significantly explained the variance in the medial:lateral cartilage thickness ratio. Higher KAM impulse was associated with lower medial:lateral cartilage thickness ratio (r = −0.56) in the nontraumatic OA group, while this relationship was weaker in the posttraumatic OA group (r = −0.30, Figure 1). KAM impulse was not significantly associated with medial tibial plateau cartilage thickness (p = 0.06). The explained variance (R2) in cartilage thickness for the regression models ranged from 28% to 47%, depending on the regions evaluated (Table 2).
3.3 Peak KEM
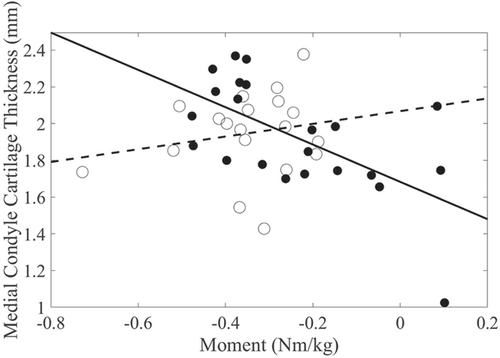
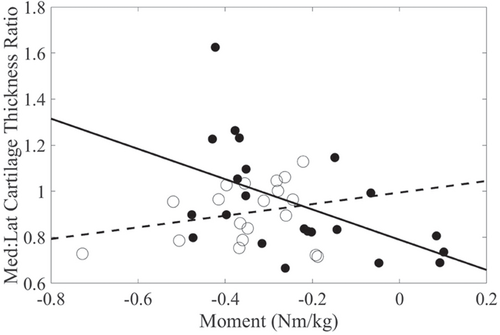
Peak KEM, OA group, and their interaction significantly explained the variance in medial femoral condyle thickness. Peak KEM was negatively associated with medial femoral condyle cartilage thickness (r = −0.61) in the nontraumatic OA group, while this relationship was found to be weaker in the posttraumatic OA group (r = 0.10, Figure 2). Additionally, higher peak KEM was associated with lower medial:lateral cartilage thickness. The relationship with KEM was stronger in the nontraumatic (r = −0.51) than the posttraumatic OA group (r = 0.25, Figure 3). Peak KEM was not significantly associated with medial tibial plateau, lateral tibial plateau, or lateral femoral condyle cartilage thickness measures. The explained variance (R2) in cartilage thickness for the regression models ranged from 20% to 46%, depending on the regions evaluated (Table 2).
3.4 Peak KFM
There were no statistically significant associations between peak KFM and regional cartilage thickness. OA group and their interaction did not significantly explain the variance in cartilage thickness (Table 2).
4 DISCUSSION
This study demonstrated that there is a relationship between knee mechanics during gait and cartilage thickness. Higher KAM values were related to lower medial compartment and higher lateral compartment cartilage thickness. Furthermore, the relationship between knee moments and cartilage thickness differed between nontraumatic and posttraumatic knee OA subtypes for the KAM impulse and peak KEM. Considering that the relationship between mechanical factors and knee OA progression differ between these OA subtypes, perhaps treatments that target these mechanical factors (e.g., tibial osteotomy) to slow OA progression should also differ.
The inverse relationship between the peak KAM and medial compartment cartilage thickness for both OA groups suggests that the peak KAM relates to medial tibiofemoral OA progression. This is consistent with previous studies examining the relationship between peak KAM and radiographic knee OA severity,8 radiographic knee OA progression,3 and cartilage thickness loss determined using MRI.10 However, other studies have demonstrated conflicting results.9, 36 This discrepancy could be explained by differences in the regions of cartilage thickness measured and the distribution of OA changes in the participants. Nonetheless, our data are concordant with the fact that an increase in medial knee joint loading has been shown to play a role in medial tibiofemoral OA progression.3 Given the KAM is a proxy for medial to lateral tibiofemoral compartment loading, a higher peak KAM would imply greater loading on the medial compartment. This could be detrimental in individuals with altered gait kinematics (i.e., individuals with knee OA or post knee injury), as a shift can occur in the contact location to cartilage regions not normally conditioned to increased loading. The inability of these cartilage regions to adapt to changes in load bearing could lead to degenerative changes in the medial tibiofemoral compartment.6 We also found a higher KAM impulse to be negatively associated with the medial:lateral cartilage thickness ratio in the nontraumatic OA group, while this relationship was weaker in the posttraumatic OA group. Our findings could be explained by several factors. First, the relationship between the KAM impulse and cartilage thickness measures may vary based on the OA subtype. This would indicate that the influence of cumulative medial knee joint loading (i.e., KAM impulse) on articular cartilage, rather than the medial knee joint load magnitude (i.e., peak KAM), may differ between patients with nontraumatic and posttraumatic knee OA. Second, participants in the nontraumatic knee OA group had a significantly greater BMI than participants in the posttraumatic knee OA group. Previous research would suggest that higher dynamic knee joint loading and KAM impulse, but not peak KAM, is a significant risk factor for medial tibial cartilage volume loss.9 As a result, the altered loading patterns37 and increased tibiofemoral compressive and shear contact forces and dynamic loads during gait38 seen in patients with knee OA with a higher BMI may further contribute to medial knee OA progression. However, BMI was accounted for in statistical analyses. Lastly, the KAM impulse has been shown to be more sensitive at distinguishing between radiographic knee OA severities.39 Consequently, our results could be due to the fact that the nontraumatic OA group had a greater proportion of participants with moderate to severe radiographic knee OA. Although, radiographic disease severity was accounted for in our analyses. Longitudinal research is needed to better understand the potential influence of the peak KAM and KAM impulse on tibiofemoral cartilage thickness loss between individuals with nontraumatic and posttraumatic knee OA. A greater understanding of this relationship would provide valuable insight into if mechanisms affecting disease progression differ between these OA subtypes.
In addition, higher late stance KEM (i.e., more negative values) was associated with greater medial femoral condyle cartilage thickness and medial:lateral cartilage thickness ratio in the nontraumatic OA group. This relationship was weaker in the posttraumatic OA group. The history of injury could account for these findings. Participants in the posttraumatic OA group may have continued to experience persistent alterations in lower extremity neuromuscular function and movement patterns post ACL injury or reconstruction, which would ultimately alter walking kinematics and knee joint loading patterns.40-42 Alternatively, the nontraumatic OA group had a greater proportion of participants with moderate or severe OA. This could potentially account for our findings, as greater OA severity has been associated with diminished late stance KEM;14, 43 although severity was accounted for in the analyses. Our findings may suggest that the role of the peak KEM on MRI measures of tibiofemoral cartilage thickness differs between individuals with nontraumatic and posttraumatic knee OA.
Lastly, the KFM was not associated with any regions of cartilage thickness. Longitudinal studies examining the relationship between baseline KFM and cartilage thickness measures demonstrate conflicting results.10, 13 Further research is needed to better understand the relationship between KEM/KFM and measures of cartilage thickness in individuals with nontraumatic and posttraumatic knee OA.
This study has limitations. First, this study was cross-sectional and does not allow for the inference of causal relationships. Second, between-group differences in sex and BMI may have confounded the results. However, these factors were accounted for in the analyses. Third, the posttraumatic knee OA group consisted of both participants with a ruptured ACL and participants with a surgically reconstructed ACL to increase the sample size. However, this is less of a concern, given ACL reconstruction does not decrease knee OA prevalence,44 nor fully restore normal knee joint kinematics when compared with ACL-deficient knees.45 Lastly, our results are not generalizable to patients with other traumatic knee injuries (i.e., posterior cruciate ligament tear, meniscal tear in isolation, etc.).
To conclude, relationships existed between peak KAM, KAM impulse, and peak KEM during gait with regional tibiofemoral cartilage thickness. In addition, the relationship between KAM impulse and peak KEM with regional tibiofemoral cartilage thickness was dependent on the OA subtype (nontraumatic vs. posttraumatic knee OA). The influence of knee joint moments on regional cartilage thickness in patients with nontraumatic and posttraumatic knee OA had not been previously examined. As a result, our findings provide new insight into the pathomechanics of knee OA and support the hypothesis that the potential influence of mechanical knee loading on articular cartilage differs between patients with nontraumatic and posttraumatic knee OA. This has important implications for clinical practice. For instance, addressing alterations in walking kinematics and knee joint loading patterns between patients with non-traumatic and posttraumatic knee OA may warrant different treatment approaches and should be explored further. Future research should also examine the longitudinal influence of gait kinematics and kinetics on indicators of disease progression in both knee OA subtypes.
ACKNOWLEDGMENTS
The authors would like to thank Larissa Fedorowich, Mohan Patel, and Antonys Melek for assistance with data collection, as well as Dr. François Abram for magnetic resonance imaging (MRI) processing. Drs. John Antoniou (Jewish General Hospital), Moreno Morelli (St. Mary's Hospital), and Paul Martineau (Montreal General Hospital) from Montreal, Canada assisted with participant recruitment. This study was supported by the Canadian Foundation for Innovation which provided funding for the infrastructure (Robbins, grant #31903), the Canadian Institutes of Health Research (grant number ONM 137376), and the Arthritis Society (grant number YIO-14-096). Shawn Robbins is supported by the Arthritis Society (grant number YIS-14-065) and the Fonds de recherche du Québec – Santé (grant number 33107). The funding sources had no role in this study.
CONFLICT OF INTERESTS
Jean-Pierre Pelletier and Johanne Martel-Pelletier are shareholders in ArthroLab Inc; company assessing the magnetic resonance imaging (MRI) images. Mr. Teoli provides continuing education courses on knee osteoarthritis best practice for rehabilitation professionals. The remaining authors have nothing to disclose.
AUTHOR CONTRIBUTIONS
Anthony Teoli was responsible for data acquisition, data processing, statistical analysis, data interpretation, as well as drafting and revising the article. Melissa Cloutier-Gendron was responsible for data acquisition, data processing, statistical analysis, and revising the article. Shirley Yin Kay Ho was responsible for data acquisition, data processing, statistical analysis, and revising the article. Susan Gu was responsible for data acquisition, data processing, statistical analysis, and revising the article. Jean-Pierre Pelletier was responsible for study design, magnetic resonance imaging (MRI) data processing, and interpretation, and revising the article. Johanne Martel-Pelletier was responsible for study design, MRI data processing and interpretation, and revising the article. Shawn Robbins was responsible for obtaining funding, study conception and design, data acquisition, data processing, statistical analysis, data interpretation, as well as drafting and revising the article. All authors have read and approved the final submitted manuscript.




