Distinct expression trend of signature antigens of Staphylococcus aureus osteomyelitis correlated with clinical outcomes
Abstract
The major limitations of clinical outcome predictions of osteomyelitis mediated by Staphylococcus aureus (S. aureus) are not specific and definitive. To this end, current studies aim to investigate host immune responses of trend changes of the iron-regulated surface determinant (Isd) of IsdA, IsdB, IsdH, cell wall-modifying proteins of amidase (Amd) and glucosaminidase (Gmd), and secreted virulence factor of chemotaxis inhibitory protein S. aureus (CHIPS) and staphylococcal complement inhibitor (SCIN) longitudinally to discover their correlationship with clinical outcomes. A total of 55 patients with confirmed S. aureus infection of the long bone by clinical and laboratory methods were recruited for the study. Whole blood was collected at 0, 6, 12 months for the serum that was used to test IsdA, IsdB, IsdH, Gmd, Amd, CHIPS, and SCIN using a customized Luminex assay after clinical standard care parameters were collected. The patients were then divided into two groups: (1) infection controlled versus (2) adverse outcome based on clinical criteria for statistical analysis. We found that standard clinical parameters were unable to distinguish therapeutic outcomes. Significant overexpression of all antigens was confirmed in infection patients at 0-, 6-, and 12-month time points. A distinct expression trend and dynamic changes of IsdB, Amd, Gmd, and CHIPS were observed between infection controlled and adverse outcome patients, while the IsdA, IsdH, SCIN remained demonstrated no statistical significance. We conclude that dynamic changes of specific antigens could predict clinical outcomes of S. aureus osteomyelitis. Clinical Relevance: The trend changes of host immune responses to S. aureus specific antigens of IsdB, Gmd, Amd, and CHIPS could predict clinical outcomes of S. aureus osteomyelitis.
1 INTRODUCTION
Staphylococcus aureus (S. aureus) is a notorious bacterium that causes orthopedic and device-related infections (ODRIs) and blood–borne osteomyelitis. It is reported that approximately 112,000 ODRIs occur annually in the United States, accounting for approximately 34% of S. aureus infections.1-3 It has the characteristics of a long treatment cycle, a high cost, frequent relapse, and poor treatment success rates. Current treatment strategies for bone infections are limited to thorough debridement, removal of implants, and the systemic or local application of antibiotics.4 However, due to the long-term use of antibiotics, it is becoming more difficult to effectively treat bone infections, which will not only lead to an imbalance of intestinal flora and an increase in opportunistic infections but also promotes the formation of bacterial resistance.5 Accurate monitoring of the dynamic changes of infectious bacteria can help us to determine the effectiveness of clinical treatment. Although erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), procalcitonin, polymerase chain reaction/electrospray ionization-mass spectrometry, and their serum markers, such as interleukin (IL)-6, IL-10, and interferon-γ, may indicate the persistence of bacterial infections to a certain extent,6, 7 the main limitations of clinical response monitoring and clinical monitoring of recurrent infections of trauma-related long bone infections medicated by S. aureus are uncertain. A promising evaluation for clinical monitoring of the treatment outcomes of this infection is to track the host immune response, which has been previously established.8, 9 Our previous studies have shown that the specific antigens of S. aureus have the diagnostic value of cell wall-modification proteins amidase (Amd) and glucosaminidase (Gmd), iron-regulated surface determinants (Isds) of IsdA, IsdB, and IsdH, and secreted virulence factor of chemotaxis inhibitory protein (CHIPS) and staphylococcal complement inhibitor (SCIN), but these have not yet been introduced into clinical practice.10, 11 Meanwhile, due to the Chinese government's regulations prohibiting the export of human materials from China, a central laboratory was established to manage and analyze Chinese specimens at Zunyi Medical University (ZMU) in China.12 On the basis of patients recruited for AO Clinical Priority Program (AOCPP) Bone Infection in China,12, 13 this study aims to study seven antigens (IsdA, IsdB, and IsdH, Amd, Gmd, CHIPS, and SCIN) associated with S. aureus bone infections, and provide an understanding of the clinical significance of serum antibody levels during the course of bone infection.
2 MATERIALS AND METHODS
2.1 Patient enrollment and microbiological analysis to identify pathogens
This study was the second phase of the 2012 AOCPP Bone Infection in China,13 and was approved by the Ethics Committees of the First Affiliated Hospital of ZMU and the Southwest Hospital of Chongqing (SHC) From January 2014 to December 2017, we enrolled 55 patients with S. aureus long bone infection (monomicrobial or polymicrobial) confirmed by clinical culture of the specimen that was collected from during surgical irrigation and debridement at the ZMU and SHC. The inclusion criteria are (1) patients aged 18 years or older; (2) confirmed oxacillin-/methicillin-sensitive Staphylococcus aureus (OSSA/MSSA) or methicillin-resistant Staphylococcus aureus (MRSA) infection involving a long bone (femur, tibia, fibula, humerus, radius, ulna, and clavicle) with one (or a combination) of the osteomyelitis, fracture fixation hardware/prosthetic joint infection, infection around an arthroplasty; (3) be able to understand the content of the informed consent form (ICF) and able to participate in the clinical investigation; (4) signed written ICF. The exclusion criteria are (1) patients who cannot give informed consent; (2) patients who cannot attend the follow-up visits; (3) recent history of substance abuse (i.e., recreational drugs, alcohol) that would preclude reliable assessment; and (4) prisoners. All patients understood the study clearly and joined voluntarily. Relative documents of ICF were signed by each individual before serum specimens were collected. The infection sites, the duration of infection before administration, and the types of infection of the involved long bone were recorded. All infection pathogenic bacteria were determined by clinical laboratory examinations at the administration hospital and confirmed again by the Joint Orthopaedic Research Center of ZMU and the University of Rochester Medical Center (URMC) at ZMU (Tables 1, 2 and Figure 1).12-14
| Infection site | Monomicrobial infection(%) | Polymicrobial infection (%) | Total (%) |
|---|---|---|---|
| Tibia and Fibulaa | 19 (34.55) | 6 (10.91) | 25 (45.45) |
| Femur | 22 (40) | 0 | 22 (40) |
| Radius and Ulna | 3 (5.45) | 0 | 3 (5.45) |
| Hip | 3 (5.45) | 0 | 3 (5.45) |
| Humerus | 1 (1.82) | 0 | 1 (1.82) |
| Knee | 1 (1.82) | 0 | 1 (1.82) |
| Total (%) | 49 (89.09) | 6 (10.91) | 55 (100) |
- a The detail of polymicrobial infection cases listed in Table 3.
| Pathogenic bacteria | No. of isolated pathogen(s) | Proportion(%) |
|---|---|---|
| Gram-positive bacteria | ||
| OSSA/MSSAa | 32 | 54.24 |
| MRSAa | 21 | 35.59 |
| Corynebacteriuma | 1 | 1.69 |
| Gram-negative bacteria | ||
| Klebsiella pneumoniaea | 2 | 3.39 |
| Pseudomonas aeruginosaa | 2 | 3.39 |
| Citrobacter freundiia | 1 | 1.69 |
| Total | 59 | 100 |
- Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus; OSSA, oxacillin-sensitive Staphylococcus aureus.
- a The detail of polymicrobial infection show in Table 3.
2.2 Serum collection and standard serum selection
Whole blood was drawn from the patients at 0 month (n = 55), 6 months (n = 39), and 12 months (n = 39) of time points, and stored in the standardized serum separator tube that was supplied by AOCPP, and was set to clot for about 2 h at room temperature. The serum was then collected after centrifugation at 12,000 rpm for 20 min at room temperature, and stored in aliquots at −80°C for later use. To generate a standard curve for further Luminex analysis, sera from four patients who underwent total hip or knee joint replacement without infection disease were combined as a noninfected control. Four of the 55 patients whose baselines showed the highest expression level of seven-candidate antigens were pooled for duplicated serial dilution of: 1:100, 1:500, 1:2500, 1:12,500, 1:62,500, 1:312,500, 1:1,562,500. As part of standard clinical care, 0- 6-, and 12-month white blood count (WBC) (n = 52, 20, 19), CRP (n = 42, 19, 19), and ESR (n = 41, 20, 19) were tested and recorded for each individual (Figure 2).
2.3 The criteria of infection controlled versus adverse outcome
Infection controlled is defined as none of the clinical signs and symptoms of active disease (fever, chills, focal pain, erythema, swelling and draining sinuses, etc.) observed at the end of 12-month follow-up and no images (radiography, computed tomography, magnetic resonance imaging, ultrasound) evidence showing the progression of bone infection. The adverse outcome is defined as failure response to therapy, as evidenced by one or more of (1) persistence of drainage; (2) a sinus tract recurrence or failure to close; (3) clinical systemic signs of infection (chills, fever, and local pain); and (4) imaging evidence of infection progression.2, 6, 15
2.4 MagPlex-Avidin magnetic beads conjugation and Luminex assay
The seven immunodominant antigens of S. aureus were provided by GenScript, include (1) cell wall-modifying proteins Amd and Gmd, (2) IsdA, IsdB, and IsdH, and (3) secreted virulence factor of CHIPS, and SCIN (GenScript, Lot# U8848CL180801/P10011801). These biotinylated antigens of S. aureus were diluted to the final concentration of 100 nM following the previous protocol developed at the URMC.11 Briefly, the final concentrations of the antigens were conjugated with magnetic beads. A total of 1,000,000 beads/ml were mixed with 50 µl equal volume per well of patient serum (10,000-fold dilution) and incubated for 2 h under sonication at room temperature. After washing three times with 100 µl of 0.05% PBST, the secondary antibody of the phycoerythrin-conjugated goat anti-human immunoglobulin G (IgG) reagent in phosphate-buffered saline-1% bovine serum albumin was added onto the plate shaker at 800 rpm for 60 min. After this was repeated three times, the plates were ready for Luminex 200TM analysis. The mean fluorescent intensity (MFI) value was arbitrary as IgG titers for further data analyses if the coefficient of variation (CV) among the replicates was less than 20%. To make sure that the data generated from the multiplex immunoassay was acceptable, the next situation was remeasured. (1) If a particular sample had bead count below 25, the sample was run again on the Luminex machine or we repeated the immunoassay for the sample until the bead count was greater than 25; (2) %CV = 100 × standard deviation/mean, a CV of less than 20% among the replicates is acceptable. (3) %difference = (FI-trimmed mean)/trimmed mean) × 100, to minimize intra-assay variability the %difference was kept below 20%.
2.5 Statistical data analyses
SAS (Version 9.4) and GraphPad Prism Software (Prism7.0) were used to perform statistical analyses. The correlation analysis linear attendance of MFI of three-time points (0-, 6-, and 12-month) of adverse outcome versus infection controlled was analyzed by mixed-effects models of type III tests of fixed effects with GLIMMIX procedure. Quantitative results were shown as means ± standard deviations, as well as the linear relationship between the dilutions ratio of STN and MFI values using Pearson correlation analysis. One-way analysis of variance (ANOVA) was used to compare the MFI of three-time points (0, 6, and 12 months), and with the Kruskal–Wallis test, multiple comparisons were performed for the duration of infection. CRP, WBC, ESR, and two-way ANOVA was used for the seven-candidate antigens expression of the baseline. A p-value of less than 0.05 was defined as statistically significant.
3 RESULTS
3.1 The lower extremity was the dominant infection site, and the type of osteomyelitis and infection pathogen of MSSA/OSSA significantly affected the duration of treatment
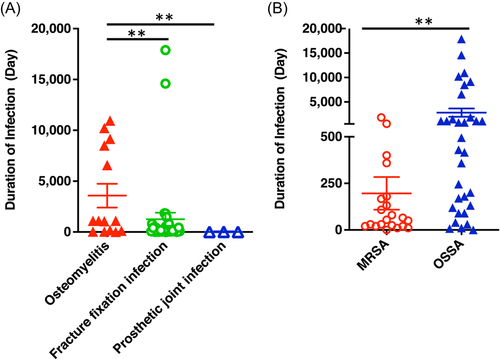
A total of 55 patients with S. aureus infection included 46 males (41.7 ± 13.2 years old) and 9 females (35.5 ± 10.5 years old). The infection sites were dominant in the lower extremities (51, 92.75%), especially the tibia and fibula 45.45% (n = 25) and the femur 40.0% (n = 22) (Table 1). Overall, a monomicrobial infection pathogen was responsible for 89.1% (n = 49) of cases, while a polymicrobial infection was seen in 10.9% (n = 6) of cases while only the tibia and fibula were involved in this study (Table 2). Among polymicrobial infections, Pseudomonas aeruginosa, Corynebacterium, Klebsiella pneumoniae, or Citrobacter freundii were combined with MRSA or MSSA (Table 3). Interestingly, the period in which a patient sought medical assistance during a fracture fixation infection and prosthetic joint infection was significantly shorter than osteomyelitis (Figure 1A), while the patients with MRSA infections received more hospital care than patients who were infected with MSSA/OSSA (Figure 1B). Considering that this group of patients was mainly recruited from the rural area in Southwest China, this may be the main reason for the different regional characteristics of bone infection in this study.
| Sites | No. of cases (%) | Pathogenic bacteria | ||
|---|---|---|---|---|
| A | B | C | ||
| Tibia and fibula (100%) | 2 (33.33%) | MRSA | Pseudomonas aeruginosa | |
| 1 (16.67%) | MRSA | OSSA/MSSA | Corynebacterium | |
| 1 (16.67%) | MRSA | Klebsiella pneumoniae | ||
| 1 (16.67%) | OSSA/MSSA | Klebsiella pneumoniae | ||
| 1 (16.67%) | OSSA/MSSA | Citrobacter freundii | ||
- Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus; OSSA, oxacillin-sensitive Staphylococcus aureus.
3.2 CRP, WBC, and ESR tests cannot distinguish between the clinical outcomes of a controlled infection and adverse outcome
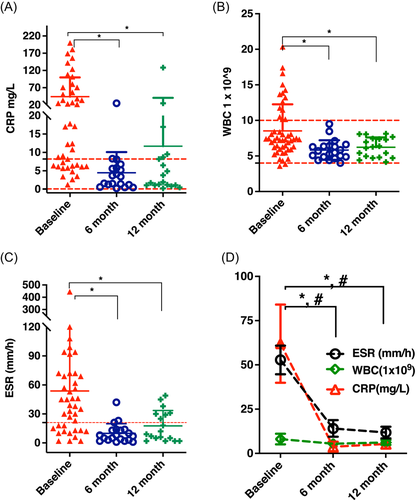
Longitudinally CRP (Figure 2A), WBC (Figure 2B), and ESR (Figure 2C) data collection showed that the 6-month and 12-month follow-up time points were significantly lower than the baseline, but none of these tests were able to distinguish between a controlled infection or adverse outcome. The clinical results of a controlled infection or adverse outcome (*, #p < 0.05) are shown in Figure 2D, may result in clinicians discharging patients before the infection has been controlled. Without intermittent clinical follow-up visits, this may put the patients at risk of recurrence of infection over time.
3.3 We successfully established a Bio-Luminex standard curve of testing antigens locally but failed to create a repeatable gold standard of Bio-Luminex of testing antigens for horizontal comparisons between three centers
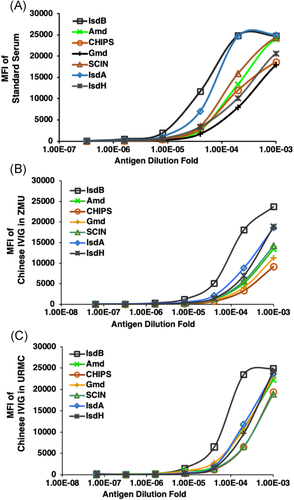
A pooled standard serum was developed from the four patients that were screened from 55 patients who expressed the highest mean fluorescent intensity (MFI) of antigens of IsdA, IsdB, IsdH, Amd, Gmd, CHIPS, and SCIN. Then, a highly repeatable standard curve was established locally at ZMU (Figure 3A). Meanwhile, the China Food and Drug Administration approved intravenous immune globulin (CIVIG) which was then employed to establish a comparable standard curve for all centers at ZMU, URMC, and Virginia Commonwealth University (VCU) due to the Chinese regulation on the exportation of human tissue. Unfortunately, the results disproved the consistency of the expression levels of each candidate antigen (Figure 3B), URMC (Figure 3C), and VCU (data not shown). Thus, we failed to generate an exact match MFI of candidate antigens by using CIVIG. Therefore, the ZMU, URMC, and VCU were forced to analyze the data separately (Figure 3B,C).
3.4 Significant overexpression of IsdA, IsdB, IsdH, Amd, Gmd, CHIPS, and SCIN in the infected patient baseline that compared with noninfected patient and CIVIG
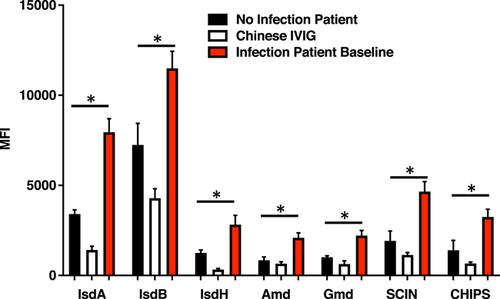
By using the customized standard serum that was pooled from the four patients, the dilution of 1:20,000 was applied for all three groups of infected patient baseline, noninfected patient, and CIVIG to perform all seven antigens of IsdA, IsdB, IsdH, Amd, Gmd, CHIPS, and SCIN testing. The results indicated that when compared with NIP, there was significantly overexpression of IsdA (2.3-fold), IsdB (1.6-folds), IsdH (2.3-fold), Amd (2.5-fold), Gmd (2.2-fold), CHIPS (2.3-fold), and SCIN (2.4-fold). When we compared with CIVIG, the results were significantly higher: IsdA (5.6-fold), IsdB (2.7-fold), IsdH (8.5-fold, Amd (3.2-fold), Gmd (3.5-fold), CHIPS (4.9-fold), and SCIN (4.1-fold) (two-way ANOVA, *p < 0.0001) (Figure 4). These findings indicated that all seven-candidate antigens are potential immunological signatures for clinical outcomes of therapy.
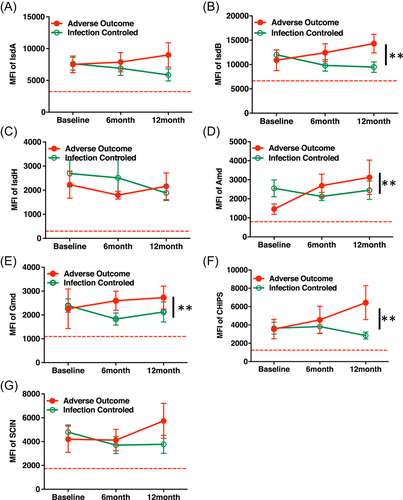
3.5 Distinct longitudinal expression trend of IsdB, Amd, Gmd, and CHIPS were observed between infection-controlled patients versus adverse outcome patients
On the basis of clinical criteria of chronic osteomyelitis therapy,2, 6 the clinical results of the current study were divided into an infection-controlled group and an adverse outcome group. In follow-up time points, the longitudinal expression trend of each individual antigen IsdB, Amd, Gmd, and CHIPS showed a significantly distinct increase in the -adverse outcome group, while showing a significant decreasing trend in the infection-controlled group (**p < 0.05). However, this was not observed in the IsdA, IsdH, and SCIN groups (Figure 5A–G). We further found that distinct MFI of IsdB, cell wall-modifying protein Amd and Gmd, and secreted virulence factor CHIPS were confirmed by using the difference of the 12 months follow up from the baseline between infection controlled patients versus adverse outcome patients (Data not shown).
4 DISCUSSION
4.1 The regional differences in bacterial infections may be related to the geographical location of patients, regional climate characteristics, and medical conditions, where it may be necessary to test the genetic characteristics of pathogens for confirmation
The patients admitted in this study were mainly from rural areas of Southwest China. These areas are located at an altitude of 1000–1600 m, with an average annual temperature of 12.6–13.1°C. It is often raining while being hot and humid at the same time. As a result, the relative humidity is high, and the climate types are three-dimensional and diverse. It belongs to the humid monsoon region of the central subtropical plateau, and is the low-value area of perennial solar radiation and is an area susceptible to bacteria.16 Before 2011, comprehensive healthcare insurance had not yet been fully implemented in China, and some patients had not received proper medical care due to economic reasons, which played a decisive role in the prolongation of the bone infection.17 On the basis of data collected in our studies, the bone infections caused by S. aureus have obvious regional characteristics.13, 14 Most of these patients had lived in mountainous, far from the cities and had not been exposed to hospital-derived S. aureus. This may be the main reason why the strains that infected patients are different from other regions.18, 19 All specimens were collected from bone biopsy and nasal swab have undergone genetic testing and with pending data analysis.
4.2 A distinct overexpression trend of IsdB, Amd, Gmd, and CHIPS was observed in adverse outcome patients
Existing clinical indicators for monitoring infection cannot distinguish whether the treatment is effective, and there is an urgent need to find sensitive and effective biochemical signature markers. As shown in the results, to monitor treatment outcome of bone infection, CRP, WBC, and ESR testing are the current clinical standard tests and tend to decrease at the 6- and 12-month time points. Unfortunately, all these changes cannot distinguish clinical outcomes of infection-controlled or adverse outcomes which represent a significant limitation and demands sensitive signature markers to surveil treatment outcomes. The S. aureus genome can encode about 2700 proteins, including cytoplasm, cell membrane, cell surface, and secreted proteins.20-22 Among the many proteins, cell surface and secreted proteins have a high chance of being recognized by the host immune system with resultant antibodies against S. aureus.23-25 Utilizing a multiantigen Luminex immunoassay with specific antibodies against S. aureus, the humoral immune response can be measured in chronic orthopedic infections and have shown promising value.26-28 Nishitani et al.11 screened 14 proteins of S. aureus and concluded that measurement of the host antibody response is a predictor of ongoing infection that may prove to have prognostic value. Our results show that all seven antigens were overexpressed in infected patients compared that those of noninfected patients: IsdA (2.3-fold), IsdB (1.6-fold), IsdH (2.3-fold), Amd (2.5-fold), Gmd (2.2-fold), CHIPS (2.3-fold), and SCIN (2.4-fold). However, only IsdB, Amd, Gmd, and CHIPS showed significant overexpression longitudinally in the adverse outcomes group versus infection-controlled group by using mixed-effects models of type III tests of fixed effects with GLIMMIX procedure. We noticed that the expression level of Gmd in this study was higher in the adverse outcomes group, which is slightly different from those patients' serum collected from North American and Europe.10 Further study to illustrate the mechanism is warranted.
In summary, the current clinical standard of care tests is unable to distinguish therapeutic outcomes of S. aureus osteomyelitis. Significantly, overexpression of antigens IsdB, cell wall-modifying protein Amd and Gmd, and secreted virulence factor CHIPS at 0-, 6-, 12-month time points and their distinct overexpression trend could be the signature markers of adverse outcome of S. aureus osteomyelitis.
4.3 Limitations
(1) The most of patients who enrolled in the study were from rural areas, and current data did not include the majority of patients with a traumatic bone infection in China's urban area. (2) Some patients missed follow-up time points of 6th and 12th months. Some patients who delayed 6-months follow-up to 8th months were classified as 6th-month specimens. Several patients who were late for the 12th-month visit by about 1 month were considered 12 months. (3) The intravenous immunoglobulin for clinical use is prepared from the serum of between 1000 and 15000 donors per batch,29 which represents an average level of candidate antigens (IsdA, B, and H, SCIN, CHIPS) in a population. Because the sample size of four noninfection patients is too small to represent a broad population as the CIVIG does, this could result in a higher expiration level of signature antigens mentioned above. (4) Also, the sample size calculation by an a priori power analysis still lacks because randomization is not the same.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China 81760400 (Jiachen Peng), NIH NIAMS P30 AR069655, P50 AR07200, and AO Trauma Clinical Priority Program (Chao Xie, Stephen L. Kates, and Jiachen Peng), AO Trauma Clinical Priority Program Fellowship (Gowrishankar Muthukrishnan), and NIH NIAID R21 AI119646 (John L. Daiss).
AUTHOR CONTRIBUTIONS
All authors have contributed to the research design, and/or the acquisition, analysis, or interpretation of data; drafting the paper or revising it critically; and approval of the submitted and final versions.




