Analgesic effects and arthritic changes following tramadol administration in a rat hip osteoarthritis model
Abstract
We investigated the analgesic effects of tramadol and the arthritic changes following tramadol administration in the rat hip osteoarthritis (OA) model using mono-iodoacetate (MIA). The right hip joints of male Sprague–Dawley rats (n = 5 rats/group) in the Sham group were injected with 25 μl of sterile saline and 1% of fluorogold (FG) retrograde neurotracer. In the MIA + Vehicle and MIA + Tramadol groups, FG and 25 μl of sterile saline with 0.5 mg of MIA were injected into the right hip joint. The MIA + Vehicle and MIA + Tramadol groups were administered daily for 4 weeks, either sterile saline (10 mg/kg, intraperitoneal [i.p.]) or tramadol (10 mg/kg, i.p.). We assessed hyperalgesia every week after MIA administration. Histopathological changes and immunoreactive neurons for calcitonin gene-related peptide (CGRP) in dorsal root ganglia (DRG) were evaluated after 4 weeks of treatment. MIA injection into the hip joint led to mechanical hyperalgesia (p < 0.01), which was significantly reduced by tramadol administration (p < 0.01). Furthermore, daily i.p injection of tramadol significantly suppressed CGRP expression in DRG (p < 0.0001). MIA + Vehicle and MIA + Tramadol groups showed significant cartilage reduction and degeneration compared to the Sham group (p < 0.0001). Interestingly, OA changes significantly progressed in the MIA + Tramadol group compared to the MIA + Vehicle group (p < 0.0001).
1 INTRODUCTION
Osteoarthritis (OA) is highly prevalent worldwide; it is a leading cause of disability and negatively impact the physical and mental well-being of affected patients.1 Pain is the commonest symptom of OA and occurs more often than other symptoms such as stiffness or disability.2 In patients with OA pain, joint pathology does not always reflect the degree of pain. Reportedly, up to 40% of patients with radiographic OA findings have no pain, whereas patients with slight OA indications in radiographic examinations experience severe to debilitating pain.3 However, the mechanism of OA-associated pain has not yet been fully understood. Various animal models have been reported for knee OA, such as models induced by anterior cruciate ligament transection4 and partial medial meniscectomy.5 Mono-iodoacetate (MIA) is frequently used to chemically induce OA in rat models via intra-articular injection to the knee.6, 7 Since there are only a few surgically induced hip joint models, an animal model using MIA has recently been developed. It has been reported that an intra-articular MIA injection to the hip joint8 led to the expression of calcitonin gene-related peptide (CGRP), which functions as a mediator of peripheral neurogenic inflammation,9 and activation of transcription factor 3, which is usually regarded as a neuronal damage marker10-12 expressed late in the dorsal root ganglia (DRG) of the rat hip, thus reflecting the involvement of both neurogenic inflammation and local nerve damage in the rat OA model. It has also been reported that duloxetine exerts a pain-relieving effect on neuropathic pain in this animal model.13
Tramadol is a weak opioid that acts on µ-opioid receptors, with a lower affinity for the receptor than morphine.14 Tramadol is widely recognized and used for relieving pain in OA.15, 16 Animal studies have demonstrated the pain-relieving effect of tramadol in an MIA-induced knee OA model.14, 17 In contrast, studies on the effect of opioid administration on arthritic changes report that OA progressed in three patients with the use of transdermal administration of strong opioid fentanyl over a relatively short period.18
Therefore, there is a hypothesis that excessive pain relief promotes arthritic changes. Although tramadol is clinically used more frequently than potent opioids in OA treatment, to the best of our knowledge, no study has reported whether the excessive use of tramadol for pain relief accelerates OA progression. Therefore, the purpose of this study was to investigate the pain-relieving effects of tramadol and the progression of osteoarthritic changes using a rat hip OA model induced by MIA.
2 METHODS
2.1 Ethical approval
All animal testing protocols were reviewed and approved by the ethics committee of the Chiba University Hospital. All animal experiments adhered to the National Institutes of Health guidelines for the management and use of laboratory animals.
2.2 Intra-articular injection of MIA and retrograde neurotracing
We used 20, 6-week-old male Sprague-Dawley rats weighing 250–300 g (CLEA). The rats were housed in a semibarrier system with a controlled environment (12 h light/dark cycle, temperature: 21°C–23°C, and humidity: 45%–65%). All rats were given water and food ad libitum and were fed a standard rodent chow diet (CRF-1; Oriental Yeast Co., Ltd.). Based on previously published research,19 all rats were anesthetized with an intraperitoneal (i.p.) injection of 0.3 mg/kg of medetomidine (Nippon Zenyaku Kogyo Co., Ltd.), 4.0 mg/kg of midazolam (Maruishi Pharmaceutical. Co., Ltd.), and 5.0 mg/kg of butorphanol (Meiji Seika Pharma. Co., Ltd.) and treated aseptically throughout the experiments. The control (Sham) group comprised five rats, which received 25 µl of sterile saline injection into their right hip joints. The experimental group comprised 10 rats, which received 0.5 mg of MIA injection into their right hip joints using a 27-gauge needle.8, 20 The remaining five rats were assigned to the Sham + Tramadol group (n = 5). Sham and the 10 experimental rats received 1% fluorogold (FG) retrograde neurotracer injections into their right hip joints. Two weeks after MIA injection, the 10 experimental rats were randomly placed into treatment groups that received either saline (MIA + Vehicle group, n = 5: 10 mg/kg, i.p.) or tramadol (MIA + Tramadol group, n = 5: 10 mg/kg, i.p.) daily for 4 weeks. The rats in the Sham group were only administered the vehicle. The rats in the Sham + Tramadol group were administered tramadol (10 mg/kg, i.p.) daily for 4 weeks to evaluate the effects of i.p. administration of tramadol on the hip joints. Tramadol was dissolved in 0.5 ml saline, and its dose was determined based on previous studies.21, 22
2.3 Behavioral tests
We evaluated mechanical cutaneous plantar sensitivity using the von Frey assay every week for 4 weeks after treatment in a behavioral study. Five rats in each of the three groups (Sham, MIA + Vehicle, and MIA + Tramadol) were subject to behavioral testing. The rats were randomly selected and allowed to adapt to the test chamber for 1 h before performing behavioral tests, so the experimenter was blinded when assessing mechanical sensitivity. Baseline thresholds were acquired before MIA induction. Two weeks after injection of MIA or sterile saline, behavioral testing was performed weekly for 4 weeks. Calibrated von Frey filaments (Monofilament kit; Smith & Nephew) were applied for 4 s or until withdrawal (whichever occurred first) and the 50% paw withdrawal threshold (PWT, g) was calculated.23 The stimulus intensity ranged from 1 to 60 g, corresponding to the number of filaments (4.08, 4.17, 4.31, 4.56, 4.74, 4.93, 5.07, 5.18, 5.46, and 5.88). For each animal, the actual filaments used within the previously mentioned series were identified based on the lowest filament to elicit a positive response, followed by five consecutive stimulations using the up-down method.24, 25 The filament range and average spacing were incorporated into individual threshold calculations, along with the response pattern. Mechanical hypersensitivity on the plantar surface area of the right (ipsilateral) hind paw was assessed using a wire mesh observation cage. Data are presented as the 50% PWT for each group ± standard error of the mean.
2.4 Immunohistochemical expression of CGRP
After the transcardiac perfusion with 0.9% saline, followed by 500 ml of 4% paraformaldehyde in phosphate buffer (0.1 M, pH 7.4), the right DRG in the three groups (Sham, MIA + Vehicle, and MIA + Tramadol) from the L4 levels were resected 4 weeks after tramadol or vehicle administration. DRG specimens were immersed in phosphate-buffered paraformaldehyde overnight at 4°C. They were frozen in liquid nitrogen after storage in 0.01 M phosphate-buffered saline (PBS) consisting of 20% sucrose for 20 h at 4°C. Using a cryostat, the DRG were sliced into 10-µm-thick sections (CM3050S; Leica Microsystems). Consequently, sections were mounted on poly-l-lysine-coated slides. The specimens were then treated with a nonspecific binding site-blocking solution comprising PBS with 0.3% Triton X-100% and 3% skim milk for 90 min at room temperature. Specimens were processed for CGRP immunohistochemistry using a rabbit antibody against CGRP (1:1000; Chemicon, Temecula). After incubation with the diluted antibody for 20 h at 4°C, the DRG sections were incubated with Alexa Fluor 488-conjugated goat antirabbit IgG (for CGRP immunoreactivity, 1:1000; Molecular Probes). After each step, the sections were rinsed three times with PBS. The immunostained sections were observed using a fluorescence microscope (Olympus) in a treatment-blinded manner. For each DRG section, blinded observers counted the numbers of FG-labeled (only) neurons, the numbers of FG-labeled neurons with CGRP-immunoreactivity (ir) per 0.45 mm2 in 10 randomly selected fields at ×400 magnification using a counting grid. The proportions of FG-labeled DRG neurons with CGRP-IR among all FG-labeled DRG neurons were then determined.
The numbers of FG-labeled CGRP-immunoreactive (ir) DRG neurons were counted by blinded observers, and their proportion relative to the total number of FG-labeled DRG neurons was calculated for each DRG sample.
2.5 Histopathological findings
For histological evaluation, samples from all groups were obtained to assess OA progression after MIA administration (after 4 weeks of tramadol or vehicle administration). The rats were anesthetized intraperitoneally with 0.3 mg/kg medetomidine, 4.0 mg/kg midazolam, and 5.0 mg/kg butorphanol. Next, they were perfused transcardially with 0.9% saline, followed by 500 ml of 4% paraformaldehyde in phosphate buffer fixative (0.1 M, pH 7.4). Soft tissues around the right hip joint, such as the cartilage, synovium, and capsule, were resected. The resected limb was cut in the middle of the femur, and the center of the femoral head was immersed in 10% neutral buffered formalin for 3 days. The specimens were continuously demineralized with the reagent K-CX (FALMA) for 30 h and 5% sodium sulfate for 16 h, and then embedded in paraffin to prepare coronary sections.
The samples were continuously sectioned in 8 µm at the maximum diameter of the femoral head. Fifteen sections in each group were stained using hematoxylin and eosin, safranin O, and toluidine blue (five sections each). Osteoarthritic changes were evaluated using the Osteoarthritis Research Society International (OARSI) histopathology scoring system.25 Two hip surgeons experienced in basic experiments were blindly evaluating and scoring. For each joint, we scored a typical slice of the maximum diameter of the femoral head. Each sample was evaluated by the depth of change (grading) and width (staging) of OA, and the score was finally expressed by multiplying the depth and width. The average scores for each group were compared between groups.
2.6 Statistical analysis
Statistical analyses were performed using GraphPad Prism 8 software (GraphPad Software). The PWT, OARSI scores, and proportions of CGRP-ir FG-labeled neurons in the DRG among groups were compared using two-way analysis of variance, followed by Tukey's multiple comparison test. Statistical significance was set at p < 0.05.
3 RESULTS
3.1 Behavioral tests
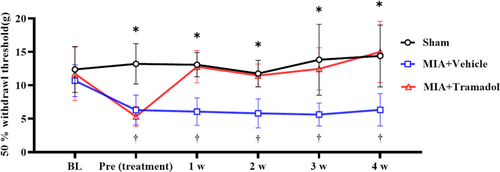
Compared to the Sham group, the behavioral pain tests showed that rats in the MIA + Vehicle group showed significant mechanical hypersensitivity every week for 4 weeks after treatment (p < 0.01; Figure 1). In contrast, the MIA + Tramadol group showed significant improvement compared to the MIA + Vehicle group every week after the i.p. administration of tramadol (p < 0.01).
3.2 Immunohistochemical expression of CGRP
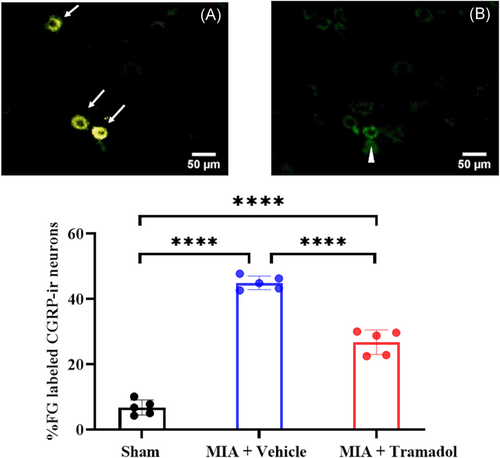
After 4 weeks of tramadol or vehicle administration, the MIA + Vehicle and MIA + Tramadol groups presented significantly higher expression levels of FG-labeled CGRP-ir DRG neurons in L4 than the Sham group (p < 0.0001; Figure 2). Furthermore, the proportion of FG-labeled CGRP-ir DRG neurons in the MIA + Tramadol group was significantly lower than that in the MIA + Vehicle group (p < 0.0001).
3.3 Histopathological findings
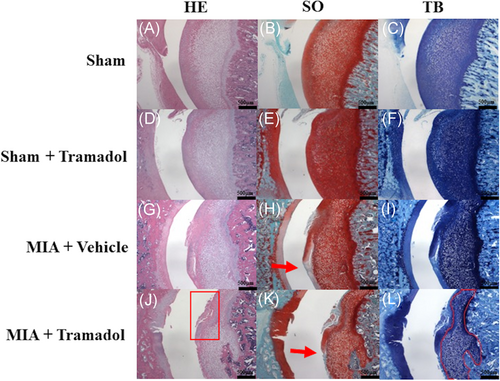
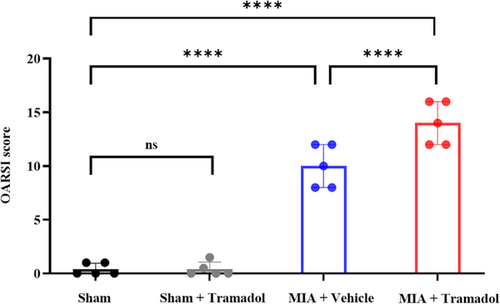
The MIA + Vehicle and MIA + Tramadol groups presented progressive OA changes, whereas the Sham and Sham + Tramadol groups maintained a normal appearance (Figure 3). The OARSI score was higher in the MIA + Vehicle group than in the Sham group (p < 0.0001; Figure 4). Furthermore, compared to the MIA + Vehicle group, OA changes were significantly more pronounced in the MIA + Tramadol group (p < 0.0001; Figure 4).
4 DISCUSSION
In this study, mechanical hypersensitivity was observed in the MIA + Vehicle group. The expression of CGRP in the DRG was significantly higher in the MIA + Vehicle group than in the Sham group. Histologically, progressive arthritic changes were observed in the 0.5 mg MIA + Vehicle group than in the Sham group. Furthermore, the MIA + Tramadol group showed lower mechanical hypersensitivity than that in the MIA + Vehicle group, and CGRP expression in DRG was suppressed. Histologically, advanced arthritic changes were observed in the MIA + Tramadol group than the MIA + Vehicle group. While these results established the pain-relieving effect of intraperitoneal tramadol, they were also associated with the progression of OA changes. Tramadol is a centrally acting analgesic with many modes of action. It acts on serotonergic and noradrenergic nociception, while its metabolite O-desmethyltramadol acts on the µ-opioid receptor.26 Tramadol also acts as a serotonin-norepinephrine reuptake inhibitor (SNRI).27 Tramadol exhibits concentration-dependent analgesic effects in the pain induced by the tail-flick test and the hot plate test that measure heat nociception latency28 and pain measured by the Randall-Sllito-typed pressure nociception threshold.29 Moreover, the effectiveness of tramadol has also been confirmed in other pain behaviors caused by tissue inflammation, such as an inflammatory pain model induced by complete Freund's adjuvant, and in neuropathic pain due to peripheral nerve injuries, such as chronic constriction injury model, and spinal nerve ligation model.30, 31 In the present study, the MIA + Vehicle group had increased mechanical hypersensitivity compared with that in the Sham group, and the MIA + Tramadol group showed improved mechanical hypersensitivity relative to that in the MIA + Vehicle group. These findings indicate that the hip pain induced by administration of 0.5 mg MIA in rats showed mechanical hypersensitivity, and it was suppressed by the administration of tramadol. In past literature, an increase of mechanical hypersensitivity was observed in a knee OA model induced by 2.0 mg of MIA, and there was an increased expression of ATF-3, which is a neuropathic marker.32, 33 Recent studies reported that the rat hip OA model induced by 2.0 mg of MIA exhibits peripheral inflammation, local nerve damage, and central sensitization, thus leading to chronic pain.13, 34, 35 Regarding tramadol administration in the MIA model, Rachel et al.14 reported that tramadol administration improved mechanical hypersensitivity in the knee of MIA-induced OA model animals. Ishikawa et al.17 similarly reported that tramadol administration improved the gait paradigm in a rat knee OA model induced by MIA. Prasant et al reported tramadol produced a complete dose-dependent reversal of the reduced grip force in the knee of the MIA-induced OA model.36 This is consistent with the improvement of walking pain in OA patients.37 Our results showed that tramadol was effective against hyperalgesia in the rat hip OA model induced by 0.5 mg MIA-administration, similar to the pain phenotype seen with 2.0 mg MIA-administration in the hip OA model in rats. Therefore, it was suggested that the rats induced by MIA injection had their pain suppressed by the administration of tramadol, and the affected limbs may strongly contact the ground while walking. In the mechanism of action of tramadol, articular chondrocytes have been reported to express opioid receptors.38 Thus, in intra-articular administration of tramadol, it is considered that tramadol acts directly on the opioid receptor of chondrocytes and exerts an analgesic effect.39 However, the current study found that intraperitoneal administration of tramadol had a significant analgesic effect on the MIA-treated group, which resulted in chondrocyte death. Therefore, it is considered that the pharmacological effect of tramadol is due to the action from systemic administration to the primary sensory nerve endings and the dorsal horn of the spinal cord rather than the direct action of chondrocyte on opioid receptors. Since local articular cells such as chondrocytes have not been evaluated in this study, further studies are needed in the future.
In the MIA + Vehicle group, the expression of CGRP-ir DRG neurons was significantly higher than that in the Sham and MIA + Tramadol groups. DRG neurons are considered to be responsible for acute inflammatory pain.35 CGRP reportedly reflects acute inflammatory pain40 in the knee of an MIA-induced OA rat model.41, 42 These findings indicate that local inflammation occurred in the hip of an MIA-induced OA model rat, and CGRP expression in the DRG was suppressed by tramadol administration, which is consistent with our study findings that tramadol administration reduced CGRP expression. In addition, immunohistochemistry of peripheral nerve biopsies harvested from patients with Morton's neuroma, which results in neuropathic pain, showed an increased amount of CGRP in patients compared to controls.43 Schou et al.44 performed a meta-analysis of CGRP in terms of its role in chronic pain, which indicated that CGRP is involved in neuropathic and chronic pain in addition to inflammation-induced pain. In the current study, tramadol effectively reduced the proportion of CGRP-ir DRG neurons and associated mechanical hypersensitivity significantly in the rat hip OA model. Therefore, it is possible that this study model involves elements of chronic pain and neuropathic pain in addition to inflammatory pain. However, further investigations are necessary to ascertain these inferences.
In this study, the MIA + Vehicle group showed OA-associated changes, such as degeneration of articular cartilage and decreased cartilage matrix 6 weeks after MIA injection with a mean OARSI score of 10.0 ± 2.0. Kawarai et al.13 reported that OA-associated changes, such as degeneration and fibrosis, subchondral bone collapse, and decreased cartilage matrix were observed 4 weeks after administration of 2.0 mg of MIA and the mean OARSI score was 22.7 ± 1.9. The rat hip OA model induced by a 0.5 mg MIA injection in this study showed a comparatively mild pathological phenotype. Therefore, we decided to use this mild OA model to compare the degree of OA change. While tramadol suppressed mechanical hypersensitivity and local inflammation in the rat hip OA model, histologically, the MIA + Tramadol group demonstrated OA progression compared to the MIA + Vehicle group. In this study, we observed slight synovial hyperplasia in both the MIA + Vehicle group and the MIA + Tramadol group, but there was no significant difference. No synovitis was seen in the Sham and Sham + Tramadol group. Therefore, it could not be said that the tramadol suppressed synovitis or that synovitis progressed. Furthermore, although the OARSI score was used to evaluate the degree of OA degeneration in this study, the OARSI score does not include the evaluation of synovium. That is why synovium was not included in the evaluation items, and the synovial tissue was not shown in the figure.
The use of opioids for OA pain has previously been reported. Tapentadol and oxycodone treatments were effective for managing moderate-to-severe chronic OA-related knee and hip pain.45, 46 In contrast, concerning adverse events, such as constipation, somnolence, nausea, vomiting, dizziness, and itching from opioid use for treating chronic noncancer pain, including OA associated with opioid use, were reported by Kalso et al.47 However, in their study, no progression of joint deformities was reported. Recently, Fujii et al.18 reported that potent opioids resulted in OA progression in a relatively short time. Their results suggest that although OA patients do not experience pain due to the strong analgesic effects of opioids, joint destruction and progressive OA change, such as Charcot's joints, could occur. Tanezumab, a monoclonal antibody against nerve growth factor (NGF) with pain-relieving effects, has demonstrated efficacy in a pivotal 16-week dose-titration phase 3 study for OA pain.48 The use of anti-NGF antibodies increased the number of cases requiring joint replacement surgery,48 which may also be due to the excessive pain-relieving effect, resulting in OA progression. Although tramadol is widely used for OA treatment, its involvement in the progression of joint deformities is not completely understood. Regarding the direct effect of tramadol on articular chondrocytes, previous reports have reported that tramadol's cytotoxicity promotes OA in intra-articular administration.49 There are no reports of in existing animal or human experiments intraperitoneal administration promotes OA as in this study. Furthermore, since no progression of OA was observed in the Sham + Tramadol group in this study, the effect of intraperitoneal administration of tramadol on joints is unlikely. Thus, tramadol promoted OA-associated changes in a rat hip OA model, while no effect of tramadol itself causing joint destruction was observed in this study. Considering the previous reports that OA is progressed by drugs that have a strong analgesic effect, such as anti-NGF antibodies in clinical studies, it is possible that OA is progressed by the increase of activity due to the analgesic effect of administration methods except for intra-articular administration.
Nevertheless, this study has several limitations. In this study, tramadol administration reduced pain in the right limb, increasing weight-bearing on the rats' right hip joint, subsequently affecting OA progression. However, it is unclear whether the rats' activity and weight-bearing on the right limb actually increased. In other words, this study does not indicate that the amount of activity increased due to the pain-relieving effect and changes in the progression of OA. However, it was suggested that the pain-relieving effect might be related to OA deformations. Second, the effect of the interaction between tramadol and MIA administration on articular chondrocytes has not been considered. More detailed data will be required in future studies. Third, we did not evaluate the difference in the progress of OA depending on the dose or the change over time. Fourth, the follow-up period was short. In this study, histological evaluation was performed 6 weeks after MIA administration (4 weeks after daily tramadol administration). The OA changes progressed in a relatively short time due to the administration of tramadol. However, it is necessary to investigate whether joint deformities progress with the long-term administration of tramadol.
In conclusion, tramadol suppressed mechanical hyperalgesia and CGRP expression at the L4 level in the DRG of rats with MIA-induced hip OA. These results imply that tramadol is effective in the conservative treatment of patients who cannot undergo surgery due to various conditions, such as age and other complications. However, it was shown that while chronic tramadol treatment has a pain-relieving effect in rats with hip OA, it may also contribute to progressive OA changes. Although tramadol has excellent pain-relieving effects and is therefore used in OA treatment, there may be a potential risk of developing joint deformities. Therefore, orthopedic surgeons should consider the possibility of progressive joint deformities when prescribing tramadol to OA patients.
STATEMENT OF CLINICAL SIGNIFICANCE
Results showed that chronic tramadol treatment has an analgesic effect on hip OA rats while likely contributing to progressive OA changes.
ACKNOWLEDGMENTS
This study was supported by JSPS KAKENHI Grant Number 19K18487. The other authors did not receive any funding or financial support that may be perceived to have biased the study.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Keijiro Kanno contributed to the original idea, literature review, data collection, statistical analysis, data interpretation, manuscript writing, preparation of figures, revision, and approval of final work. Miyako Suzuki-Narita, Yuya Kawarai, Shigeo Hagiwara, Satoshi Yoh, and Junichi Nakamura contributed to the original idea, data interpretation, manuscript revision. Sumihisa Orita and Kazuhide Inage participated in the study design and helped create this study model. Takane Suzuki contributed to the literature review and data interpretation. Seiji Ohtori contributed to data interpretation and approval of the final work. All authors have read and approved the final submitted manuscript.




