Investigation of multiple populations highlight VEGFA polymorphisms to modulate anterior cruciate ligament injury
All authors have read and approved the final submitted manuscript.
Abstract
Polymorphisms in VEGFA and KDR encoding proteins have been associated with anterior cruciate ligament (ACL) injury risk. We leveraged a collective sample from Sweden, Poland, and Australia to investigate the association of functional polymorphisms in VEGFA and KDR with susceptibility to ACL injury risk. Using a case–control genetic association approach, polymorphisms in VEGFA and KDR were genotyped and haplotypes inferred from 765 controls, and 912 cases clinically diagnosed with ACL rupture. For VEGFA, there was a significant overrepresentation of the rs2010963 CC genotype (p = 0.0001, false discovery rate [FDR]: p = 0.001, odds ratio [OR]: 2.16, 95% confidence interval [CI]: 1.47–3.19) in the combined ACL group (18%) compared to the combined control group (11%). The VEGFA (rs699947 C/A, rs1570360 G/A, rs2010963 G/C) A-A-G haplotype was significantly (p = 0.010, OR: 0.85, 95% CI: 0.69–1.05) underrepresented in the combined ACL group (23%) compared to the combined control group (28%). In addition, the A-G-G construct was significantly (p = 0.036, OR: 0.81, 95% CI: 0.64–1.02) underrepresented in the combined ACL group (12%) compared to the combined CON group (16%). Our findings support the association of the VEGFA rs2010963 CC genotype with increased risk and (ii) the VEGFA A-A-G haplotype with a reduced risk, and are in alignment with the a priori hypothesis. Collectively identifying a genetic interval within VEGFA to be implicated in ACL risk modulation and highlight further the importance of vascular regulation in ligament biology.
1 INTRODUCTION
Blood supply to the anterior cruciate ligament (ACL) is via the middle genicular artery, and it is innervated by mechanoreceptors from the tibial nerve.1 The ACL is poorly vascularised, contributing to its poor healing capacity, and postsurgical inferior biomechanical properties in comparison to the native tissue.2 Angiogenesis, the addition of new vasculature from existing blood vessels is controlled by several growth factors and cytokines. It is hypothesized that angiogenesis can be triggered by biological and/or mechanical stimuli to promote remodeling of both tendon and ligament. This has been supported by studies identifying increased proangiogenic expression protein profiles such as the A-isoform of vascular endothelial growth factor (VEGFA) in tendon cells3-5 and within specimens from ruptured tendons and ligaments, degenerative tendons and ligament reconstructive surgery.6-9 When exploring functional partners regulating the extracellular matrix (ECM) of tendon and ligament, the components of angiogenesis is placed centrally within this network.10 VEGFA is the primary regulator of angiogenesis, and its activity is further regulated by binding to its receptor, the kinase insert-domain receptor (KDR).11
Evidence associating VEGFA and KDR with the risk of ACL ruptures is growing12-14 with several studies implicating gene variants in the risk profile of musculoskeletal soft tissue injuries, such as Achilles tendinopathy15 and patellar and rotator cuff tendinopathy.16 Previous genetic association studies investigating VEGFA and KDR polymorphisms with ACL rupture12, 14 have been limited by small sample sizes and subsequently power, making it difficult to establish an association with a phenotype to be reflective of a biological consequence.17 It is therefore not surprising that previous VEGFA and KDR association studies have been conflicted by the reports of contrasting genotype associations in independent populations.12-14
The current study therefore aimed to investigate the association of the previously implicated functional VEGFA (rs699947 C/A, rs1570360 G/A, and rs2010963 G/C) and KDR (rs2071559 G/A and rs1870377 T/A) polymorphisms18-21 with susceptibility to ACL rupture in three additional independent populations (Sweden, Poland, and Australia).12-14 Second, the study aimed to investigate the distribution of these functionally implicated loci in a combined analyses with the cumulative data for the cohorts previously published for South Africa12 and Poland.14 The a priori hypothesis tested was based on the reported functional consequence of the genotypes such that (i) the rs699947 CC, rs1570360 GG20 and rs2010963 CC22 genotypes associated with an increased VEGF expression, would be associated with increased risk and that (ii) the functional inferred VEGFA A-A-G haplotype, associated with a decreased VEGF expression,18 would be associated with a reduced risk of ACL injury and (iii) the KDR G-A inferred haplotype constructed from rs2071559 and rs1870377, associated with reduced KDR transcription,23 with an increased risk of ACL rupture.
2 METHODS
2.1 Participant characteristics
This study followed a case–control genetic association, comprised of three populations (Sweden, Poland and Australia). All participants from the individual cohorts completed questionnaires from the respective research centers detailing participant personal details, ancestry, lifestyle habits, occupational details, sporting history (sports played, number of years, playing level, frequency), details of ACL injury, history of other ligament or tendon injury, and medical history. Consent was obtained according to the declaration of Helsinki. This study was approved by the Research Ethics Committee of the Faculty of Health Sciences, University of Cape Town, South Africa (HREC 269/2014, 110/2018 and 622/2015, R025/2016), the Regional Ethical Review Board in Umeå, Sweden (dnr. 2011-200-31 M), Bioethics Committee for Clinical Research, Regional Medical Chamber, Gdansk, Poland (KB-8/16) and Epworth Hospital (HREC approval: 57012) Victoria, Australia.
The inclusion criteria for the cases included individuals with a clinical diagnoses of an ACL based on physical examination, and confirmed by either magnetic resonance imaging or arthroscopy. Only ACL ruptures resulting from a noncontact mechanism were included. For the control participants, inclusion criteria included participation in regular sporting activites, participating in similar sports to cases, had no history of ACL injury or other ligament and tendon injuries, and were within a similar age category as the ACL rupture group. All participants participated in regular sporting activities, primarily at a recreational level. The type of sport and years of participation are provided in the Supporting Information data for the Swedish (cases and controls), Polish (cases and controls) and Australian (cases only) participants.
The Swedish cohort comprised of 116 control (SWE-CON) and 95 ACL (SWE-ACL) participants recruited between 2011 and 2013 from orthopaedic clinics in two hospitals in the cities of Umeå: Västerbotten and Luleå: Norrbotten as previously described.24 The Polish cohort comprised 149 control (POL-CON) and 127 ACL (POL-ACL) participants recruited from the Galen Orthopedics Clinic in Poland between 2008 and 2018. The Australian cohort consisted of 83 control (AUS-CON) and 342 ACL (AUS-ACL) participants. The control participants were recruited from the Genes and Skeletal Muscle Adaptive Response to Training (Gene SMART) cohort.25 These individuals were from the student and staff populations from universities and local communities. For the ACL group, participants were recruited between 2006 and 2018 from Epworth Richmond hospital in Melbourne Australia.
For the previously published South African cohort, the case participants were recruited between 2006 and 2012 from the Sports Science Orthopaedic Clinic in Cape Town, South Africa, and control participants from sporting clubs and gyms within the Cape Town area as described in Rahim et al.12 Lastly, for the previously published Polish cohort, the participants were recruited between 2009 and 2016 with male case participants recruited from Polish soccer leagues, and controls from similar soccer teams. Female cases were recruited from either soccer teams and included amateur skiers, and the female controls were recruited from sports clubs and wellness centers as described in Lulińska-Kuklik et al.14
Type of sports participation for all Swedish (Table S2a) and Polish (Table S2b) cases and controls, as well as Australian case participants (Table S2c) was categorized into contact sports, noncontact jumping sports, noncontact nonjumping sports, and skiing sports (with a noncontact mechanism of injury), as previously defined.26 Participation years for Swedish participants are described in Table S2a, while data describing participation years for Polish controls and all Australian participants was unavailable.
Level of sport was classified into three groups (elite, national and recreational) as previously categorized for Swedish27 and Australian28 participants. The majority (76%) of Swedish case participants were recreational, with 6% elite, and the remaining 18% competing at a national level. Similarly, most of the Swedish controls were recreational (83%), while 8% were elite, and 8% competing at a national level (Table S2d). Data describing the level of sport in Polish participants was missing. For the Australian case participants, 98% were recreational athletes, with 2% of participants competing at a national level (Table S2d). Type of sport and years of participation was unavailable for the Australian controls, however participants were deemed moderately trained (VO2peak 35–60 ml min−1 kg−1),25 participating in physical activity at a recreational level (Table S2d).
Sports participation data for the South African cohort was previously described in Rahim et al.12 Type of sport was categorized into contact, noncontact jumping, noncontact, nonjumping, and skiing sports (with a noncontact mechanism of injury) with participation years described. Briefly, female participants were matched for all sporting activity. Male participants were matched for participation in noncontact jumping sports and skiing sports. However, significantly more male cases participated in contact sports and significantly more male controls participated in noncontact, nonjumping sports.12 Level of sport was not documented.
Similarly, data for the published Polish cohort was previously described in Lulinska-Kuklik et al.,14 where type of sport, level of exposure, and sporting level are documented. Briefly, all male participants were matched for type of sport, level, and frequency of exposure. Additionally, sports participation data for female case and control participants were comparable as described by the authors.14
For the combined cohort analyses, genotype data from the Swedish, Polish an Australian samples collected in this study were pooled with the genotyping data from previously published study groups from South Africa (CON: 227 ACL: 126)12 and Poland (CON: 190 ACL: 222)14 with approval from the respective authors. KDR polymorphisms were not previously investigated in the published Polish study group14 so for the current study, a total of 1677 samples (Combined CON: 765 and Combined ACL: 912) were investigated for the VEGFA polymorphisms and a total of 1265 samples (Combined CON: 575 and Combined ACL: 690) for the KDR polymorphisms.
2.2 DNA isolation and genotyping
For the cohort from Sweden, genomic DNA was extracted from venous blood using a rapid nonenzymatic ethanol precipitation as previously described by Lahiri and Nurnberger29 with slight modifications.30 For Poland, DNA was extracted from oral epithelial cells using a Gen Elute Mammalian Genomic DNA Miniprep Kit (Sigma) according to the manufacturer's recommendations, and for Australia, DNA was isolated from a venous blood aliquot using a sequenced extraction technique (FlexiGene DNA Kit, Qiagen P/L) or the MagSep Blood gDNA kit with the epMotion M5073 automated pipetting system (Eppendorf).
Participants were genotyped for five functional single nucleotide polymorphisms (SNPs) (VEGFA: rs699947 C/A, rs1570360 G/A rs201963 G/C and KDR: rs2071559 G/A and rs1870377 T/A) using standard polymerase chain reaction (PCR)-based technology for genotyping as previously described.12 For the Swedish cohort only, restriction fragment length polymorphism (RFLP) analysis was used to genotype VEGFA rs699947 (BglII) and KDR rs1870377 (AluI) and custom-designed fluorescence-based TaqMan PCR assays (Applied Biosystems) used to genotype VEGFA rs1570360 (assay ID: C___1647379_10) VEGFA rs2010963 (C___8311614_10) and KDR rs2071559 (C__15869271_10). TaqMan PCR assays were used to genotype all five SNPs in the Polish and Australian cohorts (assay ID rs699947: C___8311602_10 and rs1870377: C__11895315_20). The TaqMan genotyping PCR reactions were conducted using the Applied Biosystems QuantStudio Real-Time PCR system and the Applied Biosystems QuantStudio Real-Time PCR software (Applied Biosystems). The manufacturer's instructions were followed. Negative controls (no DNA) and five repeat samples (known genotypes) were included as quality control measures for each 96-well plate. Genotypes were confirmed by two independent investigators (DF and MR) with an average 98.7% call rate, and laboratory work was conducted at the University of Cape Town.
2.3 Statistical analyses
Power calculations were calculated using QUANTO v1.2.4 (http://biostats.usc.edu/software). For the Swedish cohort, assuming minor allele frequencies between 0.2 and 0.5, a sample size of 85 cases would detect an allelic odds ratio (OR) of 2.0 and greater, at a power of 80% and a significance level of 5%. For the Polish and Australian cohorts, assuming minor allele frequencies between 0.2 and 0.5, a sample size of 118 cases and greater would detect an OR of 1.8 and greater, at a power of 80% and a significance level of 5%. The statistical program R (R Development Core Team, 2010) was used. Participant descriptive statistics were compared using a one-way analysis of variance to determine any significant differences between the mean characteristics of the CON and ACL groups. The R packages genetics31 and SNPassoc32 were used to analyse differences in genotype and allele frequencies between groups, and to calculate Hardy–Weinberg equilibrium (HWE) probabilities. Inferred haplotypes were constructed using the genotype data. Gene–gene interactions for VEFGA-KDR were explored using the combined allele analyses from the genotype data. The R package haplo. stats33 was used, adjusting for confounding variables where appropriate, for the individual and collective cohorts. For Sweden, age and type of sport was a considered confounder, for Poland age, body mass and type of sport, for Australia age and sex, and for the combined cohort, age, body mass index (BMI) and country were included in the adjustment model. For the combined cohort, χ2 tests were used to compare genotype frequency distributions. Sex is a known intrinsic risk factor for ACL rupture susceptibility, and previous research stratified by sex.13 However, because of reduced power, stratification by sex was not included for the independent cohort analyses. Statistical significance was accepted when p < 0.05 and the false discovery rate (FDR) procedure was used to adjust for multiple comparisons using the method applied for multiple testing under dependency.34
3 RESULTS
3.1 Participant characteristics
Participants in the Swedish cohort were previously described.24 The SWE-CON and SWE-ACL groups were matched for participation in noncontact jumping sports (Table S2a). However, a significantly higher (p < 0.001) proportion of SWE-CON participants (72%, n = 66) participated in noncontact nonjumping sports, compared to the SWE-ACL participants (6%, n = 5) and no SWE-CON individuals participated in contact sports compared to 68% (n = 62) of SWE-ACL participants (p < 0.001). No differences in years of sport participation between controls and ACL cases was noted for any of the sporting modalities (Table S2a), and no differences in the sporting level was observed between groups (Table S2d). Furthermore, Swedish participants with the KDR rs2071559 AA genotype were heavier (76.8 ± 16.6 kg, n = 42) than participants with the GG genotype (69.3 ± 9.7 kg, n = 49, p = 0.034) and had greater BMIs (25.4 ± 3.5 kg m2, n = 42) than those with the GG genotype (23.7 ± 2.6 kg m2, n = 49, p = 0.018) (Table S3a). No other genotype effects were noted for sex, age, height, body mass, or BMI.
Polish control participants differed significantly in age, body mass and BMI compared to the ACL case group (Table S1). The POL-CON group were younger (21.0 ± 1.8 years, n = 149) than the POL-ACL group (31.4 ± 10.1 years, n = 127, p < 0.001). Even when adjusted for age, the POL-CON group also had a significantly lower body mass (72.6 ± 12.0 kg, n = 149) with a corresponding lower BMI (22.8 ± 2.4 kg m2, n = 149) compared to the POL-ACL group (body mass: 79.0 ± 14.9 kg, n = 126, p < 0.001; BMI: 25.1 ± 4.1 kg m2, n = 123, p < 0.001). Polish participants were matched for participation in contact sports (Table S2b). However, significantly more controls participated in noncontact, nonjumping sports (p = 0.009) with no controls participating in noncontact jumping sports (p < 0.001). For genotype effects, the KDR rs1870377 AA genotype was significantly underrepresented in Polish male participants (56%, n = 25) compared to the AT genotype (81%, n = 114, p = 0.023) and individuals with the AT genotype were heavier (77.6 kg ± 15.3, n = 113) than those with the TT (73.5 kg ± 11.4, n = 129) or AA genotypes (71.7 kg ± 11.8, n = 25). No further significant genotype effects were noted on sex, age, height, body mass and BMI (Table S3b).
Australian participants were unmatched for age, sex, height, and body mass (Table S1). The AUS-CON group was significantly (p < 0.001) older (31.0 ± 8.3 years, n = 82) than the AUS-ACL group (25.2 ± 9.4 years, n = 269) significantly (p < 0.001) taller (180.0 ± 0.1 cm, n = 82) than the AUS-ACL group (175.2 ± 0.1 cm, n = 268) and significantly (p < 0.001) heavier (81.6 ± 12.0 kg, n = 82) than the AUS-ACL group (77.5 ± 14.7 kg, n = 268). All the AUS-CON samples were male, with 85% (n = 245) of the AUS-ACL participants participating in contact sports, while 4% (n = 11) and 11% (n = 32) participated in noncontact jumping and noncontact, nonjumping sports, respectively (Table S2c). No differences in sporting level was noted between cases and controls (Table S2d).
For genotype effects, Australian participants with the VEGFA rs1570360 AA genotype were significantly taller than participants with the AG genotype. No further effect of genotype was evident for sex, age, sex, body mass, or BMI for any of the investigated polymorphisms (Table S3c).
3.1.1 Genotype and allele frequencies
VEGFA rs2010963 (G/C) genotype frequency was significantly (p < 0.001, FDR: p < 0.001) different between the SWE-CON and SWE-ACL groups (Table S4). The GG genotype was significantly underrepresented (p = 0.001, OR: 2.8, 95% CI: 1.45–5.41) in the SWE-ACL group (17%) compared to the SWE-CON group (36%) and the CC genotype was significantly over-represented (p < 0.001, OR: 6.6, 95% CI: 3.20–13.55) in the SWE-ACL group (43%) compared to the SWE-CON group (10%). In addition, the rs2010963 C allele was significantly over-represented (p < 0.001, FDR: p < 0.001, OR: 2.9, 95% CI: 1.92–4.42) in the SWE-ACL group (63%) compared to the SWE-CON group (37%). No other significant associations were noted for the Swedish cohort (Table S4). No significant differences in the genotype or allele frequency distributions between the CON and ACL samples were noted for the Polish (Table S5) or Australian (Table S6) cohorts.
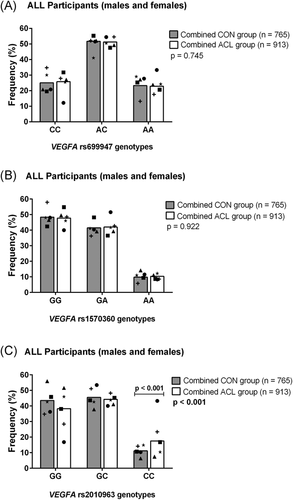
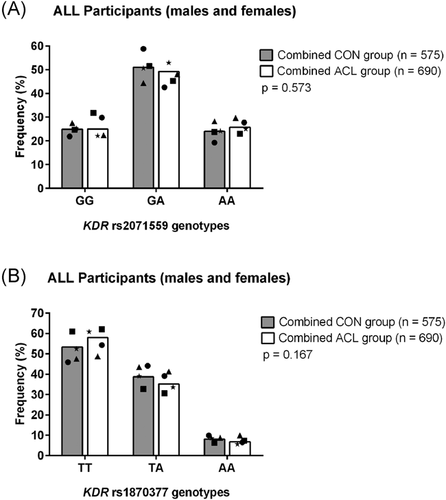
For the combined analysis, there was a significant difference in genotype frequencies for the VEGFA rs2010963 (G/C) polymorphism (Figure 1C). The rs2010963 CC genotype was significantly over-represented (p = 0.0001, FDR: p = 0.001, OR: 2.16, 95% CI: 1.47–3.19) in the combined ACL group (18%) compared to the combined CON group (11%). Moreover, the rs2010963 CC genotype was still significantly different between cases and controls when the SWE-ACL group was removed from the analysis (Figure S3). No further significant differences in genotype or allele frequency distributions were observed between the combined CON and ACL group for any of the other investigated VEGFA (Figure 1A,B) and KDR (Figure 2A,B) polymorphisms, and all polymophisms were in HWE.
Inferred haplotypes were constructed using the genotype data of the three VEGFA SNPs. Four inferred haplotypes were significantly associated with ACL rupture risk in the Swedish cohort (Figure S1). More specifically, the A-A-G and A-G-G inferred haplotypes constructed from rs699947 (A/C) rs1570360 (G/A) and rs2010963 (G/C) were significantly underrepresented in the SWE-ACL group compared to the SWE-CON group (A-A-G: p = 0.005, SWE-CON 31% vs. SWE-ACL 6% and A-G-G: p = 0.048, SWE-CON 18% vs. SWE-ACL 7%). Additionally, the A-G-C haplotype was significantly over-represented in the SWE-ACL group in comparison to the SWE-CON group (A-G-C: p = 0.002, SWE-CON group 3% vs. SWE-ACL group 19%). No further differences in the inferred haplotype distributions were noted for the Polish and Australian cohorts (Figure S1).
For the combined cohort the VEGFA A-A-G haplotype was significantly (p = 0.010, OR: 0.85, 95% CI: 0.69–1.05) underrepresented in the combined ACL (23%) compared to the combined CON (28%) group and similarly, the A-G-G haplotype was significantly (p = 0.036, OR: 0.81, 95% CI: 0.64–1.02) underrepresented in the combined ACL (12%) compared to the combined CON (16%) group (Figure 3A). Additionally, in a reduced interval analysis (VEGFA rs1570360 G/A–rs2010963 G/C) both the -G-G and -A-G inferred haplotypes were significantly underrepresented (-G-G: p = 0.031, OR: 1.00 and -A-G: p = 0.024, OR: 0.98, 95% CI: 0.82–1.18) in the combined ACL group, compared to the combined CON group (Figure 3C). While the -G-C haplotype was significantly over-represented (p = 0.012, OR: 1.18, 95% CI: 0.99–1.40) in the combined ACL group, when compared to the combined CON group (Figure 3C).
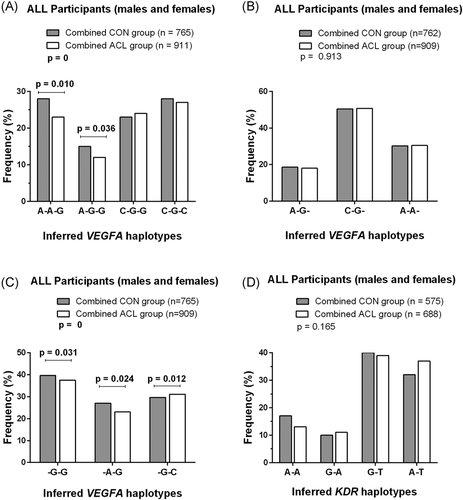
For the KDR gene, four inferred haplotypes were constructed from the two (rs2071559 G/A, rs1870377 T/A) polymorphisms, however no significant differences were observed in the genotype frequency distribution for any of the independent (Figure S1) or combined cohorts investigated (Figure 3D).
The interaction of VEGFA (rs699947 A/C and rs2010963 G/C) and KDR (rs2071559 G/A and rs1870377 T/A) (Figure S2) showed a significant under-representation of the inferred A-G-A-A (p = 0.005, OR: 0.51, 95% CI: 0.30–0.87) and A-G-G-A (p = 0.018, OR: 0.93, 95% CI: 0.54–1.60) haplotypes in the combined ACL (4% and 5%, respectively) compared to the combined CON (9% and 6%, respectively) group.
4 DISCUSSION
Part of the elucidation of ACL rupture risk maybe through increasing our understanding of the factors contributing to the disrupture of the vasculature. Unraveling this process in soft tissues such as ligaments, may increase our knowledge of both injury and healing mechanisms35 in addition to graft ligamentization after ACL reconstructive surgery.36 VEGFA and KDR are two key proteins initiating angiogenesis. This study aimed to investigate five functional polymorphisms within VEGFA and KDR with susceptibility to ACL rupture in a large cohort of samples.
The main findings from the collective cohort of 1677 samples, included the (i) association of the VEGFA rs2010963 CC genotype with increased risk, (ii) the association of the VEGFA A-A-G haplotype (rs699947 A/C-rs1570360 G/A-rs2010963 G/C) with a reduced risk, which are both in alignment with the a priori hypothesis, and (iii) the KDR genotype and haplotype analyses illustrated that it is highly unlikely that the investigated KDR sequences are associated with modulating ACL injury risk.
Furthermore, this study noted similar findings to previous genetic studies investigating small samples sizes, when analysing the independent cohorts whereby associations seemed to be noted only in one cohort (e.g., Sweden). It was also interesting to note that the VEGFA A-G-G inferred haplotype was previously implicated with increased Achilles tendinopathy risk in a combined South African Caucasian and British study group.15 This alternate risk allele association between ligament and tendon injury is a thought-provoking finding, and one could hypothesize these differences relate to tissue vascularity, and differences in biomechanical properties which are influenced by the regional and load sensitive expression of ECM components.37 Although both tendons and ligaments are poorly vascularized, ligaments have a slightly improved blood supply38 and it may be that a predisposition to a lower VEGF expression may affect these tissues differently because of their specific healing-load dynamics.
The VEGFA rs2010963 CC genotype has been associated with increased VEGFA gene expression.22 Increased VEGFA upregulates the expression of matrix metalloproteinases which are key mediators of ECM turnover during ligament repair.39 However, research has shown that the overexpression of VEGFA reduces the biomechanical strength of the tendon graft in the early stages of an ACL ligament reconstruction, but is essential in the later phases of ligamentization.36 Furthermore, in addition to the rs2010963 CC genotype, the rs699947 CC, rs15706630 GG genotypes were also associated with increase in VEGFA expression20 and therefore the alternate alleles with reduced VEGFA expression.
The data from this study and previous work12, 14 suggest that VEGFA is associated with altered risk of ACL injury, but there remains much complexity around VEGFA gene expression, and its effects on the remodeling continuum.
We therefore hypothesize that the “Goldilocks affect” is still plausible40 whereby a finely tuned homeostatic feedback regulation of turnover of ligament ECM components is required for optimal repair responses. More recently, Willard et al.41 and Suijkerbuijk et al.24 have shown functional evidence linking a genetic contribution to the expression of ECM components in a susceptibility model.
VEGFA activity relies on a key receptor: KDR, and binding of the receptor is necessary for downstream angiogenesis signaling. For the independent participant groups (Swedish, Polish, and Australian) and the combined cohort analysis, no associations were identified for any of the KDR polymorphisms or inferred haplotypes. These observations are therefore in agreement with the previously reported lack of independent associations noted for KDR and the risk of ACL rupture in a Caucasian study group.12 In contrast, the previously reported inferred KDR haplotype association with increased risk of ACL rupture12 was not observed in this study, and may represent a type I error. KDR is vital for effective VEGFA signaling and altered KDR binding affinity impacts the ability of VEGFA to function efficiently. The functional evidence suggests that the KDR rs2071559 G allele is associated with reduced KDR transcription,19 resulting in lower levels of KDR and consequently reduced VEGF activity.21 The KDR rs1870377 A allele specifically results in an altered binding recognition site for VEGFA which may impair VEGFA binding efficacy19 and can thereby influence downstream VEGFA signaling.
This study explored interactions between VEGFA-KDR and the associations noted were exclusively resulting from the alleles inherited at the VEGFA locus and risk was independent of the KDR alleles inherited. From the collective data set, the inferred haplotypes identified for the VEGFA locus highlights a reduced genetic interval between rs1570360 G/A and rs2010963 G/C to harbor potential functional motifs related to ACL risk susceptibility.
This study represents one of the largest studies investigating the angiogenesis loci for ACL risk susceptibility. One of the limitations of the study was matching participants for confounders, specifically type of sport and frequency of exposure. The authors acknowledge that confounders between the individual cohorts differ and should be considered when pooling cohorts from independent research centers. Going forward, sports participation data in particular should be more uniformly collected, to facilitate the identification of potential confounders and to allow for there adjustment in the analyses. Furthermore, the cases and control numbers were not balanced within the Australian cohort and in particular the control participants sampling would need to increase to facilitate understanding of the frequencies of the variants in this group with reference to the case group. However, when the collective cohort was evaluated, the numbers were more balanced between the groups. In addition, the participants recruited for the independent cohorts were selected from both hospitals and general population sampling.
The data suggest greater variability in the frequency distributions at the functional VEGFA polymorphisms between the cohorts. This is most likely not a surprising observation for a biologically relevant genetic interval implicated in modulating a complex phenotype such as ACL ruptures. Future collaborative work requires rigidity in matching participants for sports participation data including type of sport, years of participation, and level of play to determine exposure, as nongenetic factors such as sporting types and frequency of participation do play a large role in the risk of these injuries. Prospective cohort analyses is required to evaluate the clinical relevance of these genotypes and their contribution to the potential biomechanical properties of ligament and tendon.
5 CONCLUSION
VEGFA and KDR are key components in the angiogenesis pathway and altered forms of their genes have downstream effects on their expression. Exploring cohorts from different geographical regions have assisted in supporting the growing body of evidence implicating the VEGFA locus in ACL susceptibility. Characterization of the functional biological effects of the VEGFA genetic susceptibility, may in future assist in unraveling the influence of an individual's soft tissue vasculature on the healing and regeneration capacity of ligaments and tendons. To further explore this genetic locus and surrounding gene–gene interactions, large initiatives such as the Athlome Consortium are required (PMID: 26715623) and our future research aims to employ an unbiased approach through whole genome sequencing.
ACKNOWLEDGMENTS
K.G.N. is a consultant for Zimmer. Additionally, he received grants from both Zimmer and Link. Also, he is a speaker for Zimmer, Smith&Nephew and DePuy. No further declarations for any of the other authors. This study was funded in part by funds from the National Research Foundation (NRF) of South Africa (Grant: CPRR160426163211) and the University of Cape Town, South Africa. In Sweden, financial support was obtained from the Swedish research Council (Grants Nos. K2011-69X-21877-01-6, K2014-99X-21876-04-4), Västerbotten County Council (Grant No. ALF VLL548501 and Strategic funding VLL-358901; Project No.7002795). For Poland, the study was supported by the National Science Centre of Poland (No. UMO-2016/21/B/NZ7/01068). In Australia, funding was received from the Perpetual Trustees Grant and the Epworth Research Institute Grant. M.A.M.S and M.R were financially supported by the European Union funded project RUBICON H2020-MSCA-RISE-2015–690850, in addition MR was funded by the Harry Crossley Postdoctoral fellowship. D.C.F was funded by the NRF South African Doctoral Scholarship, and in part by the University of Cape Town.
AUTHOR CONTRIBUTIONS
Daneil C. Feldmann, Masouda Rahim, Malcolm Collins, Alison V. September: Substantial contributions to research design, or the acquisition, analysis or interpretation of data. Daneil C. Feldmann, Mathijs A.M. Suijkerbuijk, Emile R. Chimusa, Malcolm Collins, Alison V. September: Drafting the paper or revising it critically. Daneil C. Feldmann, Masouda Rahim, Mathijs A.M. Suijkerbuijk, Mary-Jessica N. Laguette, Paweł Cieszczyk, Krzysztof Ficek, Kinga Huminska-Lisowska, Charlotte K. Häger, Evalena Stattin, Kjell G. Nilsson, Javier Alvarez-Rumero, Nir Eynon, Julian Feller, Oren Tirosh, Michael Posthumus, Emile R. Chimusa, Malcolm Collins, Alison V. September: Approval of the submitted and final versions; Mathijs A.M. Suijkerbuijk, Assisting with laboratory experiments; Paweł Cieszczyk, Krzysztof Ficek, Kinga Huminska-Lisowska, Charlotte K. Häger, Evalena Stattin, Kjell G. Nilsson, Javier Alvarez-Rumero, Nir Eynon, Julian Feller, Oren Tirosh, Michael Posthumus: Acquisition of clinical material