Pulsed electromagnetic field therapy alters early healing in a rat model of rotator cuff injury and repair: Potential mechanisms
Abstract
Rotator cuff repair failure remains common due to poor tendon healing, particularly at the enthesis. We previously showed that pulsed electromagnetic field (PEMF) therapy improved the mechanical properties of the rat supraspinatus tendon postoperatively. However, little is known about the mechanisms behind PEMF-dependent contributions to improved healing in this injury model. The objective of this study was to determine the influence of PEMF treatment on tendon gene expression and cell composition, as well as bone microarchitecture and dynamic bone metabolism during early stages of healing. We hypothesized that PEMF treatment would amplify tendon-healing related signaling pathways while mitigating inflammation and improve bone metabolism at the repair site. Rats underwent rotator cuff injury and repair followed by assignment to either control (non-PEMF) or PEMF treatment groups. Gene and protein expression as well as tendon and bone histological assessments were performed 3, 7, 14, 21, and 28 days after injury. Gene expression data demonstrated an upregulation in the bone morphogenetic protein 2 signaling pathway and increases in pro-osteogenic genes at the insertion, supporting important processes to re-establish the tendon-bone interface. PEMF also downregulated genes related to a fibrotic healing response. Anti-inflammatory effects were demonstrated by both gene expression and macrophage phenotype. PEMF significantly increased the rate of kinetic bone formation directly adjacent to the tendon enthesis as well as the number of cuboidal surface osteoblasts (active osteoblasts) in the humeral head. This study has provided insight into how PEMF affects cellular and molecular processes in the supraspinatus tendon and adjacent bone after injury and repair.
1 INTRODUCTION
Rotator cuff tears affect millions of individuals each year.1, 2 Due to associated pain and loss of function, these injuries often require surgical intervention.1, 2 Surgical and rehabilitation protocol refinement has improved postoperative results, yet repair failure remains common due to poor healing of the tendon, particularly at the enthesis.2 Therefore, several therapeutics have been utilized in conjunction with surgical repair to improve outcomes. Noninvasive, adjunctive techniques are of interest as they do not interfere with surgical, immobilization, or physical therapy protocols.
Pulsed electromagnetic field (PEMF) therapy has been shown to enhance orthopedic healing processes and is approved by the US Food and Drug Administration (FDA) to treat fracture nonunion and augment spine fusion surgery.3, 4 In two studies utilizing the rat model of rotator cuff and repair, PEMF improved mechanical properties of the rat supraspinatus tendon postoperatively.5, 6 Although these improvements were consistent over a range of treatment durations and electromagnetic frequencies, 1 h of PhysioStim PEMF (Orthofix Medical, Inc.) led to over 100% increases in both tendon stiffness and modulus compared to non-PEMF controls at 4 weeks postinjury and repair.5, 6 While PEMF-treated tendon properties were still much lower than native, uninjured values,5 these clear improvements in mechanical strength could reduce the incidence of early repair failure.7 Increased bone volume fraction, trabecular thickness, and bone mineral density were also noted at the enthesis in the proximal humerus with PEMF treatment, supporting the role of PEMF in bone formation activation.5 Repaired supraspinatus tendons treated with PEMF demonstrated increased expression of Type I collagen and fibronectin and improved collagen organization.6 Interestingly, these positive effects were not observed with PEMF administration following midsubstance repair of the rat Achilles tendon.8 Together, these findings suggest that PEMF treatment alters the native biological healing response at the supraspinatus enthesis following injury and tendon-to-bone repair.
However, these alterations in composition and tissue structure could be downstream of many physiological responses to PEMF, including changes in signaling pathways and inflammation that regulate cell metabolism, production of matrix components, and matrix turnover. A number of key signaling pathways have been identified as part of the native tendon healing response. Transforming growth factor β (TGFβ) signaling plays a major role in driving neonatal tendon regeneration and is involved in recruitment of cells to the injury site.9 Several members of the TGFβ superfamily are known players in robust tendon healing, including bone morphogenetic protein (BMP) 2 enhancement of bone-tendon integration and BMP4 and 7 regulation of tenocyte differentiation.10, 11 The mTOR signaling pathway also regulates collagen fibril formation and alignment,12 which are crucial for re-establishing force transmission between muscle and bone. This pathway also stimulates tenocyte differentiation and has been shown to be involved in exosome-mediated tendon regeneration.13, 14 The Wnt/β-catenin signaling pathway regulates anabolic bone formation; localized activation has successfully enhanced rotator cuff healing in a previous animal study.15 Further, inflammation during tendon healing follows a temporal pattern and must be specifically modulated; early inflammation is necessary for healing but chronic inflammation diminishes tendon properties. Therefore, the establishment of a short inflammatory period is important but it must culminate to allow later stages of cell proliferation and tissue remodeling.16, 17
Interestingly, all of the pathways mentioned are known to be altered with PEMF exposure. Rat osteoblasts treated with PEMF responded similarly to cells treated with BMP2,18 and mTOR signaling was activated in mouse fibroblasts exposed to PEMF.19 These and a number of other studies support the concept that many cell types respond to PEMF exposure analogously to soluble growth factor exposure. Further, a series of human cell culture experiments demonstrated PEMF mediation of inflammatory cytokine expression,20 and PEMF treatment has been associated with pain relief in clinical studies following surgical procedures.21, 22
Although our previous studies indicate a positive effect of PEMF on both soft tissue and bony properties during tendon-to-bone healing, little is known about the possible contributions of PEMF to biologically improved healing in this model. We speculated that early cell-level changes could result in the significant improvements in tissue mechanics seen at 4 and 8 weeks postinjury in our previous studies.5, 6 Therefore, the objective of this study was to determine the influence of PEMF treatment on tendon gene expression and cell composition, as well as bone microarchitecture and metabolism during early stages of healing. We hypothesized that PEMF treatment would amplify tendon-healing related signaling pathways while mitigating inflammation, as well as improve bone metabolism at the site of repair.
2 METHODS
2.1 Study design and surgical procedures
All animal procedures were approved by the University of Pennsylvania Institutional Animal Care and Use Committee (#805274). A total of 106 adult male Sprague–Dawley rats (400–450 g, approximately 4–5 months of age) were used. This age replicates previous rotator cuff studies and is associated with slow or no skeletal growth. Animals were housed with 12-h light/dark cycles and received standard rat chow ad libitum. All animals underwent bilateral supraspinatus injury and repair, as previously described,5, 6, 23-25 followed by systemic exposure to PhysioStim PEMF (Orthofix Medical, Inc.) for 1 h daily (PEMF, n = 53) starting postoperative Day 1 and continuing until euthanasia. Control animals did not receive PEMF therapy (non-PEMF, n = 53). All animals received a 3-day regimen of buprenorphine post-op. Animals were euthanized via carbon dioxide at 3, 7, 14, 21, and 28 days postinjury and repair. These time points were chosen to cover stages of healing including inflammation, proliferation, and early remodeling. Additionally, previous studies identified alterations in protein expression and tendon mechanical properties as early as 4 weeks postsurgery.5, 6, 17 All animals were used for at least two assays (two shoulders from a single animal were never assigned to the same assay). Animals were randomly assigned within treatment groups for either (1) tendon gene expression, tendon protein expression, and microcomputed-tomography (µCT) analyses or (2) tendon (paraffin embedded) and bone (plastic embedded) histology.
2.2 PEMF exposure
Animals receiving PEMF were exposed systemically using the commercial PEMF signal PhysioStim (Orthofix Medical, Inc.); this signal has a maximum amplitude of 1.10 mT and a fundamental frequency of 3.85 kHz. This signal and treatment duration (1 h per day) were chosen based on positive results from our previous studies.6 The day after surgery, animal cages were placed on custom-built PEMF racks (Orthofix Medical, Inc.), which include an electromagnetic coil for each module. A uniform electromagnetic field was produced across the module volume, sized to hold a standard rat cage.
2.3 Gene expression
At the time of euthanasia, right supraspinatus tendons (n = 5/group/time point) were dissected and detached from the humerus at the insertion. The supraspinatus muscle was removed. The tendon was cut into two parts, with the proximal 2 mm designated as the insertion, and remaining tendon designated as the midsubstance, including the myotendinous portion. Humerii from these limbs were wrapped in phosphate-buffered saline soaked gauze and stored at −20°C for µCT analysis (see below). Tendon samples were frozen in liquid nitrogen and stored at −80°C. RNA was isolated using a TRIspin method. Briefly, specimens were thawed in TRIzol (Life Technologies Inc.) and then physically chopped using microscissors followed by pipette pestling for approximately 5–10 min or until the tissue was integrated. Following phase separation, the aqueous phase was processed via fractionation on RNA Clean and Concentrator—5 columns (Zymo Research). RNA concentrations were measured using a NanoDrop (ThermoScientific) and quality was checked via Bioanalyzer System (Agilent). RIN values of RNA used in this study were all over 7. The Penn Molecular Profiling Core performed specific target amplification followed by quantitative polymerase chain reaction for target genes (Table S1) using the Fluidigm BioMark HD Platform. Housekeeping genes were GAPDH and Ppib, both of which were stably expressed between groups at each time point. Samples with Ct value outliers of reference genes were removed from analysis. ΔΔCt values were calculated at each time point to determine fold change between gene of interest and housekeeping gene, and then fold change between non-PEMF controls and PEMF-treated samples. ΔΔCt outliers were also removed (2.2x interquartile range method).26, 27 After outlier removal, the minimum n for each group/time point was 3. ΔΔCt values were used to calculate fold change differences between controls and PEMF-treated samples at each time point (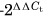 ), allowing the determination of time-specific effects of PEMF.
), allowing the determination of time-specific effects of PEMF.
2.4 Protein expression
At the time of euthanasia, left supraspinatus tendons (n = 5/group/time point) were dissected in an identical fashion as the right tendons used for gene expression. Wet weights of each sample were recorded. Tendons were frozen in liquid nitrogen and stored at −80°C. Samples were chopped with microdissection scissors in radioimmunoprecipitation assay buffer, followed by homogenization using a 3 mm Polytron Generator probe (VWR) for 10 × 15 s pulses. Samples were incubated on ice followed by centrifugation. Protein quantity was measured using a BCA assay (ThermoFisher). Supernatant was stored at −80°C. Based on gene expression findings, Milliplex Multiplex Assays (Millipore) were chosen to measure target protein concentrations (Rat TGFβ 3-Plex, Rat Vascular Injury Panel, and Rat Cytokine/Chemokine SemiCustom Panel).28 The Penn Human Immunology Core performed these assays using the Luminex flow-cytometry based platform, according to manufacturer's instructions. A quantity of 50 µg of total protein was analyzed using each of the magnetic bead assays to measure the abundance of each protein included in the panel, allowing for the concentration of up to 15 analytes to be measured in a single sample. Mean fluorescence intensity values of analytes were measured in a MAGPIX system (Luminex). Total concentration of target protein in each sample is reported as ng/ml based on standard curve values.
2.5 Tendon and enthesis histology
Animals assigned for histology were injected with 5-ethynyl-2'-deoxyuridine (EdU; 3 mg/kg) 24 h before euthanasia. At the time of euthanasia, right humeral-tendon-muscle complexes (n = 5/group/time point except for the 28 day time point, at which n = 8/group) were dissected out and processed with paraffin. Samples were bisected in the coronal plane and 7 µm sections were collected from the tendon center. H&E: Sections were stained with hematoxylin and eosin. Slides were scanned using a Zeiss Axio Scan.Z1 and regions of interest (ROI) were imaged at ×200 magnification. Cell shape and cellularity at both the tendon insertion and midsubstance were semiquantitatively evaluated by three blinded graders using a custom grading scale as previously described (split by regions of interest but evaluated against samples from all time points).6, 24, 29 SafO: Adjacent sections were stained with Safranin O and Fast Green to identify changes in matrix composition. Enthesis fibrocartilage was imaged at ×200 magnification, encompassing rounded fibrocartilage cells at the tendon-bone interface. Adjacent regions of the proximal tendon proper were also imaged. Safranin O-positive staining areas were quantified using ImageJ (NIH, v1.52). ROI were color thresholded for Safranin-positive red staining and quantified as a percentage of the ROI. No more than one sample per group was excluded for poor quality tissue at the enthesis. EdU: Immunohistochemical staining was performed to assess cell proliferation. Sections were permeabilized and stained with the Click and Go Reaction Buffer Kit (Click Chemistry #1263) with AlexaFluor647 Azide and cover slipped with 4′,6-diamidino-2-phenylindole (DAPI) mountant. Slides were imaged with DAPI and Cy5 filters using an AxioZoom fluorescent microscope (Zeiss). ROI in the tendon insertion and midsubstance were imaged at ×100 magnification. Cy5+ cells were counted using ImageJ. On average 1–2 samples per group had poor quality staining (high background, etc.) and were excluded from quantification. Macrophage Immunohistochemistry: Sections from 14 to 28-day time points were immunohistochemically stained for CD68 and CD163 (M1 and M2 macrophages, respectively30). Heat-induced antigen retrieval was performed with sodium citrate buffer, followed by primary antibody incubation (anti-CD68 Abcam ab31630 1:300; anti-CD163 Abcam ab182422 1:400) overnight at 4°C. Biotinylated secondary antibody was detected using diaminobenzidine. ROI at the tendon insertion and midsubstance were imaged at ×200 magnification and % positive pixels were quantified using a custom MATLAB code. For all histological measurements, at least two ROI were chosen from two to three slides selected from comparable depths through the tendons. On average 1–2 samples per group had poor quality staining (high background, etc.) and were excluded from quantification.
2.6 µCT scans
Humerii dissected for tendon gene expression (described above) (n = 5/group/time point) were μCT scanned (VivaCT80, Scanco) at 10.5 μm resolution (70 kVP energy, 400 ms integration time). Specifically, the trabecular bone contained between the articular surface and the proximal humeral growth plate within the greater tuberosity was analyzed by manually segmenting sequential coronal plane slices to specify analysis to trabecular bone excluding surrounding cortical bone and humeral growth plate, as well as establishing the region within the greater tuberosity using the anatomical neck as a landmark. Global thresholding was applied to all images, and standard parameters of trabecular microarchitecture were measured.
2.7 Bone histomorphometry
Animals assigned for histology for the 21 and 28-day time points (n = 5/group at 21 days, n = 8/group at 28 days) were injected with calcein (Sigma, 10 mg/kg) 7 and 14 days before euthanasia. At the time of euthanasia, left humeral heads were harvested and cleaned of soft tissues. The medullary cavity was opened and the majority of the diaphysis was removed. Samples were fixed and dehydrated in ethanol, followed by processing into methyl methacrylate (MMA), embedding in PMMA, and coronal sectioning at 5 µm onto gelatin-coated slides. Derived kinetic indices: Slides were coverslipped with DAPI mountant and fluorescently imaged with FITC (for calcein) and DAPI filters. ROI were chosen at ×200 magnification in the epiphysis. Derived kinetic indices were calculated by measuring lengths of bone surface area and distance between calcein labels (total bone surface [BS]; calcein-positive mineralizing surface [MS]; interlabel distance, Ir.L.Th) using ImageJ (NIH). These parameters were used to calculate percent mineralizing surface, mineral apposition rate (MAR), and bone formation rate (BFR). On average 1–2 samples per group had poor quality staining (high background, etc.) and were excluded from quantification. Osteoblast/Osteoclast counts: Slides were stained with Goldner's Trichrome for bone cell analysis. ROI were imaged at ×500 magnification in the epiphysis. Surface osteoblasts and osteoclasts were counted manually. Cell identification was based on descriptions/examples from literature of location and morphology. All measurements were made using 2–3 slides per sample and 3–5 ROI chosen from each slide; measurements were averaged for each sample. On average 1–2 samples per group had poor quality staining (high background, etc.) and were excluded from quantification. All counts and measurements were by three blinded reviewers.
2.8 Statistics
Statistical comparisons were made using GraphPad Prism 7 (GraphPad Software). Comparisons were made between PEMF and non-PEMF groups over time using two-way analysis of variances (ANOVAs with Bonferroni post-hoc tests for pairwise comparisons. Gene expression data was log transformed before statistical analysis. Due to the large number of comparisons, false discovery for gene expression data is possible; for unbiased presentation, all comparisons (ANOVA and pairwise) with p < 0.05 are disclosed. Semiquantitative histological assessments (cell shape and cell density) were compared with non-parametric two-way ANOVA (Kruskal Wallis). No statistical comparisons were made between tissue types (insertion vs. mid-substance). Significance was set at p < 0.05. Significant ANOVAs are reported as p values in the results and in figure legends, with specification for time or treatment variable. Data is presented as mean ± SD or median ± interquartile range as specified in figure legend.
3 RESULTS
3.1 Gene expression
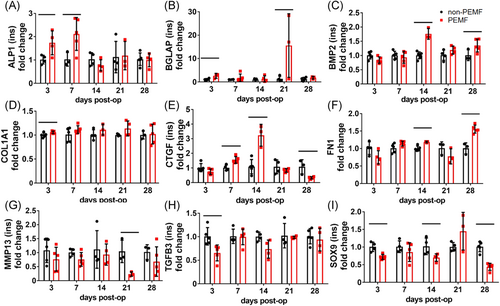
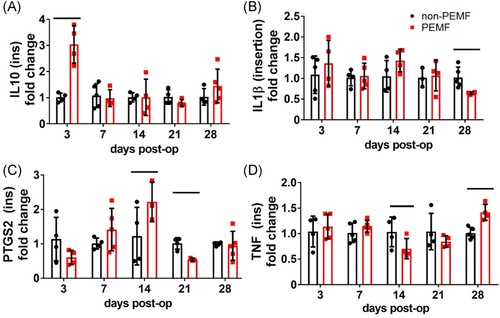
At the tendon insertion, PEMF treatment significantly altered a number of genes related to BMP signaling across time points. Compared to non-PEMF controls, tendons exposed to PEMF demonstrated increased BMP target Alp1 (Figure 1A, p = 0.04), increased downstream target Bglap (Figure 1B, p = 0.007), increased the Bmp2 signaling molecule (Figure 1C, p = 0.0003), and increased downstream target Col1a1 (Figure 1D, p = 0.03). Additionally, many genes related to the TGFβ signaling pathway were modulated by PEMF exposure. PEMF increased downstream growth factor Ctgf early and decreased Ctgf late (Figure 1E, p = 0.02), increased upstream regulator Fn1 (Figure 1F, p = 0.05), decreased downstream (downregulated) target Mmp13 (Figure 1G, p = 0.006), decreased signaling molecule Tgfb3 (Figure 1H, p = 0.009), and decreased downstream cartilage marker Sox9 (Figure 1I, p = 0.008). Interestingly, PEMF also impacted several genes associated with inflammation, increasing anti-inflammatory cytokine Il10 (Figure 2A, p = 0.0005), decreasing proinflammatory cytokine Il1b at 28 days postinjury (Figure 2B), and altering Ptgs2 (Figure 2C) and Tnf expression (Figure 2D). There were no significant differences identified in the expression of other target genes at the tendon insertion (Figures S1 and S2).
At the tendon midsubstance, PEMF treatment had similar significant effects on a number of genes related to the TGFβ signaling pathway. PEMF increased downstream targets Ctgf (Figure S3A, p = 0.002), Gdf5 (Figure S3B, p = 0.006), Mmp13 (Figure S3C, p = 0.005), Postn (Figure S3D, p = 0.01), Scx at 7 days postinjury (Figure S3E), and Tnc (Figure S3F, p = 0.009). Select genes involved in inflammatory processes were also altered with PEMF at the tendon midsubstance, including increased Ptgs2 (Figure S3G, p = 0.04), increased Tac1 (Figure S3H, p = 0.001), and decreased Tnf (Figure S3I, p = 0.04). Genes related to the mTOR signaling pathway were also uniquely altered in the midsubstance, with PEMF exposure leading to increased expression of downstream matrix protein Fn1 at mid- to late-time points (Figure S4A, p = 0.002), decreased expression of regulatory protein Rptor across time points (Figure S4B, p = 0.05), and decreased downstream transcription factor Srebf1 expression at early time points (Figure S4C, p = 0.0009). There were no significant differences identified in the expression of other target genes at the tendon midsubstance, with the exception of Wnt receptor Lrp5 (Figures S5 and S6).
3.2 Protein expression
At the tendon insertion, protein expression was not significantly altered by PEMF treatment across all time points for any target measured. Although the expression of TGFβ1 was consistent across time points (Figure S7A), there were time-dependent changes in the expression of TGFβ2 and TGFβ3 signaling molecules (Figure S7B,C, p < 0.0001, for both). At 3 days postinjury, monocyte chemoattractant protein 1 (MCP-1) was decreased in PEMF-treated tendons (Figure S7D, p = 0.04). The expression of VEGF and TIMP-1 were also time-dependent (Figure S7E,F, p < 0.0001 for both). Anti-inflammatory cytokine interleukin-10 (IL-10) was increased in PEMF-treated tendons at 21 days postinjury (Figure S7G, p = 0.003), but PEMF did not have any effect on IL-1β or IL-17A (Figure S7H,I).
Similar to the insertion, the expression of TGFβ1 was consistent in the midsubstance across time points (Figure S8A) and there were time-dependent changes in the expression of TGFβ2 and TGFβ3 signaling molecules (Figure S8B,C, p < 0.0001 for both). MCP-1 levels dropped below detectable levels for non-PEMF controls at day 14 and beyond but were consistently detectable in PEMF samples across all time points (Figure S8D). Expression of VEGF was time dependent (Figure S8E, p = 0.0002). TIMP-1, an MMP inhibitor, was decreased in PEMF-treated tendons at 7 days postinjury (Figure S8F, p = 0.01). No measured cytokine was altered by PEMF exposure (Figure S8G–I).
There were no differences in the expression of caveolin-1 at the insertion or midsubstance, or in the expression of connective tissue growth factor (CTGF) at the insertion (Figures S7J,K and S8J). Several proteins were not expressed above detectable level (CTGF in the midsubstance, IL-6 in both regions, IL-4 in both regions); Pai-1 data was unreliable due to low bead counts.
3.3 Histology of tendon and enthesis fibrocartilage
There were no differences in cell density or cell shape in either the tendon insertion or midsubstance with PEMF treatment compared to non-PEMF controls (Figure S9). Cell shape and cellularity changed dependent of time in the midsubstance for both groups (cellularity, p = 0.001; cell shape, p < 0.0001); these metrics did not significantly change in the insertion. Positive EdU staining was also not different between control and PEMF-treated groups, but cell proliferation was highly time-dependent, diminishing across time for both regions of interest (Figure S10A,B, p < 0.0001). Representative images from postinjury Day 7 are shown in Figure S10C.
Quantification of positive immunohistochemical staining at the tendon insertion demonstrated CD68 + macrophages were unaltered by PEMF (Figure 3A), while CD163 + macrophages were significantly increased by PEMF across time (p = 0.01, Figure 3B). The ratio of M1 to M2 macrophages at the insertion was also decreased with PEMF (p = 0.03, Figure 3C). In the tendon midsubstance, PEMF decreased CD68 + cells (p = 0.02, Figure 3D) and increased CD163 + macrophages (p = 0.01, Figure 3E), leading to a significantly decreased ratio of M1 to M2 macrophages across time (p = 0.02, Figure 3F). Representative images from postinjury Days 14 and 28 are shown in Figure S11.
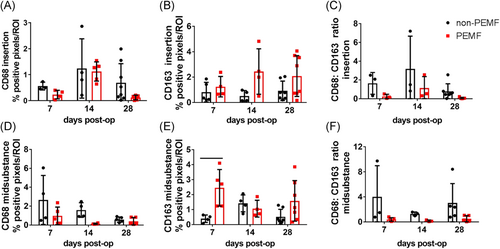
There were no differences in the quantity of positive Safranin O (proteoglycan content) staining of the enthesis fibrocartilage between treatment groups (Figure S12); this metric did demonstrate a significant variability within groups.
3.4 µCT
Bone properties of the greater tuberosity of the humerus measured by µCT were largely unaffected by PEMF exposure. No differences were seen in bone mineral content (Figure S13A), bone mineral density (Figure S13B), connectivity density (Figure S13C), bone volume/total volume (BV/TV) (Figure S13D), trabecular thickness (Figure S13E), or trabecular number (not shown). Trabecular separation was increased by PEMF (p = 0.05, Figure S13F).
3.5 Bone histomorphometry
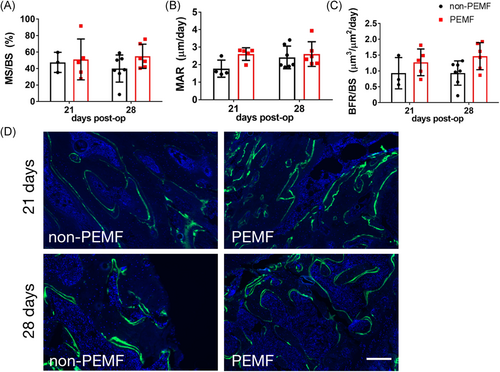
Double calcein labeling of the humeral tuberosity was quantified to compare dynamic bone metabolism. The ratio of mineralizing surface to bone surface (MS/BS) was not affected by PEMF exposure (Figure 4A), nor was mineral apposition rate (MAR, distance between labels per day) (Figure 4B). PEMF exposure significantly increased bone formation rate per bone surface (BFR/BS) across time points (Figure 4C, p = 0.04). Representative calcein double-labeling images of humeral tuberosity trabecular bone surfaces at 21 and 28 days postinjury for both groups are shown in Figure 4D.
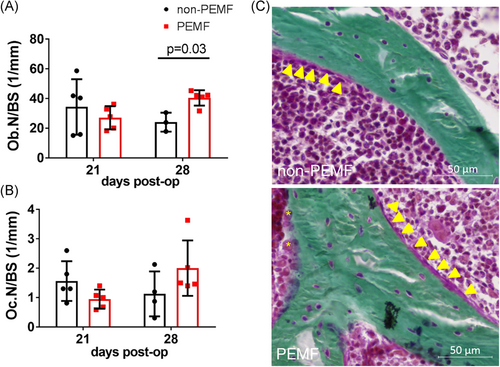
Calcified sections of humeral tuberosity stained with Goldner's Trichrome allowed for measurement of the number of osteoblasts (Ob.N/BS) and the number of osteoclasts (Oc.N/BS). The number of osteoblasts on mineralizing bone surface was significantly increased with PEMF treatment at 28 days postinjury (Figure 5A). Osteoclast number was not affected by treatment across time points (Figure 5B). Representative images of the humeral tuberosity at 28 days postinjury for both groups are shown in Figure 5C.
4 DISCUSSION
Although previous studies support the use of PEMF to enhance tendon-to-bone repair, biological processes and molecular mechanisms associated with improved repair strength remained unclear. To optimize therapeutic interventions, identifying molecular pathways related to enhanced tendon healing is critical. This study demonstrated specific molecular and cellular changes within repaired supraspinatus tendons following PEMF treatment compared to controls.
First, gene expression data suggests upregulation in members of the BMP2 signaling pathway, including BMP2 ligand and CTGF. Increases in signaling may stimulate increased collagen and fibronectin expression seen in the tendon insertion throughout early healing. PEMF similarly induces expression of procollagen and other BMP2 targets in rat osteoblast cultures.18, 29, 30 Previous immunohistochemistry supports increased quantities of collagen and fibronectin proteins in PEMF-treated tendons at 4 weeks6; both are important components of the healing response and re-establish tendon tissue structure after injury.31 Interestingly, expression of both TGFβ3 ligand and MMP13 were decreased; these proteins are involved in a mechanically inferior fibrotic healing response.32 These results support a downregulation in the fibrotic response with PEMF, which corresponds with decreased tendon cross-sectional area after 4 weeks of treatment shown in previous studies.5, 6 Taken together, gene expression data supports the hypothesis that PEMF treatment amplifies transcriptional activation of important molecular pathways, such as TGFβ signaling, involved in tendon healing.
We also hypothesized that PEMF would mitigate inflammation. Several studies have detailed the anti-inflammatory effect of PEMF on other musculoskeletal tissues including intervertebral disc.33, 34 Indeed, PEMF had an anti-inflammatory effect in our model, upregulating expression of the anti-inflammatory cytokine Il10 in both the tendon insertion (confirmed at the protein level) and midsubstance, and downregulating proinflammatory cytokines Il1β and TNF-α. However, Ptgs2, which encodes proinflammatory cyclooxygenase-2 (COX2), was upregulated in both tendon regions at day 14 postinjury but downregulated at Day 21. During tendon healing, the presence of an inflammatory response is crucial for initiating a healing response; however, chronic inflammation can impede ideal tissue structure reorganization.17 PEMF may play a role in balancing helpful early inflammation and detrimental late inflammation. Several studies have associated the presence of proinflammatory M1 macrophages with ECM degradation and other markers of the inflammatory response. Shifts toward an M1 phenotype correlate with poor healing. M2 macrophages play a contrasting role in promoting matrix deposition and restoration of normal tissue.35 Recruitment of M2 macrophages enhanced tendon microarchitecture recovery in ovine tendon lesions.36 Immunohistochemical staining demonstrated that at both the supraspinatus insertion and midsubstance, the ratio of M1 (CD68+) to M2 (CD163+) macrophages was significantly decreased across time points, suggesting a shift toward the anti-inflammatory M2 macrophage phenotype with PEMF. PEMF induced a similar shift in macrophage phenotype in cultured human tendon cells.37 Taken together, gene expression and immune cell marker localization may suggest a reduction in inflammation with PEMF treatment.
Our last hypothesis was driven by the primary clinical use of PEMF, fracture healing and spinal fusion,38, 39 as well as our previous studies demonstrating improved bone microarchitecture in the rat rotator cuff model after 16 weeks of PEMF treatment.5 Additionally, the lack of improved healing in Achilles tendon midsubstance injury models suggests that PEMF plays a specific role in tendon-to-bone healing rather than tendon-to-tendon healing.8 These models differ in many ways including tendon injury location, surgical repair procedures, loading mechanics, and vascularity, all of which may impact PEMF efficacy. Specifically, we proposed that PEMF treatment in our model would increase early bone metabolism at the repair site. PEMF increased the expression of several pro-osteogenic genes at the insertion, including alkaline phosphatase (Alp1), Bglap (osteocalcin), and Col1a. This suggests that PEMF could support important processes to re-establish the tendon–bone interface. PEMF also significantly increased the rate of kinetic bone formation adjacent to the tendon enthesis as well as the number of active osteoblasts in the humeral head. While bone microarchitecture was not significantly altered within the time course of this study, this was expected as our previous study also demonstrated little to no differences in the humeral head until the 16-week treatment time point.5 Increases in early bone metabolism support increased bone volume at the insertion at later stages. Taken together, gene expression and dynamic bone metabolism data suggest some increases in bone formation via PEMF exposure. Clinical studies correlate decreased humeral trabecular bone volume with age. Such degenerative changes of the humerus have been associated with the occurrence of rotator cuff tears,40 and the association of rotator cuff tear with age is also well-established.41 The use of PEMF to address this aspect of rotator cuff integrity could be invaluable.
We were not able to draw clear conclusions regarding other pathways interrogated by gene expression, including mTOR and Wnt. Future studies will build on gene expression and protein quantification data, as it may provide further insight into native healing processes. There were interesting temporal patterns in the expression of many target genes.
Our study was not able to identify differences in tendon cell morphology, density/cellularity, or proliferation. We also did not measure a difference in the area of proteoglycan-rich fibrocartilage at the enthesis. This data suggests that increased cell organization, tenocyte proliferation, and fibrocartilage formation may not be processes involved in tendon-to-bone healing after rotator cuff repair when augmented with PEMF. Of note, although the study was not powered to address regional comparisons, qualitative regional differences in gene expression and cell morphology support the need to assess tendon responses to injury and/or therapeutic applications regionally. Tendon cell heterogeneity has only recently begun to be characterized and should be considered further in future studies.42
This study is not without limitations. Although the rat rotator cuff model of acute supraspinatus injury and immediate repair is well-established and has been used extensively in previous publications and in our previous work using PEMF, it does not represent the most common clinical presentation, which is a chronic condition. However, early repair failure can occur after acute tears,7 and identifying and augmenting targets involved in early healing processes are important for re-establishing tendon-to-bone strength in both the acute and chronic patient populations. Our gene expression assessment required a large number of comparisons, with likelihood of some false positives. Further, we took a targeted approach to the evaluation of gene and protein expression; whole transcriptome and proteome analyses would likely provide more information. We selected our protein expression assay based on our limited sample volume; however, issues with sample stickiness led to low bead counts and read-out variability, and protein targets were limited by commercial availability of immunoassays. Therefore, we were able to relate very little of our gene expression data to the protein level. The use of immunohistochemistry to detect immune cells limits characterization of all tendon cell types, but was chosen to allow additional histological studies to be performed on the same samples. Additionally, animals were assigned to a single time point, leading to the inability to perform paired analyses across time points. The use of one or more longitudinal metrics, such as assessment of collagen synthesis via microdialysis could be useful. In this study, PEMF therapy was delivered systemically to match our previous study designs; future studies could assess if and how localized PEMF exposure may uniquely affect healing.
Previous work showing improved rotator cuff healing with PEMF, along with the noninvasive nature and history of clinical safety, supported the initiation of a PEMF clinical trial for use after rotator cuff repair. This study provides important biological insight into how PEMF affects cellular and molecular processes in the supraspinatus tendon after injury. The increased expression of genes that regulate production and organization of tendon extracellular matrix, shift towards an anti-inflammatory environment, and augmented humeral head bone formation observed in this study further support the potential promising use of PEMF as an adjuvant treatment to clinical improve rotator cuff repair healing and outcomes.
ACKNOWLEDGMENTS
We thank Dr. Matthew Counihan, Dr. Yaping Ye, Dr. Nathaniel Dyment, the Penn Molecular Profiling Facility, the Penn Human Immunology Core, and the PCMD MicroCT Imaging and Histology Cores for their assistance. We disclose that Orthofix Medical, Inc. employs Nianli Zhang and employed Erik Waldorff and James Ryaby during the time this study was completed. This study was funded by Orthofix Medical, Inc. and the NIH/NIAMS supported Penn Center for Musculoskeletal Disorders Grant (5P30AR069619).
AUTHOR CONTRIBUTIONS
Julianne Huegel contributed to research design, data acquisition, analysis, interpretation, and drafting and editing the manuscript. Peter Y. W. Chan and Harina Raja contributed to data acquisition, analysis, and critical review of the manuscript. Stephanie N. Weiss and Courtney A. Nuss contributed to animal procedures, data acquisition, and critical review of the manuscript. Erik I. Waldorff, Nianli Zhang, James T. Ryaby, Louis J. Soslowsky, and Andrew F. Kuntz contributed to research design, interpretation, and critical review of the manuscript. All authors have read and approved the final manuscript for submission.