Intraoperative knee kinematics measured by computer-assisted navigation and intraoperative ligament balance have the potential to predict postoperative knee kinematics
Abstract
This study was designed to analyze the effects of type of activity and cruciate ligament resection on knee kinematics and ligament balance after total knee arthroplasty (TKA), and to determine if intraoperative passive kinematics are associated with active kinematics. Fresh-frozen human cadaveric knees were examined. The knees were mounted on a quadriceps-driven simulator. Cruciate-retaining (CR-TKA) and posterior-substituting (PS-TKA) TKA was performed using a contemporary knee system. Active flexion (closed-kinetic chain [CKC] and open-kinetic-chain [OKC]) and passive flexion were analyzed by recording the knee kinematics using a specifically developed application of an imageless navigation system. An electronic ligament balancer was used to measure the tibiofemoral gap under constant distraction pressure. The femur rotated externally relative to the tibia during passive and active CKC flexion. The femur translated anteriorly from 10° to 50° of flexion after TKA. Beyond 50° of flexion, the femur translated posteriorly in all surgical conditions. The femoral location during active CKC flexion was posterior relative to that during active OKC. Femoral rotation and translation during passive knee flexion correlated significantly with that during active knee flexion. Posterior tilt of the electronic ligament balancer was greater with CR-TKA than with PS-TKA and correlated significantly with the anteroposterior position of the femur. Statement of Clinical Significance: Intraoperative knee kinematics measured by computer-assisted navigation and intraoperative ligament balance have the potential to predict postoperative knee kinematics.
1 INTRODUCTION
Enhancements in implant design and surgical techniques have increased survivorship after total knee arthroplasty (TKA). Clinical outcomes after TKA have been linked to many factors, including component orientation and limb alignment.1-5 Advances such as computer navigation have reduced the risk of outliers in alignment of TKA.6-9 However, patient satisfaction after TKA has not been consistent.10-14
A recent study found that in vivo fluoroscopic knee kinematics correlated with patient-reported outcomes.15 Therefore, evaluation of knee kinematics after TKA may provide valuable insights into improving patient satisfaction. Studies have reported differences in knee kinematics between weight-bearing and non-weight-bearing activities, as well as between early-flexion and high-flexion during squatting.16-19 Knee flexion varies by type of activity of daily living: for example flexion of 50°–60° is typically needed during stair climbing, while rising from a chair may require flexion beyond 90°.20, 21 Therefore, to assess the impact on activities of daily living, it is relevant to investigate kinematics during weight-bearing and non-weight-bearing activities over the full range of flexion.
Computer-assisted navigation systems increase accuracy in implant placement and reduce outliers in TKA.6-9 However, intraoperative kinematics are measured during navigation under passive conditions with the patient under anesthesia. The relevance of intraoperative passive kinematics to active kinematics is largely unknown. Furthermore, cruciate ligament sacrifice or substitution, mediolateral (ML) collateral ligament balance, and imbalance in flexion-extension gaps can affect both active and passive kinematics. Many studies have compared the kinematics between cruciate-retaining total knee arthroplasty (CR-TKA) and posterior stabilized TKA (PS-TKA).22-26 Ligament imbalance has been linked to knee instability.27, 28 However, the relationship between kinematics and ligament balance remains unknown.
- (1)
The type of activity and cruciate ligament resection will significantly affect knee kinematics and ligament balance.
- (2)
Passive kinematics measured intraoperatively will be correlated with active kinematics measured postoperatively.
2 METHODS
A total of five fresh-frozen human cadaver knees were examined. Four male and one female donors with average age of 58 ± 15.7 years at the time of death, were used in this study. Computed tomography (CT) scans of the knees were obtained to measure preoperative alignment and rule out significant anatomic defects.
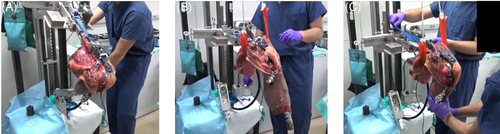
Specimens were thawed overnight until they reached room temperature and tissues were moistened with phosphate-buffered saline during the testing. The knees were mounted on a quadriceps-driven simulator based on the Oxford knee rig design.29, 30 The tibial rod was attached to the ankle-joint assembly on the rig, which had 3° of rotational freedom, while its translation was constrained. The femoral rod was attached to the hip-joint assembly on the rig. The hip-joint assembly was allowed freedom in flexion-extension and varus-valgus angulations, with vertical motion guided by two parallel cylindrical bars. The location of the hip joint was offset laterally to account for the anatomic valgus (5.9 ± 0.8°) of the cadaver knees (as measured on CT scans). The quadriceps tendon was attached to an electric motor via a nylon strap sutured to the quadriceps tendon. Two active flexion activities, closed-kinetic chain (CKC) and open-kinetic-chain (OKC), and one passive flexion-extension activity were performed. Active CKC simulated squatting. The hip load was adjusted to generate a peak moment of approximately 40 Nm, which was close to that reported during in vivo knee flexion activity.20, 31 An electric motor applied load to the quadriceps tendon to extend the knee from 110° to 10° (Figure 1A). Active OKC simulated extending the knee against gravity in a seated position. The hip was flexed to 110° and the thigh was supported. The ankle was unlocked with the lower leg hanging free against gravity. An electric motor applied load to the quadriceps tendon to extend the knee (Figure 1B). To simulate passive intraoperative kinematics, the surgeon manually flexed and extended the knee while the thigh was supported (Figure 1C).
The surgical procedures were performed using a contemporary knee system (Truliant; Exactech). An imageless navigation system (ExactechGPS; Blue-Ortho) was used to align the cutting guides. The mechanical axis of the femur was determined by circumducting the thigh to calculate the center of the femoral head and by digitizing the the femoral knee joint center at the intercondylar notch. The mechanical axis of the tibia was determined by digitizing the middle of the intercondylar eminence and the medial and lateral malleoli. After resecting the anterior cruciate ligament (ACL), the proximal tibia was cut at 10 mm below the lateral compartment with 0° varus. Measured resection was used to make femoral cuts; the distal femoral cut was made at 0° to the mechanical axis and the posterior femoral cut was made parallel to the surgical epicondylar axis. A tibial insert was selected with thickness that allowed passive extension to 0°. The patella was not resurfaced.
Knee kinematics were recorded using a specifically developed application of the imageless navigation system (Figure 1) with an accuracy of ±1 mm or ±1°.6 Four conditions: Normal (before any bony resection), CR-TKA, CR-TKA-PCLrelease (after posterior cruciate ligament release), and PS-TKA were compared. The capsule was not closed after each implantation and the patella did not visibly subluxate during testing.
An electronic ligament balancer (XO1; Exactech)32 was used to measure ligament balance during passive flexion. The performance and accuracy of the electronic ligament balancer has been previously described.27, 32 The balancer consists of a top and bottom plate separated by bellows that are inflated to the appropriate pressure (nominally 20 psi). The bottom plate of the balancer rests on the tibial cut surface while the top plate articulates with the femoral condyles. The balance measures the tibiofemoral gap as well as the angle of tilt between the top plate and the bottom plate through the arc of flexion. The data were wirelessly transmitted to a computer display.
The following variables were measured: axial femoral rotation relative to the tibia, femoral anteroposterior (AP) translation, tibiofemoral varus, and ligament balance. Internal rotation of the femur relative to the tibia was denoted as positive. Anterior location of femur relative to tibial mechanical axis was denoted as positive.
3 STATISTICAL ANALYSES
The results were analyzed using SPSS version 24 (IBM Corp.). Repeated measured analysis of variance (ANOVA) and post hoc pairwise comparison (after Bonferroni correction) were used to analyze the differences in axial femoral rotation angle, femoral AP translation, tibiofemoral varus, and ligament balance among three types of activities and four ligament conditions. Pearson's correlation coefficient was used to analyze the correlation between the kinematics and ligament balance, and between passive and active kinematics. A p < 0.05 was considered statistically significant.
4 RESULTS
4.1 Knee kinematics changes with type of activity
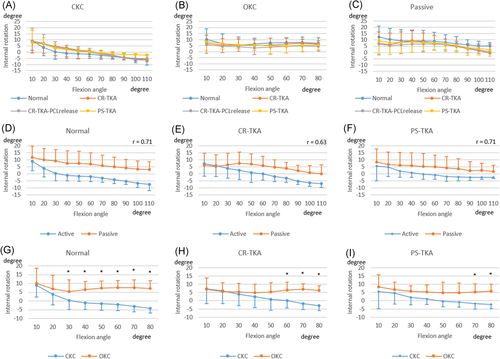
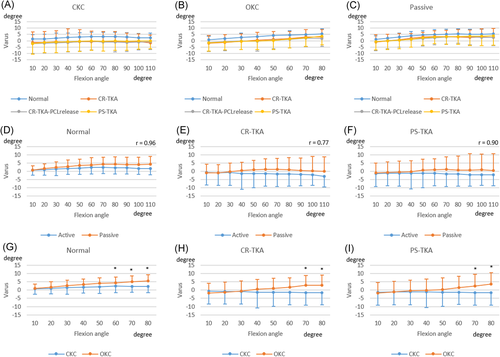
In general, the femur tended to rotate externally during active CKC flexion and passive flexion compared with active OKC flexion (Figure 2). Axial femoral rotation during passive flexion was moderately correlated with that during active CKC flexion (R = 0.63–0.71, p < 0.01). However, there were significant differences in femoral rotation between active OKC and active CKC. Within each activity there were no differences in femoral rotation between surgical conditions.
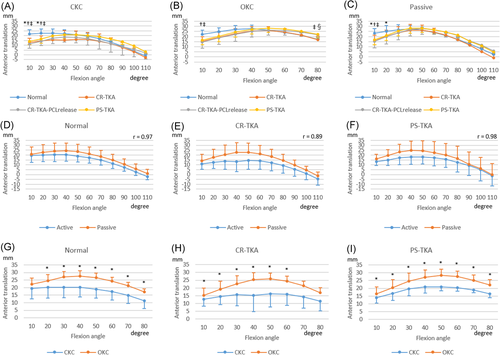
The femur translated anteriorly from 10° to 50° of flexion in both TKA conditions (Figure 3). Beyond 50° of flexion, the femur translated posteriorly in all conditions. The femoral AP location during active CKC flexion was posterior to that during OKC for all surgical conditions. Femoral AP translation during passive flexion was highly correlated with AP translation during active CKC flexion (R = 0.89–0.98, p < 0.01).
Preoperatively, the knee was in varus during extension and moved into further varus with flexion especially during passive flexion (Figure 4). Tibiofemoral varus was reduced in extension postoperatively. Tibiofemoral varus during passive flexion was highly correlated with that during active CKC flexion (r = 0.77–0.96, p < 0.01). The knees during OKC moved into more varus with flexion than that during CKC.
4.2 Knee ligament balance correlates with knee kinematics
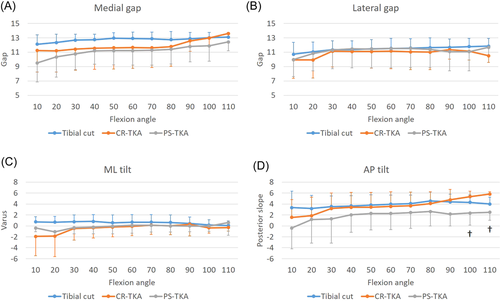
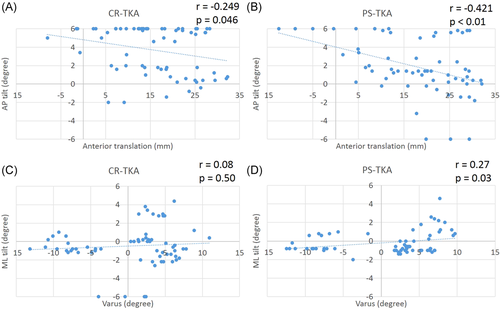
There were no significant differences between the surgical conditions (tibial cut, CR-TKA, and PS-TKA) in medial and lateral gap, and ML tilt measured by the electronic balancer. On the other hand, the posterior tilt after CR-TKA was greater than that after PS-TKA beyond 100° of flexion (Figure 5). There was a significant correlation between the posterior tilt and the femoral AP position in CR-TKA knees (r = −0.25, p = 0.046) and PS-TKA knees (r = −0.42, p < 0.01). After PS-TKA the ML imbalance correlated with tibiofemoral varus (Figure 6, r = 0.27, p = 0.03).
4.3 The effects of cruciate ligament resection
In early flexion, for all activities, the femur in normal knees was more anteriorly located than in knees after CR-TKA, CR-TKA-PCL release, or PS-TKA (Figure 3). Femoral rollback with further flexion was similar in all conditions. However, at 80° of active OKC flexion, release of the PCL resulted in a more anterior femoral location (Figure 3). In all conditions, cruciate ligament resection did not generate significant differences in tibiofemoral axial rotation or varus angulation (Figures 2 and 4).
5 DISCUSSION
We analyzed associations between intraoperative passive kinematics with active kinematics. Tibiofemoral axial rotation, AP translation, and varus angulation during passive knee flexion correlated significantly with active CKC knee flexion. Cruciate ligament status affected knee kinematics. We also found weak correlations between ligament balance in the AP and ML directions and knee kinematics after TKA.
Knee motion and laxity has been studied in cadavers since the 1960s.33 The ranges of secondary motions (such as AP translation and axial rotation) are loosely coupled with flexion and constrained by soft-tissues and loading.33 We and others have reported extensively on knee kinematics using the Oxford knee rig.29, 34-37 This rig has also been recommended to study kinematics under different surgical conditions as it facilitates a repeated measures comparison of kinematics before and after multiple surgeries.38
The femur during the active OKC flexion was located more anteriorly than that during active CKC flexion in normal and TKA knees. In vivo, weight-bearing activities in normal subjects have generated significantly different patterns of secondary motions compared to non-weight-bearing activities.38 In vivo kinematics between weight-bearing (CKC) knee flexion and non-weight-bearing (OKC) knee flexion were also different after TKA.16, 17, 39 Our results of cadaver kinematics are consistent with these in vivo reports, which further supports and validates our cadaver model. Given the differences in the implant designs analyzed, these findings suggest that weight-bearing can significantly affect active knee kinematics, and is likely to be independent of implant type.
While there were kinematic differences between the two types of active flexion, passive knee kinematics correlated significantly with active CKC knee flexion. Passive knee flexion is often used as an intraoperative kinematic evaluation.40-42 However, the relevance of passive knee flexion to postoperative active knee kinematics has not been established. Our findings that the intraoperative kinematics were correlated with postoperative kinematics suggest that intraoperative kinematics could be used to predict postoperative kinematics. This relationship between intraoperative and postoperative kinematics, if validated by clinical studies, opens the potential for targeting more desirable outcomes.
The results of this study indicate that ligament balance can affect knee kinematics. The posterior tilt of the top plate of the electronic ligament balancer indicates greater posterior soft-tissue tightness or a more posterior location of the center of contact pressure, which is supported by our finding that posterior tilt correlated with femoral AP position. The mediolateral tilt is a measure of balance in the mediolateral direction and did not correlate with tibiofemoral varus in the CR-TKA conditions. However, the mediolateral balance correlated with tibiofemoral varus in the PS-TKA condition, suggesting that the intact PCL contributes to mediolateral soft-tissue balance. These findings open the potential for tuning ligament balance to target postoperative kinematics. The lower strength of correlation between ligament balance and kinematics could be due to the fact that we tested cadaver knees without severe osteoarthritis or anatomic deformity. In addition, the articular geometry of the implant design may play a relatively larger role than ligament balance in determining knee kinematics.
Cruciate ligament status also affected knee kinematics. The femur in ACL-deficient knees starts in a relatively more posterior position in early flexion.43 Also, in all activities in our study the femur was more posteriorly located after CR-TKA near extension. During active OKC flexion, the femur after PCL resection was more anteriorly located relative to normal knees and CR-TKA knees at 80° of flexion. These findings are consistent with the general consensus that PCL sacrifice reduces posterior rollback.45
Knee kinematics are driven by complex interactions between type of activity, ligament status, implant design and alignment, and soft-tissue balance. A recent study correlated in vivo fluoroscopic knee kinematics with clinical outcomes.15 The results of our study suggest that intraoperative kinematics measured by computer-assisted navigation and intraoperative ligament balance have the potential to drive postoperative knee kinematics and therefore improve clinical outcomes. A follow-up study is required to confirm this hypothesis by analyzing the change in kinematics driven by specific change in ligament balance. These results open the possibility for applications where ligament balance could be integrated with surgical navigation.
The limitations of this study need to be acknowledged. This was a cadaver study conducted using an artificial test rig and our conclusions need to be validated by clinical testing. Sequential surgery and loading could have influenced the kinematics. However, we and others have reported extensively on similar cadaver studies29, 34 and the Oxford rig has been recommended for feasibility in testing multiple conditions in the same specimen.38 Passive flexion was manually performed to simulate intraoperative kinematics which may have issues with reproducible moments. Only one TKA design was tested. Other designs might result in different kinematics and ligament balance. However, analysis of several TKA designs have reported in vivo kinematics similar to those measured in our study.16, 17, 39
ACKNOWLEDGMENTS
Exactech, Inc. provided surgical navigation, surgical equipment, and trial implants. Laurent Angibaud is an employee of Exactech, Inc., Darryl D'Lima is a consultant for Exactech, Inc.
CONFLICT OF INTERESTS
Laurent Angibaud is an employee of Exactech, Inc; Darryl D'Lima is a consultant for Exactech, Inc.
AUTHOR CONTRIBUTIONS
Kenichi Kono, Darryl D. D'Lima, Tetsuya Tomita generated the research design; Kenichi Kono, Darryl D. D'Lima, Laurent Angibaud, Tetsuya Tomita, Sakae Tanaka drafted the paper or revised it critically; Kenichi Kono, Erik W. Dorthe, Laurent Angibaud were involved in the acquisition and analysis of data; Kenichi Kono, Darryl D. D'Lima, Tetsuya Tomita were involved in the interpretation of data; Laurent Angibaud, Tetsuya Tomita, Sakae Tanaka approved the final version. All authors have read and approved the final submitted manuscript.