Pathological changes of frozen shoulder in rat model and the therapeutic effect of PPAR-γ agonist
Jinfu Chen, Junjun Zhu, Tianfei Zhu, Jiaming Cuim, and Zhenhan Deng contributed equally to this study.
Abstract
Frozen shoulder is a common shoulder disorder characterized by a gradual increase of pain and a limited range of motion. However, its pathophysiologic mechanisms remain unclear and there is no consensus as to the most effective treatment. The purpose of the study was to investigate the effect of transforming growth factor-β (TGF-β) on fibrosis and inflammatory response of the shoulder joint of rat models and to explore the therapeutic effect of the peroxisome proliferator-activated receptor-γ (PPAR-γ) agonist. In the study, the effect of PPAR-γ agonist CDDO-IM treatment on cell proliferation, migration, and extracellular matrix proteins synthesis (vimentin, α-smooth muscle actin, collagen I, and collagen III) were tested by cell proliferation test, scratches test, real-time quantitative polymerase chain reaction, and Western blot analysis. The frozen shoulder was also established on the rat model by injecting adenovirus-TGF-β1 into rats' shoulder capsule. Pathological changes of the frozen shoulder tissue of the experimental group and PPAR-γ agonist treatment group were evaluated. The stiffness of joints of the three groups was tested. Inflammatory mediators' expression including cyclooxygenase-1, interleukin-1β, and tumor necrosis factor-α of the shoulder was tested by enzyme-linked immunosorbent assay, and the expression of extracellular matrix proteins was evaluated by hematoxylin and eosin staining and immunohistochemistry. The results showed that pathological changes of the frozen shoulder in the rat model include an abnormal proliferation of fibroblasts, infiltration of inflammatory cells, and disorder of fibrous structure, while rosiglitazone reduced the severity of the frozen shoulder in the treatment group. Clinically, PPAR-γ agonists may be a promising target for the treatment of the frozen shoulder.
1 INTRODUCTION
Frozen shoulder is a progressive disease of shoulder joints characterized by pain and a limited range of motion. Although the etiology is unknown, it usually occurs in three stages within a duration of around 12 to 40 months (30 months on average): pain, freezing, and thawing.1-4 Some patients could resume joint movement after this period, but a significant number of patients had limited shoulder movement that affected their daily work and life. The most significant clinical presentation of the frozen shoulder is the limitation of active and passive movement of the shoulder joint in all directions, especially external rotation.5, 6 Despite a large number of relevant studies, the etiology and pathological mechanisms leading to the development of frozen shoulder are still poorly understood, and there is no consensus on the best treatment method.
Transforming growth factor-β (TGF-β) is a multifunctional cytokine, which plays a decisive role in the expression of extracellular matrix proteins, cell proliferation, differentiation and apoptosis, and cellular immunity.7-9 It can promote the formation of the extracellular matrix, fibroblast synthesis of collagen and vimentin, α-smooth muscle actin (α-SMA), and the expression of extracellular matrix protein specificity receptor.10 Research shows that TGF-β plays a key role in liver fibrosis11 and pulmonary interstitial fibrosis.12 Furthermore, studies have found increased expression of TGF-β and its receptor in the shoulder capsule in patients with primary or secondary frozen shoulder, suggesting that TGF-β can also cause joint capsule fibrosis, and may be involved in the origin and development of frozen shoulder.13 In addition, recent studies have shown that the overexpression of inflammatory cytokines in the joint capsule has a significant contribution to the pathogenesis of frozen shoulder.14-16 It has been reported that inflammatory mediators such as interleukin-1α (IL-1α), IL-1β, IL-6, tumor necrosis factor-α (TNF-α), cyclooxygenase-1 (COX-1), cyclooxygenase-2 (COX-2) were rich in the inflammation and collagen decomposition of frozen shoulder.17-19
Previous studies have shown that the ligand of peroxisome proliferator-activated receptor-γ (PPAR-γ) plays an important role in anti-inflammatory, immunity, and glycolipid metabolism by affecting nuclear factor-κB (NF-κB), activator protein-1 (AP-1), JAK/STAT, which further regulates the expression of downstream related genes. Simultaneously PPAR-γ is effective in preventing fibrosis of skin, kidney, and cornea.20-23 However, the effectiveness of the PPAR-γ agonist in treating frozen shoulder is still unknown and yet to be studied. The rat frozen shoulder model is similar to human anatomy and highly repeatable.24-26 According to the report by Soslowsky et al, the rats' forelimbs at 90° of forward flexion and 0° of abduction was analogous to 0° of forward flexion and 90° of abduction in humans.27 Therefore, the purpose of the study was to investigate the anti-fibrotic and anti-inflammatory effects of the PPAR-γ agonist in the rat model of frozen shoulder. We hypothesized that PPAR-γ agonist was effective in inhibiting human skin fibroblast (HSF) fibrosis, and had positive therapeutic effects in the rat model of frozen shoulder.
2 METHODS
2.1 Cell viability test
The HSFs were resuspended in growth medium (GM) which contained Dulbecco's modified Eagle's medium (Gibco), 10% fetal bovine serum, 100 aU/ml penicillin, and 100 mg/ml streptomycin (all from Hyclone). The cells were seeded into a 96-well plate at a density of 1 × 104 per well in the controlled conditions of 37°C and 5% CO2. After cell adhesion, the cells were treated with TGF-β1 (10 ng/ml),28, 29 CDDO-IM (20 nM),30 and TGF-β1 (10 ng/ml) + CDDO-IM (20 nM) in serum-free media. Vehicle-treated cells were used as controls. The cells collected at each time point (0, 24, 48, and 72 h) were added to the MTS solution (Promega; cat. no. G3582) at a ratio of 1/10. That is, we added 10 μl test solution to 100 μl culture solution. After 4 h of incubation, the optical absorbance was read at 450 nm on a microplate reader (Thermo Fisher Scientific, multiscan MK3).
2.2 Cell migration test
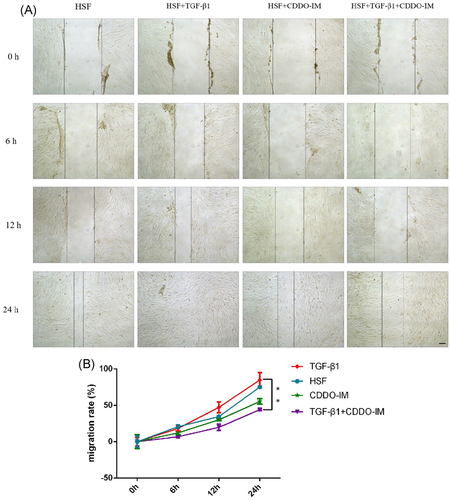
The HSFs were seeded into six-well plates and incubated for 24 h. Furthermore, scratch tissues were induced with a pipette tip (10 μl), suspended cells were washed two to three times with phosfate-buffered saline. The cells were treated with TGF-β1 (10 ng/ml), CDDO-IM (20 nM), and TGF-β1 (10 ng/ml) + CDDO-IM (20 nM) in serum-free media. Vehicle-treated cells were used as controls. The tissues were monitored using phase-contrast microscopy and photographed at 0, 6, 12, and 24 h. Image pro-plus 6.0 software was used to determine the scratch width of any eight sites at the same time points in each group, and calculate the cell migration rate = (0h − other time points)/0 h to reflect the migration ability of HSF in each group.
2.3 Real-time polymerase chain reaction (PCR)
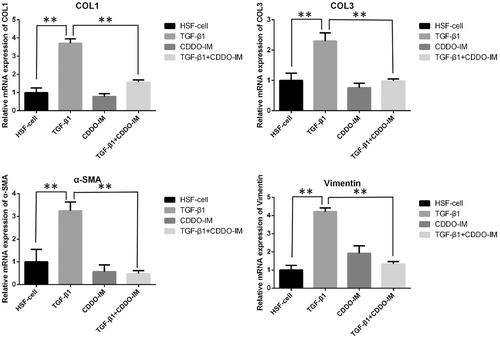
Cells were collected and RNA was extracted using TRIzol reagent (Invitrogen). The purity of the extracted RNA was detected by Eppendorf BioPhotometer plus, and the ratio of OD260/OD280 was greater than 1.8. The extracted RNA was then reverse-transcribed into cDNA following the steps of the reverse transcription kit (Promega). Collagen I (COL I), Collagen III (COL III), vimentin, and α-SMA expressions were detected using the real-time PCR kit (SYBR Green qPCR SuperMix, Invitrogen), and the target gene primers are shown in Table 1. Real-time PCR reaction was performed under the following conditions: denaturation at 95°C for 5 min, 50 cycles at 95°C for 5 s, 60°C for 10 s. Quantitative PCR (qPCR) instrument: ABI PRISM® 7500 Sequence Detection System.
| Gene name | Primer sequence |
|---|---|
| COL1 | Forward: 5′-CCTGGATGCCATCAAAGTCT-3′ |
| Reverse: 5′-ACTGCAACTGGAATCCATCG-3′ | |
| COL3 | Forward: 5′-TTCAGTTTAGCTACGGCAATCC-3′ |
| Reverse: 5′-GGCCTGATCCATGTATGCAA-3′ | |
| Vimentin | Forward: 5′-GACCAGCTAACCAACGACAA-3′ |
| Reverse: 5′-GTTTTCGGCTTCCTCTCTCT-3′ | |
| α-SMA | Forward: 5′-GTGAAGCAGCTCCAGCTATG-3′ |
| Reverse: 5′-CGTCCCAGTTGGTGATGATG-3′ |
2.4 Western blot analysis
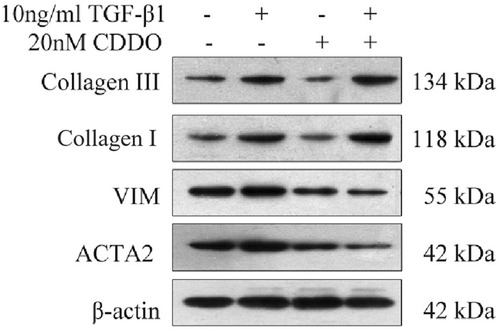
The cells were lysed on ice for 30 min in lysis buffer containing 50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 1% Nonidet P-40, and 0.1% SDS supplemented with protease inhibitors (10 mg/ml leupeptin, 10 mg/ml pepstatin A, and 10 mg/ml aprotinin). The sample was resolved on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and then electro-transferred onto nitrocellulose membranes (Whatman). The primary antibodies used were VIM (1:1000, A11952; Abcam), Col1a2 (1:1000, A5786; Abcam), anti-collagen III (1:1000, ab23445; Abcam), ACTA2 (1:1000, A7248; Abclonal). For the normalization of protein loading, secondary antibodies (Cell Signaling Technology) were used at a 1:1000 dilution. The antigen-antibody complexes were visualized using the enhanced chemiluminescence detection system (Millipore) as recommended by the manufacturer.
2.5 Animal experiment
The experiment was carried out following relevant guidelines and regulations and was approved by the Medical Ethics Committee of the First Affiliated Hospital of Shenzhen University. The Sprague-Dawley rats were purchased from Guangdong Medical Laboratory Animal Center.
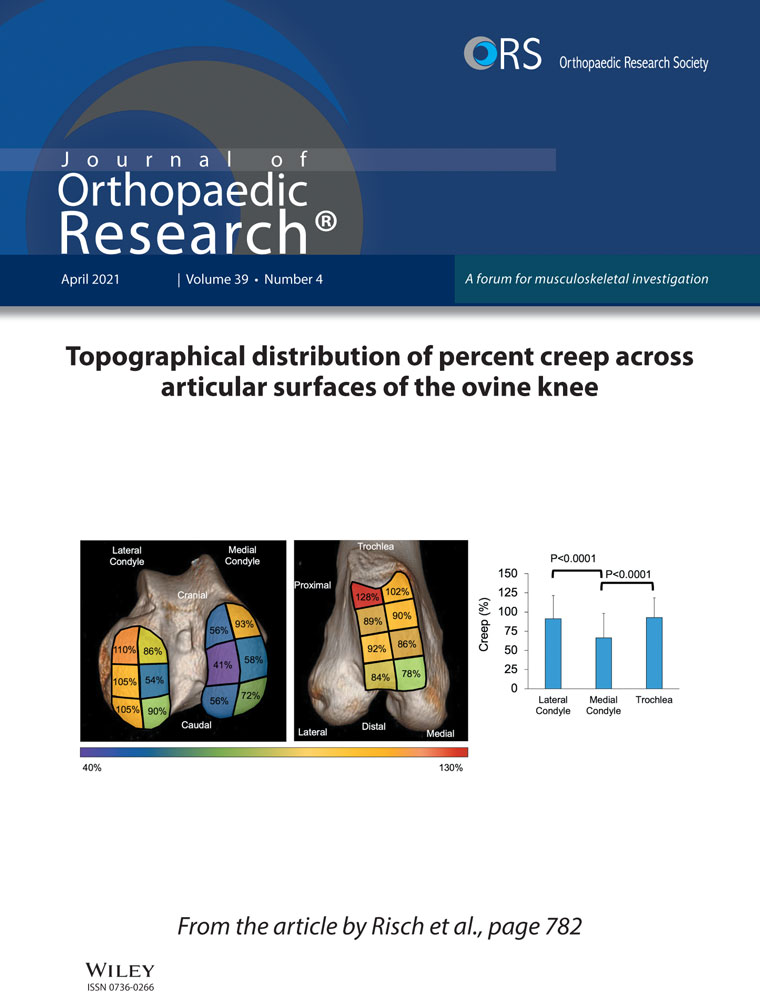
Eighteen mature Sprague-Dawley rats (20 months old and weighing 455.4 ± 2.5 g) were used in this study (Table 2), and were randomly divided into three groups: the control group (N = 6), the experimental group (N = 6), and the treatment group (N = 6). The experimental procedure was shown in Figure 1. Both experimental and treatment groups were first given intraperitoneally chloral hydrate (400 mg/kg) for anesthesia. Then, the shoulder joints were shaved, and the injection points were marked and sterilized. Under aseptic conditions, 30 μl adenovirus-TGF-β1 (1 × 1011 PFU/ml) was diluted to 100 μl and injected into the shoulder joint capsule at the insertion point by ultrasound-guided. Two weeks later, the treatment group was treated with rosiglitazone (PPAR-γ agonist) (2 mg/kg/d)31, 32 for another 2 weeks. Postoperatively, the animals in all groups were allowed normal activity and had free access to water and food.
| Group | Number | Weight (g) | Microbiological status | Ad-TGF-β1 | Rosiglitazone |
|---|---|---|---|---|---|
| Control group | C1 | 452 | SPF | × | × |
| C2 | 455 | × | × | ||
| C3 | 453 | × | × | ||
| C4 | 450 | × | × | ||
| C5 | 453 | × | × | ||
| C6 | 458 | × | × | ||
| Experimental group | E1 | 455 | √ | × | |
| E2 | 455 | √ | × | ||
| E3 | 457 | √ | × | ||
| E4 | 454 | √ | × | ||
| E5 | 456 | √ | × | ||
| E6 | 458 | √ | × | ||
| Treatment group | T1 | 460 | √ | √ | |
| T2 | 455 | √ | √ | ||
| T3 | 454 | √ | √ | ||
| T4 | 459 | √ | √ | ||
| T5 | 457 | √ | √ | ||
| T6 | 456 | √ | √ |
In the fifth week, specimens from three groups were tested for shoulder joint stiffness under anesthesia (Figure 2). Under anesthesia, rats' forelimbs were fixed at 90° of forward flexion and 0° of abduction (analogous to 0° of forward flexion and 90° of abduction in humans). The torque testing device performed passive external and internal rotation of the rat shoulder joint, as described in the previous study.33 The range of motion was set to 45° for internal and 45° for external rotation. Each rat was subjected to three passive activities at a frequency of 1/30 Hz. They were then euthanized by intraperitoneal injection of an overdose of pentobarbital sodium (150 mg/kg). The shoulder capsules were immediately removed and processed for histological evaluation and immunohistochemistry (enzyme-linked immunosorbent assay [ELISA] test, hematoxylin and eosin [H&E] staining).
2.6 Statistical analysis
All tests (biomechanical test, cell viability test, real-time PCR, cell migration test, Western blot analysis) were repeated three times. Data were expressed as mean ± standard deviation. SPSS 17.0 statistical software was used for statistical analysis of experimental data, and one-way analysis of variance was used for comparison between different groups. Statistical analyses were performed using the Tukey test. p < .05 was considered to be significant.
3 RESULTS
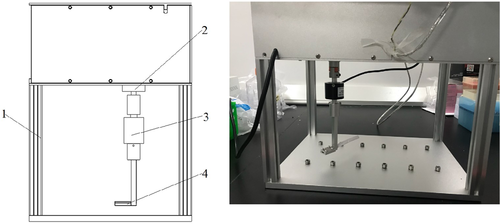
The proliferation of cells at different times (0, 24, 48, and 72 h) in each group was tested by the MTS solution. Results showed that TGF-β1 promoted the proliferation of HSF, and CDDO-IM (PPAR-γ agonist) inhibited the proliferative effect of TGF-β1. Significant differences were found in cell viability and rate of proliferation between the TGF-β1 group and each of the other groups at 72 h. Additionally, the HSF group had significantly higher cell viability and rate of proliferation than the CDDO-IM group and TGF-β1+ CDDO-IM group at 72 h (Figure 3).
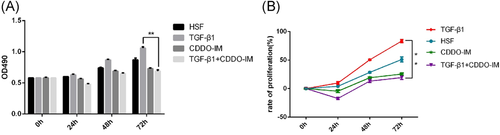
Cell migration rates at different times (0, 6, 12, and 24 h) were calculated and compared between groups (Figure 4A). The results showed that TGF-β1 promoted HSF migration, and CDDO-IM inhibited this promotion effect. Significant difference was found between the TGF-β1 group and TGF-β1 + CDDO-IM group at 24 h (Figure 4).
The result of real-time PCR showed that TGF-β1 promoted the RNA expression of HSF Collagen I, Collagen III, α-SMA, and vimentin (Figure 5). The PPAR-γ agonist treatment group showed that CDDO-IM could inhibit the high expression of RNA levels of Collagen I and Collagen III, α-SMA and vimentin RNA in TGF-β1 induced HSF (Figure 5). The Western blot analysis showed similar trends in vimentin, α-SMA as the real-time PCR results, but not as significantly different as the real-time PCR results on Collagen I, Collagen III (Figure 6). This might be due to the different timing of Western blot analysis test and the real-time PCR. In the Western blot analysis test, cells were cultured for one additional week so that enough protein could be extracted. It is possible that more Collagen I, Collagen III may have been expressed.
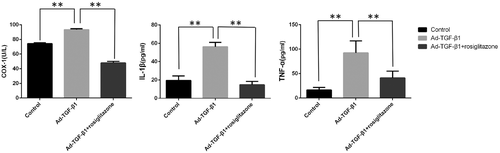
The ELISA test results showed that TGF-β1 overexpression could increase the inflammatory cytokines in the shoulder fluid of rats, while rosiglitazone treatment could reduce the production of inflammatory cytokines. The expression of COX 1, IL-1β, and TNF-α was all significantly higher than the control group and treatment group, while no significant difference was found between the control group and treatment group (Figure 7).
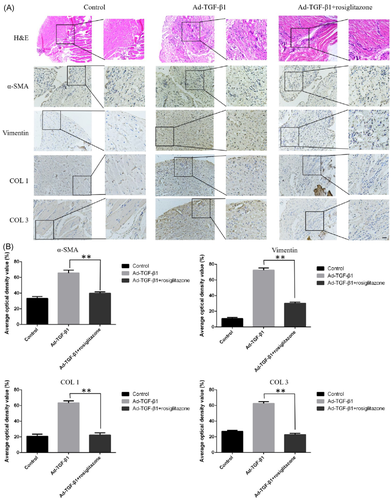
H&E staining and immunohistochemistry tests showed that in the control group, no expression of α-SMA and vimentin was observed (Figure 8). There was no proliferation of fibrocytes, and the fiber structure was orderly. In the experimental group, TGF-β1-induced proliferation of fibrocytes and inflammatory cells, neovascularization, and fiber structure disorder were observed. In the rosiglitazone treatment group, the fiber structure was comparable to the experimental group, the inflammatory response was reduced, and the expression of α-SMA and vimentin was decreased (Figure 8A). The results showed that TGF-β1 overexpression induced the inflammation of shoulder joint capsule inflammation in rats, increased matrix protein, disrupted arrangement of fibrous tissue, and fibroblast hyperplasia. Rosiglitazone treatment would inhibit the inflammation in frozen shoulders, reduce matrix protein production, and reconstruct fibrous tissue structure.
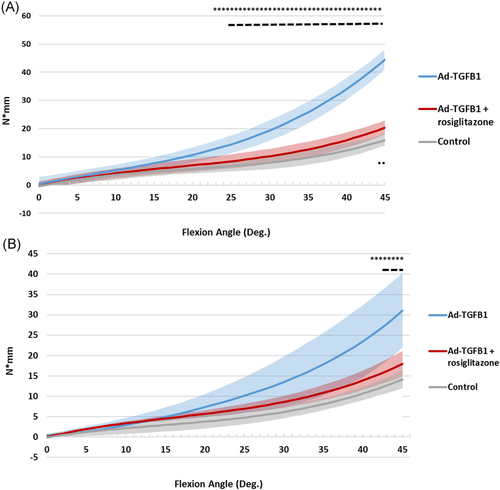
The biomechanical test showed that at the same external/internal rotation angle, the TGF-β1 induced experimental group required greater torque than the control group and the rosiglitazone treatment group. In the external rotation test, significant differences were found between the experimental group and the control group beyond 22° external rotation, and between the experimental group and the treatment group beyond 24° external rotation (Figure 9A). Significant differences were only found between the control group and the treatment group beyond 44° external rotation (Figure 9A). A similar trend was observed in the internal rotation tests (Figure 9B). Significant differences were found between the experimental group and the control group beyond 40° external rotation, between the experimental group and the treatment group beyond 42° external rotation (Figure 9A). No significant differences were found between the control group and the treatment group, suggesting that PPAR-γ agonists relieve shoulder stiffness in TGF-β1 induced frozen shoulders in rats (Figure 9).
 denotes p < .05 between Ad-TGFB1 Group and Ad-TGFB1 + rosiglitazone group,
denotes p < .05 between Ad-TGFB1 Group and Ad-TGFB1 + rosiglitazone group,  denotes p < .05 between control group and Ad-TGFB1 + rosiglitazone group). TGFB1, transforming growth factor-β [Color figure can be viewed at wileyonlinelibrary.com]
denotes p < .05 between control group and Ad-TGFB1 + rosiglitazone group). TGFB1, transforming growth factor-β [Color figure can be viewed at wileyonlinelibrary.com]4 DISCUSSION
One of the most important findings of the current study is that the typical characteristics of the frozen shoulder were observed in the rat model after applying Ad-TGF-β1 into the shoulder joints of rats. And fibrosis as well as the increase of inflammatory factors in the shoulder joint were confirmed. At present, it is believed that the contracture of shoulder capsule tissue is the main pathological change of the frozen shoulder. Its matrix is mainly composed of dense type III collagen fibers, which are rich in fibroblasts and myofibroblasts. Surgery to relieve the contracture of the shoulder capsule can reduce pain and improve shoulder joint movement.34-38 Recent studies have shown that cytokines and inflammatory cytokines play an important role in the pathogenesis of frozen shoulder.39, 40 Abnormal increases in Collagen I and Collagen III in the extracellular matrix of the frozen shoulder, mainly synthesized by fibroblasts and myofibroblasts, suggest that this condition may be abnormally regulated by cytokines.41-43 TGF-β1 is a pro-fibrotic and pro-inflammatory cytokine that plays a key role in the fibrosis of various tissues. In the current study, TGF-β1 was used to induce HSF proliferation. The results of qPCR and Western blot analysis experiments proved that TGF-β1 can cause excessive expression of Collagen I and Collagen III in the extracellular matrix. Meanwhile, RNA and protein levels of α-SMA and vimentin, markers of fibrosis, were significantly higher than those of the control group. This suggests that TGF-β1 can promote HSF proliferation and cause fibrosis. Therefore, Ad-TGF-β1 was injected into the shoulder joints of rats and successfully induced the rat frozen shoulder model. The shoulder range of motion tests showed that the experimental group had significantly limited shoulder joint motion. Meanwhile, we collected the shoulder joint fluid of rats for ELISA assay, and the results showed that TGF-β1 overexpression could cause an increase of inflammatory factors in the shoulder joint fluid of rats. We also demonstrated through immunohistochemical experiments that in the experimental group, there was a large number of fibrocytes, with disordered fiber structure, as well as the formation of new blood vessels and an increase of inflammatory cells. We believe that the occurrence of frozen shoulder is closely related to fibrosis and inflammation. The formation of a frozen shoulder involves the hardening of tissue caused by fibrosis, which is caused by inflammation.
This study also found that PPAR-γ agonists could inhibit HSF fibrosis, and had positive therapeutic effects on the rat frozen shoulder model. Recent studies have shown that PPAR-γ activation is an important mechanism to anti-fibrosis and anti-inflammatory, and involves in the negative regulation of physiological and pathological reconstruction of the extracellular matrix of connective tissue.44-46 It was demonstrated by previous studies that PPAR-γ agonists downregulate the activity of NF-κB and Smad pathways.47, 48 PPAR-γ agonists also decrease macrophages to produce various pro-inflammatory mediators, such as IL-6, TNF-α, IL-1, and inducible nitrate oxide synthase.49, 50 Studies have been focusing on whether PPAR-γ agonists inhibit the activation of AP-1, MAPKs and Smads signaling pathway by inhibiting TGF-β1, and inhibit the activation of fibroblasts, and extracellular matrix synthesis,51-53 and also whether PPAR-γ agonists can treat chronic fibrosis diseases.54, 55 At present, relevant studies have shown that PPAR-γ agonists are effective drugs against corneal fibrosis.56, 57 However, there have been no studies on the treatment of frozen shoulder fibrosis caused by TGF-β1 overexpression with PPAR-γ agonists. Our results showed that there was an inflammatory reaction in the joint capsule of the frozen shoulder with fibrosis. PPAR-γ agonists significantly inhibited the proliferation of fibroblasts, the release of inflammatory factors and the expression of fibrosis-related proteins, showing that PPAR-γ agonists inhibited the inflammation and fibrosis process. In the animal experiment, two weeks of PPAR-γ agonists medication also significantly increased shoulder range of motion when compared with the control group. This suggests that PPAR-γ agonists can delay or reverse the formation of frozen shoulder. Therefore, PPAR-γ agonists may be a promising gene target for the treatment of the frozen shoulder. Noncytotoxic and antiproliferative effects of PPAR-γ agonists (shown in cell proliferation and scratch tests) are also an additional advantage over current treatments.
Our study has several limitations. First, in the long term, the therapeutic mechanism of PPAR-γ agonist needs to be further studied, such as its actions in the Smad pathway, which is another regulatory pathway for frozen shoulder. Second, the rat model and human skin fibroblast were used in the current study. Though the human skin fibroblast is similar to frozen shoulder capsule tissues in terms of inflammatory changes and proliferation and cell types involved,19 the effectiveness of PPAR-γ agonist might still be different. Also, though the rat shoulder is anatomically similar to the human shoulder and has been used in previous studies, more clinical research are required for the PPAR-γ agonist treatment of human frozen shoulders. Third, a safer and more effective gene delivery method is yet to be investigated and improved. New methods such as the labeled adenoviral vector could demonstrate the uptake of TGF-β1 by cells and the effectiveness of the frozen shoulder model. Lastly, different treatments were used in the cell culture model and the animal model. Rosiglitazone was chosen for animal models as it is an antidiabetic drug. Its safety for animal trials was proven. In the cell culture model, we choose CDDO-IM as an alternative PPAR-γ agonist due to the lack of cellular experimental reagents for rosiglitazone at the time of the study. Whether CDDO-IM or rosiglitazone for both the cell culture model and the animal model can repeat the result of the current study will be part of our future study.
In summary, this study showed that the PPAR-γ agonist produced a positive treatment effect on the frozen shoulder. The cell culture model indicated that the PPAR-γ agonist inhibits the proliferation, migration, and abnormal fibrosis of HSF induced by TGF-β1. The rat model showed that PPAR-γ agonists reversed the joint stiffness caused by the frozen shoulder in rat, inhibited the inflammatory response, and reduced abnormal fibrosis of fibroblasts in rat frozen shoulder models. Our findings provide a novel rationale for future studies to use PPAR-γ agonists in the clinical treatment of frozen shoulders. PPAR-γ agonists, therefore, may be a promising clinical treatment for the frozen shoulder, although more further studies are required.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (81672234, 81902303, 81902558), the Shenzhen Science and Technology Project (GJHZ20180416164801042, JCYJ20180305124912336), the Shenzhen Double Chain Project for Innovation and Development Industry supported by Bureau of Industry and Information Technology of Shenzhen (201806081524201510), and the Clinical Research Project of Shenzhen Second People's Hospital (20173357201814).