Perivascular lymphocytic aggregates in hip prosthesis-associated adverse local tissue reactions demonstrate Th1 and Th2 activity and exhausted CD8+ cell responses
Abstract
Hip implants are a successful solution for osteoarthritis; however, some individuals with metal-on-metal (MoM) and metal-on-polyethylene (MoP) prosthetics develop adverse local tissue reactions (ALTRs). While MoM and MoP ALTRs are presumed to be delayed hypersensitivity reactions to corrosion products, MoM- and MoP-associated ALTRs present with different histological characteristics. We compared MoM- and MoP-associated ALTRs histopathology with cobalt and chromium levels in serum and synovial fluid. We analyzed the gene expression levels of leukocyte aggregates and synovial fluid chemokines/cytokines to resolve potential pathophysiologic differences. In addition, we classified ALTRs from 79 patients according to their leukocyte infiltrates as macrophage-dominant, mixed, and lymphocyte-dominant. Immune-related transcript profiles from lymphocyte-dominant MoM- and MoP-associated ALTR patients with perivascular lymphocytic aggregates were similar. Cell signatures indicated predominantly macrophage, Th1 and Th2 lymphocytic infiltrate, with strong exhausted CD8+ signature, and low Th17 and B cell, relative to healthy lymph nodes. Lymphocyte-dominant ALTR-associated synovial fluid contained higher levels of induced protein 10 (IP-10), interleukin-1 receptor antagonist (IL-1RN), IL-8, IL-6, IL-16, macrophage inflammatory protein 1 (MIP-1α), IL-18, MCP-2, and lower cell-attracting chemokine levels, when compared with prosthetic revisions lacking ALTRs. In addition, the higher levels of IP-10, IL-8, IL-6, MIP-1α, and MCP-2 were observed within the synovial fluid of the lymphocyte-dominant ALTRs relative to the macrophage-dominant ALTRs. Not all cytokines/chemokines were detected in the perivascular aggregate transcripts, suggesting the existence of other sources in the affected synovia. Our results support the hypothesis of common hypersensitivity pathogenesis in lymphocyte-dominant MoM and MoP ALTRs. The exhausted lymphocyte signature indicates chronic processes and an impaired immune response, although the cause of the persistent T-cell activation remains unclear. The cytokine/chemokine signature of lymphocyte-dominant-associated ATLRs may be of utility for diagnosing this more aggressive pathogenesis.
1 INTRODUCTION
Adverse local tissue reactions (ALTRs) are a major cause of hip prosthetic failure and the accompanied pain, stiffness, and swelling that reduces mobility and causes disability.1 ALTRs affect at least 10% of patients with metal-on-metal (MoM) articulations.2 While less frequent in patients with metal-on-polyethylene (MoP) articulations, they still represent a significant number of patients, as these remain the most used articulation in hip arthroplasty.2 ALTRs are presumed to be caused by a delayed hypersensitivity reaction to metallic corrosion or wear.3-6
Both MoM and MoP-associated ALTRs are histologically characterized by synovial ulceration, necrosis, and mononuclear cell infiltration.7 This immune infiltrate can vary from a diffused distribution of macrophages to the presence of organized perivascular lymphocytic aggregates that resemble tertiary lymphoid structures composed of highly organized, densely packed T and B cells.8 Perivascular lymphocytic aggregates are important activators of immunological responses in diverse maladies by inducing isotype switching and autoreactive T-cell expansion, among other functions.9, 10 ALTR patients with perivascular lymphocytic aggregates are described as presenting with type IV hypersensitivity features.11 They are the most aggressive types of ALTRs, having shorter revision times.11, 12 In the context of MoM prosthetics, ALTR patients with perivascular lymphocytic aggregates are associated with increased necrosis.12 These observations were recently confirmed by hierarchical clustering of the observation of 256 MoM ALTR biopsies.13 The analysis indicated that ALTRs could be divided into two major groups, one with higher macrophage infiltrate and particle load and the other group characterized by a larger extent of necrosis and lymphocyte aggregates but with minimal macrophagic infiltration.
Intriguingly, ALTRs associated with the failure of MoM and MoP articulations present with histological and clinical differences.7, 14 MoM-associated ALTRs usually exhibit a cystic-inflammatory pattern, while MoP-associated ALTRs tend to present with a fibro-necrotic pattern. Furthermore, descriptive studies have proposed that a higher percentage of MoM-associated ALTRs have a macrophage-dominant infiltrate when compared with MoP ALTRs. This is while a higher percentage of MoP-associated ALTRs have a lymphocyte-dominant infiltrate in comparison with MoM-associated ALTRs.11, 14 In addition, elevated serum cobalt (Co) and chromium (Cr) ion levels have been associated with ALTRs in MoM patients15; however, such a relationship has not been clearly documented in MoP ALTR patients.16 Thus, it is accepted that there are differences in patients' sensitivity to developing ALTRs.17 While ALTRs have been extensively characterized, whether any of these differences between MoM and MoP ALTRs are due to distinct immunological responses or represent varying degrees of development of the same pathology remains controversial.
The assumption that ALTRs are caused by type IV hypersensitivity reactions has been based primarily on histological observations, such as the presence of perivascular lymphocyte aggregates that are indicative of T-cell activation and cytokine production that presumably directs the subsequent immune response.18, 19 Different types of T lymphocytes are distinguished by the expression of the plasmatic membrane proteins, the cluster of differentiation (CD), CD4+, or CD8+. CD4+ T lymphocytes are also known as T helper cells (Th), which according to their cytokine secretion pattern as well as by intracellular markers, can be divided into different Th subpopulations.20 Th1 cells are characterized by the secretion of interferon-γ (IFN-γ) and the activation of the T-bet transcription factor. Th2 cells are characterized by the secretion of interleukin (IL)−4 and the activation GATA3 transcription factor. Th17 cells secrete IL-17 and the transcription factor is the retinoic acid receptor-related orphan receptor α (RORα). The last group, regulatory T cells (Tregs), are characterized by the secretion of IL-10 and Forkhead box P3 (FOXP3) as a transcription factor.20 Recently, through blood cell analysis, researchers have attempted to characterize the ALTRs immunological reactions mediated by T cells. Higher circulating Th1 and Th17 cell counts, and lower Th2 cell counts, were described in a follow-up of patients with MoM prosthetics with a high proportion of failures. Only elevated Th1 cell counts, however, were described in patients with MoP prosthetics with a lower failure rate.21 The suggestion that linked a Th17 response to a high failure rate was supported by the demonstration of a Th17 response by circulating T cells challenged with orthopedic implant-derived metal particles in vitro.22 Such a response would be consistent with the Th17-predominant infiltrate observed in different systemic hypersensitivity diseases.23, 24 Conversely, a study analyzing circulating lymphocyte populations showed a significantly lower percentage of memory T cells and Th1 cells in ALTR-affected patients compared with controls.17 The authors proposed that local sequestration of these Th1 cells would explain this phenomenon, indicating that ALTRs correspond to a predominant Th1 response in a type IV hypersensitivity setting.
Cytokines and chemokines are important drivers of inflammation-triggering endothelial activation, cell migration, and modulating the immune response. The most frequent joint diseases, such as osteoarthritis and rheumatoid arthritis, have an important inflammatory component. As such, cytokines have been extensively studied, and their effects are of paramount relevance for disease progression.25 In hip implant failure, cytokines have also been studied. An early immunohistochemistry-based study described a high correlation between IL-1b and IL-6 immunostaining and the presence of metal particles in the tissues.26 A later study compared synovial fluid cytokine concentrations from patients with failed small head MoM total hip arthroplasty with patients undergoing primary hip surgery.27 The authors found significantly higher concentrations of 14 cytokines: IL-1ß, IL-2, IL-4, IL-5, IL-6, IL-10, IL-13, IL-17, interferon (IFN-γ), IFN-γ-induced protein 10 (IP-10), and the granulocyte and macrophages colony stimulant factor (GM-CSF) in the failed MoM synovial fluid cohort; furthermore, they found an association between some of these cytokines and the presence of lymphocyte-dominant lesions. In comparison, a total RNA analysis-based study obtained from failed MoP biopsy implants reported elevated expression of IL-10, IL-6, GM-CSF, IL-8, monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein 1 (MIP-1b), macrophage-derived chemokine (MDC), and IP-10 when compared with osteoarthritic joints.28
This lack of consensus regarding the characteristics of the lymphocytic reactions associated with ALTRs drove us to assess whether the differences observed in histopathology of MoM- and MoP-associated ALTRs could be distinguished by the infiltrated lymphocytes' composition or differentiation rather than that of those in the circulation. We hypothesized that, as important drivers of inflammatory responses, specific cytokine and chemokine patterns could be identified as associated with the development of macrophage- or lymphocyte-dominant ALTRs. We demonstrated that lymphocyte aggregates from both MoM and MoP-associated lymphocyte-dominant ALTRs are characterized by a predominant macrophage, Th1 and Th2, cell differentiation pattern and with suppressed Th17 and B cell responses. We also define a strong signature of exhausted CD8+ cells. The exhaustion of CD8+ cells is a gradual deterioration of immune cell functions, such as cytokine secretion and cytotoxicity, which was determined by the presence of checkpoint inhibitors in the lymphocyte infiltrate of ALTRs.29 Finally, we describe a higher concentration of five cytokines/chemokines in the synovial fluid of lymphocyte-dominant ALTRs than macrophages-dominant ALTRs, although this is not necessarily associated with their expression in the lymphocyte aggregates. The presence of CD8+ exhaustion could represent a therapeutic target for ALTRs, while the elevated cytokines could be a predictor of hypersensitivity reactions to hip implants.
2 METHODS
2.1 Patients
All procedures were performed under the ethics approval from the University of British Columbia (H14-03050). We recruited 90 consecutive patients who underwent revision of nondual modular neck total hip arthroplasty surgery between May 2012 and December 2016: 45 patients with MoM-associated ALTRs, 34 patients with MoP-associated ATLRs, 11 MoM patients without ALTRs (control MoM), defined histologically by the absence of synovial ulceration, necrosis, or leukocyte infiltration. Two patients had bilateral implants, but samples from only one hip were considered in the analysis. Patient demographics and implant types details are provided in Tables 1 and S1 and Figure S1. Patients with infections were excluded from the study.
| Mean | Median (range) | SEM | ||
|---|---|---|---|---|
| MoM (n = 45, 20 females) | ||||
| Age (years) | 60.0 | 60.8 (29.2–74.8) | 8.8 | |
| Implantation time (months) | 84.0 | 85.0 (47–133) | 18.1 | |
| MoP (n = 34, 19 females) | ||||
| Age (years) | 69.3 | 71.2 (38.9–87.1) | 10.8 | |
| Implantation time (months) | 51.6 | 47.3 (9–107) | 23.9 | |
| Control MoM (n = 11, 7 females) | ||||
| Age (years) | 62.5 | 61.3 (39.1–85-4) | 6.5 | |
| Implantation time (months) | 95.0 | 97.0 (14–108) | 21.2 | |
- Abbreviations: MoM, metal-on-metal; MoP, metal-on-polyethylene.
2.2 Histological processing and classification of biopsied tissues
Tissue samples were obtained from tissue excised during revision surgeries and processed according to established protocols.14 Three to 10 sections from the obtained tissue from each patient were fixed in 10% v/v buffered formalin (4% final w/v formaldehyde) for 24 h and dehydrated in ascendent concentrations of ethanol (70%–100% v/v). The samples were cleared in xylene substitute (Thermo Fisher Scientific) and embedded in paraffin. Five-micron-thick sections of each sample were stained with hematoxylin-eosin (Thermo Fischer Scientific). All samples were evaluated by an experienced pathologist in bone and soft tissue (N. M.), and blindly confirmed by a researcher experienced in histopathology (F. E.). Case discrepancies were resolved by a senior pathologist in bone and soft tissue of the Vancouver General Hospital (T. N.). We defined ALTRs by the presence of synovial ulceration, subsuperficial necrosis, and leukocyte infiltration in at least one of the tissue sections.30 We divided the ALTR samples into three groups based on the Ricciardi method11: (i) macrophage-dominant pattern, with predominantly macrophagic infiltrate and the absence or minimal presence of lymphocytes, (ii) mixed infiltrate pattern, with the presence of both macrophages and lymphocytes, (iii) lymphocyte-dominant pattern, characterized by the presence of large (>500 μm diameter) perivascular aggregates. The presence of perivascular aggregates in any of the slides determined the lymphocytic characteristic of the lesion. To evaluate the presence of macrophages in the tissues, Perl's Prussian blue staining (Abcam) was also performed.31 This stain is used to indicate the presence of iron that is not forming porphyrinic groups as it is the case of hemoglobin, staining ferritin and hemosiderin.32 Macrophages present a high content of intracellular iron because of two major physiological roles (i) they use iron as an enzymatic cofactor for defense and inflammation proteins, (ii) they phagocyte senescent erythrocytes recycling iron to be used in hemopoiesis.33 The tissue slides were incubated in a solution of 2% (w/v) potassium ferrocyanide in 2% (v/v) hydrochloric acid for 20 min at room temperature. The slides were washed in distilled water and the nuclei were counterstained with neutral red, then washed in phosphate-buffered solution, dehydrated in ethanol (70%–100% v/v) and xylene substitute, and mounted with a resin-based mounting medium (Histo-Mount; Electron Microscopy Sciences).
2.3 Co and Cr concentrations in serum
Before surgery, 10 ml of peripheral blood was obtained from each patient following standardized procedures for trace metal analysis.15 Briefly, the blood was collected using a plastic (trace-element) 7-ml nonadditive, BD Vacutainer tube (Becton Dickinson), allowed to clot for 20 min, then centrifuged at 1200g for 15 min. The serum was then transferred using a polypropylene transfer pipette into a 7-ml Sarstedt polypropylene tube and stored at –20°C before analysis. Six-hundred microliters serum aliquots were transferred to 1 ml Eppendorf tubes (Millipore Sigma) and stored at −20°C until shipped on dry ice to PaLM Laboratories for measuring the concentration of Co and Cr in serum using an Element 2, high-resolution sector field inductively coupled plasma mass spectrometry (HR-SF-ICP-MS; Thermo Fisher Scientific) that has a lower limit of quantification of 0.02 μg/L for Co and 0.08 μg/L for Cr. Group comparisons were performed using a nonpaired t test, followed by Benjamini–Hochberg post hoc correction.
2.4 Gene expression analyses in paraffin-embedded tissues
To minimize contamination from nonlymphocytic tissue, we selected tissue sections with the largest perivascular lymphocytic aggregates (all with diameters >1 mm) from nine MoM ALTRs and nine MoP ALTR specimens located in the paraffin cores using a stereomicroscope. As a control group, we obtained four healthy lymph nodes (LNs): two from the genital tissues of male donors and two from the mammary glands of female donors. Total messenger RNA (mRNA) was isolated from lymphocytic aggregate punches of these specimens using a formalin-fixed paraffin-embedded RNA extraction kit (Roche Molecular Diagnostics) following the manufacturers' instructions. The RNA concentration and purity were determined using a microvolume spectrophotometer Nanodrop ND-100 (NanoDrop Technologies). The quality of the extracted RNA was confirmed by an RNA integrity number (260 nm/280 nm absorbance ratio) between 1.96 and 2.04. The RNA samples were diluted to a final concentration of 100 ng/μl. Transcript profiles for 579 genes related to immune system functions were determined on an nCounter Digital Analyzer (NanoString), using the nCounter GX Human Immunology V2 kit. Analysis of the transcript profiles was completed using the nSolver Analysis Software 3.0. Confounding factors such as age, gender, lesion size, and implantation time were assessed for differential gene expression through multivariate linear regression (Supporting Information Methods and Figure S2). Cell signature scores, obtained by derivation of annotated genes (provided by the manufacturer), which have been reported to be highly enriched and specific for different cell types, were assessed to identify those with high correlations across the samples.34, 35 In Table 2, we show the genes finally used to perform the cell score analysis. Cell signature score values are obtained by finding the mean value of log 2 of the normalized counts of the markers that are cell specific. Genes included to generate the scores must be those that correlate and behave as cell markers.35 The generation and validation of these markers are further explained in Supporting Information Methods and Figure S3. Canonical pathways and physiological functions were analyzed using Ingenuity Pathway Analysis version 01-14 (IPA; QIAGEN Inc.).
| Cell type | Molecular markers (genes) | ||||||
|---|---|---|---|---|---|---|---|
| B cells | CD19 | TNFRSF17 | MS4A1 | ||||
| CD45 | PTPRC | PTPRC | |||||
| CD8 cells | CD8B | CD8A | |||||
| Cytotoxic cellsa | KLRD1 | GZMB | KLRB1 | GZMA | GNLY | KLRK1 | PRF1 |
| Dendritic cells | CCL13 | CD209 | |||||
| Exhausted CD8 | CD244 | PTGER4 | EOMES | LAG3 | |||
| Macrophages | CD163 | ||||||
| Neutrophils | FCAR | FCGR3B | CSF3R | ||||
| NK CD56dim cells | KIR2DL3 | KIR3DL1 | KIR3DL2 | IL21R | |||
| NK cells | NCR1 | XCL1 | |||||
| T cells | CD3E | SH2D1A | CD3D | CD6 | |||
| Th1 cells | TBX21 | CXCR3 | |||||
| Th17 | IL22RA2 | CCR6 | TNFSF13B | ||||
| Th2 cells | GATA3 | CCR8 | IL10RA | ||||
| Treg | FOXP3 | ||||||
- Abbreviations: NK, natural killer; Tregs, regulatory T cells.
- a Cytotoxic cells include natural killer cells and cytotoxic T lymphocytes.
2.5 Determination of metal ions and cytokines/chemokines in synovial fluid
Synovial fluid specimens were extracted during the surgery by aspiration before opening the articular capsule. The volume of the synovial fluid obtained was highly variable (1–30 ml). This volume was usually very small for the MoP ALTR patients. The samples obtained were then kept on ice and centrifuged immediately at 1860g at 4°C for 10 min using a 5804 centrifuge (Eppendorf). Aliquots of 600 μl obtained from the supernatant were transferred immediately to trace element tubes (Becton Dickinson) and stored at −20°C until shipped on dry ice to PaLM Laboratories. The concentration of Co and Cr were then determined by ICP-MS at the PaLM Laboratories. As the synovial fluid Co and Cr concentrations were highly variable, and not normally distributed, Log (10) transformations were performed on the concentration values. To confirm whether the assumption of normality of the data distribution required by the parametric tests is met, we performed a Shapiro–Wilk test before and after the log transformations. A nonpaired t test was used to determine differences between the groups. These statistical analyses and charts were performed using the Prism 9 software (GraphPad).
After centrifugation, 75 μl aliquots of synovial fluid supernatant were separated and stored at −20°C until analyzed. The concentration of 65 cytokines, chemokines, and growth factors associated with inflammation, necrosis or fibrosis, were quantified in the synovial fluid of 13 patients with macrophage-dominant MoM ALTRs, 12 patients with lymphocyte-dominant MoM ALTRs, and 11 patients with MoM articulations undergoing revisions, but not presenting with ALTRs as a control cohort. The Bio-Plex Suspension Array System (65 plex-Eve Technologies) was used for measuring the concentration values. The obtained values of cytokine concentrations were not normally distributed; thus, we performed Log (10) transformation of the values and confirmed the normality by Shapiro–Wilk test. Then a one-way analysis of variance with a Tukey post hoc and Benjamini–Hochberg correction was used to determine the factors differentially detected between the groups.
3 RESULTS
3.1 ALTRs in MoP implants are more likely than MoM ALTRs to present with lymphocyte-dominant inflammatory infiltrates
Through histopathological observation of our samples, we found that 29% of the MoM-derived ALTR samples exhibited a lymphocyte-dominant pattern as defined by the presence of large perivascular lymphocytic aggregates (>500 µm). Forty-four percent of the MoM-derived ALTR samples, however, exhibited a mixed infiltrate pattern with evidence of macrophages and lymphocytes. This is while 27% exhibited macrophage-dominant infiltrates with no evidence of lymphocyte infiltration. Conversely, 63% of the MoP-derived ALTR samples presented with a lymphocyte-dominant pattern, 37% exhibited a mixed infiltrate pattern, and none exhibited a macrophage-dominant infiltrate pattern (Table 3).
| Implant type | Macrophage- dominant | Mixed infiltrate pattern | Lymphocyte- dominant | Not classifieda | Total |
|---|---|---|---|---|---|
| MoM | 12 | 20 | 13 | 0 | 45 |
| MoP | 0 | 7 | 12 | 15 | 34 |
- Abbreviations: ALTR, adverse local tissue reaction; MoM, metal-on-metal; MoP, metal-on-polyethylene.
- a >75% of the tissue was necrotic.
We next assessed whether the differences in macrophage- or lymphocyte-dominant ALTR histologic presentation are related to the concentrations of Co and Cr in the serum or the synovial fluid of the affected joint. We found that Co and Cr ion levels were significantly elevated in the ALTR groups as compared with those from the non-ALTR control patients. However, Co or Cr concentrations between the synovial fluid or the serum from patients with macrophage-dominant and lymphocyte-dominant ALTRs were indistinguishable (Figure 1 and Table S2).
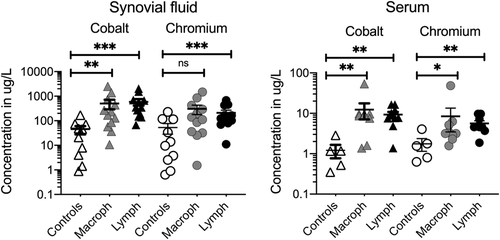
3.2 Lymphocytic response in MoM and MoP implants is highly congruent
Since lymphocyte-dominant ALTRs are considered to be more aggressive, we characterized the immune profile of lymphocyte-dominant ALTRs (representative images presented in Figure 2A,B) by analyzing the immune-related gene expression of MoM- and MoP-derived ALTR perivascular lymphocytic aggregates. Normalized to the immune-related gene expression profile of LN from healthy patients as a basal lymphoid organ transcript profile control, we found that 149, and 153, of the 579 analyzed genes were differentially expressed in MoM- and MoP-derived ALTRs, respectively (Figure 2C). Of these genes, 133 were common to both MoM- and MoP-derived ALTRs and shared a remarkably concordant pattern of differential expression (Figure 2D,E and Table S3). This is while only two of the genes were differentially expressed between lymphocytic aggregates from MoM and MoP ALTRs (Table S4). Hierarchical clustering of the samples according to their gene expression also showed a clear differentiation between ALTRs and controls but failed in segregating MoM from MoP (Figure 2F). These results indicate that the immunologic profiles of lymphocyte aggregates in MoM and MoP ALTRs are similar and suggestive of a common etiology and pathogenesis.
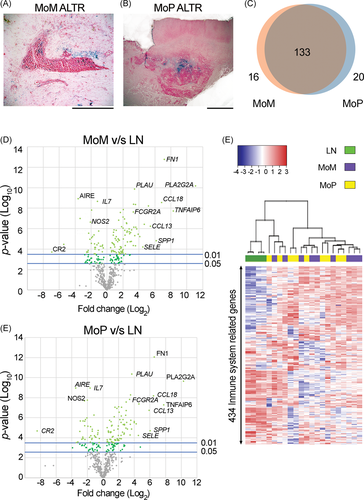
3.3 CD8+ exhaustion in Th1 and Th2 cells upregulated response is a characteristic of ALTRs' lymphocyte aggregates
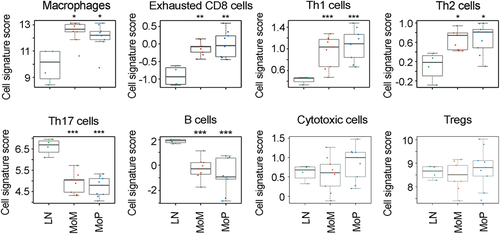
To assess the specific cell subtypes of these lymphocyte-dominant ALTR infiltrates, we compared the transcript profiles with the gene signature profiles for different immune cell subtypes described in Table 2. Relative to normal LN controls, the resulting cell scores for MoM- and MoP-derived ALTR lymphocyte infiltrates were the highest for macrophages, exhausted CD8+ cells, and Th1 and Th2 cells. On the other hand, the cells scores were the lowest for B cells and Th17 T cells, while T-Reg and cytotoxic cell scores were indistinguishable from LN controls (Figures 3, S4, and S5). Ingenuity Pathway Analysis revealed that the same canonical pathways were enriched in the lymphocyte aggregates from MoM and those from MoP ALTRs: Altered T and B cell signaling in rheumatoid arthritis, granulocyte adhesion and diapedesis, and Th1 and Th2 activation (Tables 4 and S5).
| p value | Overlap | |||
|---|---|---|---|---|
| MoM versus LN top canonical pathways | ||||
| Altered T cell and B cell signaling in rheumatoid arthritis | 1.48E−29 | 25.6% | 23/90 | |
| Granulocyte adhesion and diapedesis | 1.31E−23 | 13.4% | 24/179 | |
| Primary immunodeficiency signaling | 2.67E−19 | 29.2% | 14/48 | |
| Th1 and Th2 activation pathway | 3.54E−19 | 11.3% | 21/186 | |
| Agranulocyte adhesion and diapedesis | 5.54E−19 | 11.1% | 21/190 | |
| MoP versus LN top canonical pathways | ||||
| Altered T cell and B cell signaling in rheumatoid arthritis | 5.90E−32 | 27.8% | 25/90 | |
| Granulocyte adhesion and diapedesis | 4.03E−28 | 15.6% | 28/179 | |
| Agranulocyte adhesion and diapedesis | 2.98E−23 | 13.2% | 25/190 | |
| Th1 and Th2 activation pathway | 3.79E−22 | 12.9% | 24/186 | |
| Th1 pathway | 8.85E−20 | 14.7% | 20/136 | |
- Abbreviations: LN, lymph node; MoM, metal-on-metal; MoP, metal-on-polyethylene.
3.4 Synovial fluid from patients with ALTRs with lymphocytic aggregates has higher concentrations of specific cytokines/chemokines
We listed the 50 genes with greater fold changes in their expression in ALTRs' lymphocyte aggregates compared with controls (Table S3). We found that 10 of the most expressed genes corresponded to cytokines and chemokines. Of these, MIP-4 (76- and 41-fold increase in MoM and MoP ALTRs compared with controls, p-values 0.0017 and 0.0350, respectively), MCP-4 (46- and 48-fold increase, p-values 0.0059 and 0.0277, respectively), Interleukin-1 receptor antagonist (IL-1RN) (44- and 38-fold increase, p-values 0.0063 and 0.0199, respectively), Eotaxin-1 (18- and 17-fold increase, p-values 0.0217 and 0.0877, respectively), IL-8 (CXCL8) (15- and 14-fold increase, p-value 0.0461 and 0.0371, respectively), IP-10 (8- and 12-fold increase, p-values 0.0103 and 0.0034, respectively), MCP-3 (12- and 8-fold increase, p-values 0.0294 and 0.1003, respectively), Monokine induced by γ-interferon (MIG) (9- and 10-fold increase, p-values 0.0066 and 0.0001, respectively), and MCP-2 (6- and 7-fold increase, p-values 0.0023 and 0.0149, respectively) presented with the highest significance and increased expression compared with controls.
The finding of elevated gene expression of cytokines and chemokines in ALTRs' lymphocyte aggregates drove us to analyze whether this gene expression corresponds with the presence of these cytokines and chemokines in the synovial fluid of the affected joints. As described previously, the amount of synovial fluid obtainable from MoP ALTRs is very limited due to the characteristics of the lesions; therefore, we did not include this group in the following analysis. We analyzed the cytokine and chemokine profiles of patients with MoM implants but without ALTRs (controls, n = 11), macrophage-dominant ALTRs (n = 13), or lymphocyte-dominant ALTRs (n = 12, nine of which were used for gene expression analysis presented in Figure 1 and Table S3). Through bidimensional hierarchical clustering analysis of cytokine concentration among the samples, we observed that the groups do not present a clear cluster pattern (Figure 4).
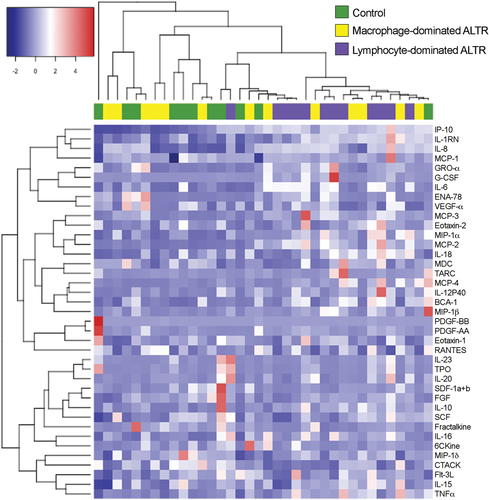
Next, we performed statistical analyses on the cytokine concentrations in the synovial fluid of the control patients (Figure 5A), patients with macrophage-dominant ALTRs (Figure 5B), and patients with lymphocyte-dominant ALTRs (Figure 5C). Our observations revealed significantly higher IP-10, IL-8, IL-6, MIP-1α, and MCP-2 levels in ALTR patients with lymphocyte-dominant ALTRs than those with macrophage-dominant lesions. In addition, elevated IP-10, IL-1RN, IL-8, IL-6, IL-16, MIP-1α, IL-18, and MCP-2, and decreased cutaneous T-cell-attracting chemokine, were present in ALTR patients with lymphocyte-dominant infiltrates relative to controls; those cytokines/chemokines showed no differences between macrophage-dominant ALTRs and controls (Figure 5D and Table S6). These observations suggest that the observed synovial fluid cytokine and chemokine patterns can distinguish lymphocyte- and macrophage-dominant ATLRs. Of note, specific signatures cytokines for Th1 response (IFN-γ), Th2 (IL-4), or Th17 (IL-17) were indistinguishable between the groups. Moreover, the concentration of these signature cytokines was below the detection limit of the system (~5 pg/ml) in most samples, suggesting a limited presence in these lymphocyte-dominant lesions.
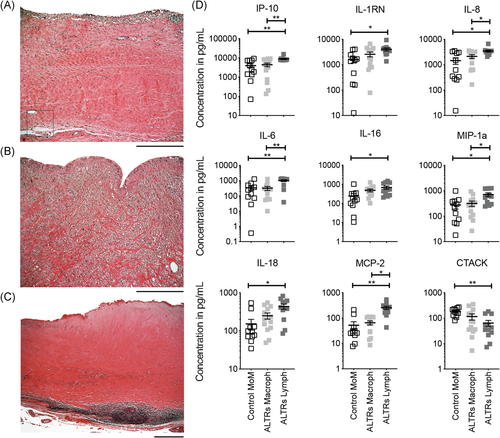
3.5 Cytokine secretion in lymphocyte-dominant ALTRs is not restricted to perivascular lymphocyte aggregates
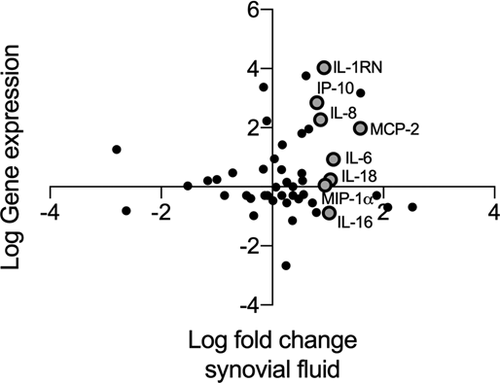
We previously described that the presence of perivascular lymphocyte aggregates is associated with higher cytokine concentrations in the synovial fluid of patients with ALTRs. To determine whether this elevated cytokine concentration is associated with their expression in lymphocyte aggregates, we compared the cytokines' gene expression in perivascular lymphocyte aggregates with their concentration in the synovial fluid. The plot of the analyzed cytokines is presented in Figure 6. In the plot, we highlight those eight cytokines that showed significantly higher concentration in the synovial fluid of patients with lymphocyte-dominant ALTRs (IP-10, IL-1RN, IL-8, IL-6, IL-16, MIP-1α, IL-18, and MCP-2). We observed that IL-1RN, IP-10, IL-8, and MCP-2 exhibited elevated expression in lymphocyte aggregates. On the other hand, cytokines, such as IL-6, IL-18, and MIP-1a, that exhibited higher concentrations in the synovial fluid, exhibited no change in their expression in lymphocyte aggregates compared with healthy LNs. This is while IL-16 was shown to have an even lower expression in ALTRs' lymphocytic aggregates than in healthy LNs.
4 DISCUSSION
ALTRs represent significant morbidity for patients with hip implants; yet, the underlying etiology remains enigmatic. While T-cell hypersensitivity has been a preeminent hypothesis for the etiology of ALTRs in MoM and MoP hip implants, the mechanisms and characteristics of this response remain unclear. In this study, through analyzing gene expression of lymphocyte aggregates in ALTRs, we determined that in both types of articulations, the reaction in lymphocyte-dominant ALTRs is highly similar. The reaction is composed of predominant Th1 and Th2 cells and a strong exhausted CD8+ cell signature, together with reduced B cell and Th17 cells relative to normal LN controls. These results suggest that these lesions are a chronic adaptive response to as yet unidentified antigens. Finally, we determined a differential cytokine pattern in ALTRs depending on the leukocytic infiltrate (lymphocytic vs. macrophagic), which is not strongly correlated with the metal ion concentrations in the synovial fluid or the plasma.
A major observation of our study is the similarity of the type of lymphocytic reaction in MoM and MoP ALTRs. It is to note that our cohort of MoP cases has a relatively short revision time (mean = 51.6 months), which is indeed shorter than those of the MoM cases (mean = 84 months). Interestingly, some of the MoP ALTRs included in the study showed highly elevated Co and Cr concentrations in synovial fluid, but not in serum, perhaps originated from the nanosized particles in the fluid that were not removed by centrifugation. The presence of ALTRs in MoP implants has been associated with the presence of corrosion in the tapered junctions. Indeed, particularly high levels of failure have been observed in patients with a modular femoral neck (with two tapered junctions), which leads to an increase in the surfaces exposed to this type of corrosion.7 The group of patients analyzed in our study correspond uniquely to the nondual modular femoral neck with only one tapered junction between femoral head and neck. However, the presence of extensive corrosion in addition to wear in the articular surface of MoPs' femoral head implants as a consequence of tribocorrosion has been recently described.6 These mechanisms of metal release are certainly related to the presence of ALTRs, and perhaps the presence of both mechanisms in MoM and MoP implants may explain the similar pathogenesis that we described in this study. However, it remains unknown as to how these elements relate to lymphocytic activation.
Several studies have previously focused on the immunological characteristics of the response to metal implants. Kolatat et al.36 found increased levels of MIG and IP-10 (two molecules related to the IFN-γ pathway) through total mRNA and cytokine analysis of ALTRs failed MoM and nonmetal on metal articulations when compared with osteolysis-related failure. Although they did not find an elevated expression or secretion of IFN-γ, their findings were suggestive of an increased Th1 response in ALTRs. The same group, however, demonstrated an increased detection of GATA-3 (Th2 transcription factor) over T-bet (Th1 transcription factor) in ALTR T cells through immunohistochemical analysis.7 A different approach has been the experimental analysis of circulating T cells. By stimulating circulating T cells from healthy donors and from well-functioning hip implants using different metal ions, Hallab et al.37 demonstrated that Cr, Ni, Co, and Ti elicit lymphocytes proliferation,37 with a differential cytokine signature indicative of a predominant Th1 switch, which is higher in patients with implants than in healthy donors. These results were later confirmed in the prospective analysis of patients who underwent hip arthroplasty. Based on the analysis, the reactivity of circulating T cells increased after 3 years of hip arthroplasty, with an elevated expression of IL-12, indicative of a Th1 predominant response.38 Our results show that both Th1 and Th2 responses are increased in ALTRs. Although in our study, we compare the expression with what suggests to be a baseline for Th1 or Th2 differentiation, such as naïve LNs, it has recently been described that the basal status of LNs shows tissue-specific differences in their T-cell populations, responses, and cytokine secretions.39, 40 Despite these differences, the presence of Th1 and Th2 responses in ALTRs is also affirmed by the early observation of CD8+ T cells as well as B cells follicular clusters being present in ATLRs.18, 30, 41
An in vitro study analyzing the T-cell response of healthy individuals to metal particle exposure showed that there are individual differences in the T-cell response. The authors defined two types of patients, those that respond with an inflammatory Th17 response, increasing their IL-17 expression and those that develop an anti-inflammatory response with decreased IL-17 expression.22 These results were also supported by a recent report that described an increase in Th1 and Th17 cells in the circulation of ALTR patients, suggesting that ALTRs are promoted by a Th17-mediated reaction.29 In our samples, we found that the synovial fluid from 6 of the 12 ALTR patients with lymphocyte-dominant infiltrates exhibited three- to fivefold elevated expression of IL23 and IL12p40, which are considered Th17-associated markers. Lymphocytic aggregates from these patients, however, did not exhibit elevated gene expression of other Th17-associated markers, such as IL-22RA2, CCR6, TNFSF13B, and IL-23. We did not observe any patients with an upregulation of the canonical Th17 marker, IL-17, and we did not observe increased IL-17 in the synovial fluid of any of the analyzed patients. Thus, our data do not support the presence of Th17 response in our ALTR cohort, which is frequently present in autoimmune and hypersensitivity diseases. Further studies are necessary to demonstrate TH17 role in ALTRs.
A major finding in our study was the presence of an exhausted CD8+ population in the ALTR-associated lymphocytic aggregates, identified by an elevated expression of exhaustion markers: LAG3, PTGER4, EOMES, B7-H3 (CD-276), and PD-1 (CD279). This previously undescribed exhausted CD8+ response is typically related to chronic inflammation, persistent stimulation, or antigen abundance.29, 42 B7-H3 is associated with hypersensitivity, autoimmunity, and is expressed by synovial macrophages in arthritis.43, 44 PD-1 expression has been used to describe “anergic” cells in rheumatoid arthritis synovial fluid and is linked to elevated IL-6 levels, which we observed in lymphocytic infiltrated ALTRs.45, 46 Remaining to be determined are the mechanisms driving CD8+ exhaustion and the role of the metal elements derived from the hip implants in these events.
Cytokines/chemokines in the synovial fluid play a role in pathogenesis and serve as diagnostic markers of osteoarthritis47, 48 and infections in hip prosthesis patients.49, 50 A previous study using total RNA extraction from tissue homogenates found higher expression of IL-6, IL-8, IP-10, MIP-1α, and MCP-3 in ALTRs than peri-implant osteolytic lesions.36 Another study found a higher concentration of IL-2, IL-4, IL-17, and IL-10 in synovial fluid of patients with MoM ALTRs compared with the synovial fluid of patients with ceramic-on-ceramic failed implants.27, 51 In our study, we directly compared cytokine/chemokine profiles of MoM ALTR-associated synovial fluids with that from patients with well-functioning prosthetics and separated them according to their histological characteristics. We demonstrated a higher concentration of cytokines/chemokines in the synovial fluid from joints with lymphocyte-dominant ALTRs compared with those with macrophage-dominant ALTRs and also when compared with the control synovial fluid from patients undergoing revisions and not exhibiting ALTRs. Cytokine/chemokine levels in the synovial fluid from joints with macrophage-dominant infiltrates, however, showed more subtle differences when compared with the control group. Interestingly, when we correlated the synovial fluid cytokine/chemokine levels with their respective transcript levels in the perivascular lymphocytic aggregates, we found a group of factors that are enriched in the synovial fluid but whose transcripts are not enriched by lymphocytes. This indicates that other cell populations also increase cytokine secretions in lymphocyte-dominant ALTRs.
5 LIMITATIONS
In this study, we approach the understanding of the immunological response of ALTRs to metal hip implants. This pathology affects a large population that may not be fully represented by our sampling. Similarly, in large lesions (up to 1 L in volume as included in this study), the observation of 5-µm sections does not necessarily allow the characterization of the condition of the whole lesion, and thus our classification of ALTRs is biased by this observation process.
We performed a gene expression analysis based on a platform that allows the identification of transcripts in paraffin-embedded tissues. Through barcoding specific sequences, this technology tags fragments of RNA that are unique to individual genes, avoiding the problem of RNA instability and fragmentation during the histological process. However, this technique limits the number of genes to be analyzed to several hundreds, and for that reason, does not allow a whole-genome analysis.
Synovial fluid presents high variability in volume and composition, and thus a larger group of patients is needed to have definitive conclusions. Furthermore, the handling and storing processes may alter the measurements due to the presence of proteases or oxidative degradation of the organic molecules. The amount of synovial fluid is regularly low and even lower in MoP groups. Many of the recruited MoP ALTRs and MoP control patients had dry joints. Thus, for the cytokine analysis, we selected only MoM controls.
Finally, we measured metal ions in the synovial fluid through ICP-MS, a technique that does not differentiate between particles or ions in solution. We analyzed the synovial fluid samples only after centrifugation. This process, however, may not remove nano-sized particles and the persistence of nano-sized particles in the fluid may affect the reported results.
6 CONCLUSION
Overall, our study supports the concept that ALTR development involves several interacting processes in which the implant type does not determine the characteristics of the specific immunological reaction. Epidemiological data and laboratory analysis of patient serums had led to the proposal that metal ions or particles are involved in the development of ALTRs. A subset of patients with MoM ALTRs shows a lymphocyte-dominant pattern, which has been proposed to correspond to hypersensitivity reactions, characterized by CD8 exhaustion and Th1 and Th2 components. This lymphocytic infiltration might be of paramount importance in orchestrating the adverse reactions in this subset of patients and perhaps associated with other phenomena observed in ALTRs, such as cell death and fibrosis. Those patients developing hypersensitivity reactions cannot be identified by cobalt and chromium ion levels in serum or synovial fluid; instead, they may be identified by elevated levels of a panel of cytokines/chemokines in the synovial fluid. T-cell exhaustion is a dysfunctional state of the immune response with paramount importance for cancer progression. Furthermore, modern treatments, including immunotherapy, are focused on overcoming this condition. Our finding of CD8+ exhaustion in ALTRs may represent an important condition of the immunological response on ALTRs, and perhaps a novel direction for ALTRs' treatment.
ACKNOWLEDGMENTS
The authors would like to thank Mrs. Raman Johal, of the Department of Orthopedics of UBC, for the patient recruitment and sample obtaining in this study. The authors also like to thank Mr. Simon Cheung and Mrs. Margaret Luk, from the Department of Pathology at the Vancouver General Hospital, for their collaboration in the sample collection and processing. To Angela Goitain from the Genetic Pathology Evaluation Centre (Vancouver) for her collaboration in RNA extraction from histological samples and RNA analysis through the nCounter system, Niloufar Benam for helping with editing this manuscript and Dr. David Hart for his contribution to the original planning of this project. This study has been partially funded by a grant of Orthopedic Research Excellence Fund (OREF), the Collaborative Health Research Projects (CHRP) jointly funded by the Natural Sciences and Engineering Research Council of Canada (NCERC), and the Canadian Institutes of Health Research (CIHR). Felipe Eltit was supported by a scholarship from the Chilean Bureau of Science and Technology (CONICYT).
AUTHOR CONTRIBUTIONS
Felipe Eltit, Tony L. Ng, Rizhi Wang, and Michael E. Cox designed the study and experiments, and collaborated with manuscript writing and editing. Felipe Eltit conducted the design, sample collection, analysis, experiments, and drafted the article. Donald S. Garbuz, Clive P. Duncan, Nelson V. Greidanus, and Bassam A. Masri contributed to the patients' recruitment, sample collection, and clinical data analysis and manuscript editing. Nelson V. Greidanus and Tony L. Ng provided with histopathological diagnosis, evaluation of biopsies, and immunohistochemistry. Anne Haegert performed the statistical analysis of the protein analysis and gene expression. Indira Medina helped to design the gene expression analysis, performed the data analysis, cell scores, and assisted with manuscript writing.




