Neo-Angiogenesis, Transplant Viability, and Molecular Analyses of Vascularized Bone Allotransplantation Surgery in a Large Animal Model
Conflict of interest: None. Grant sponsor: NIH; Grant number: AR049069.
ABSTRACT
Vascularized composite allotransplantation of bone is a possible alternative treatment for large osseous defects but requires life-long immunosuppression. Surgical induction of autogenous neo-angiogenic circulation maintains transplant viability without this requirement, providing encouraging results in small animal models [1–3]. A preliminary feasibility study in a swine tibia model demonstrated similar findings [4, 5]. This study in swine tibial allotransplantation tests its applicability in a pre-clinical large animal model. Previously, we have demonstrated bone vascularized composite allotransplantation (VCA) survival was not the result of induction of tolerance nor an incompetent immune system [1]. Fourteen tibia vascularized bone allotransplants were microsurgically transplanted orthotopically to reconstruct size-matched tibial defects in Yucatan miniature swine. Two weeks of immunosuppression was used to maintain allotransplant pedicle patency during angiogenesis from a simultaneously implanted autogenous arteriovenous bundle. The implanted arteriovenous bundle was patent in group 1 and ligated in group 2 (a neo-angiogenesis control). At twenty weeks, we quantified the neo-angiogenesis and correlated it with transplant viability, bone remodeling, and gene expression. All patent arteriovenous bundles maintained patency throughout the survival period. Micro-angiographic, osteocyte cell count and bone remodeling parameters were significantly higher than controls due to the formation of a neo-angiogenic autogenous circulation. Analysis of gene expression found maintained osteoblastic and osteoclastic activity as well as a significant increase in expression of endothelial growth factor-like 6 (EGFL-6) in the patent arteriovenous bundle group. Vascularized composite allotransplants of swine tibia maintained viability and actively remodeled over 20 weeks when short-term immunosuppression is combined with simultaneous autogenous neo-angiogenesis. © 2019 Orthopaedic Research Society. Published by Wiley Periodicals, Inc. J Orthop Res 38:288-296, 2020
Current treatment options for large bone defects are associated with significant problems and complications. Vascularized bone autografts provide a good treatment option since they contain an intrinsic blood flow and remain viable over time. When compared with another common reconstructive method using cryopreserved allograft bone, their viability enables better healing, less risk of a stress fracture, and a unique ability to remodel or even hypertrophy in response to loading.1 Vascularized bone autografts such as iliac crest and fibula are available for large defects although size and shape match with most segmental defects is poor. Stability and healing are improved when combined with a cryopreserved matched allograft segment,2 but either method requires flap harvest and resultant donor site morbidity.3-5
Transplantation of living allogenic bone, a form of vascularized composite allotransplantation (VCA) would potentially combine the benefits of living bone and the ability to closely match the specific defect morphology without donor site complications. It has seldom been performed clinically, in part because allotransplant viability requires lifelong drug immunosuppression (IS). Its risks include organ toxicity, opportunistic infection, and risk of neoplasm, ethically problematic to replace a non-life-critical structure.
Previous studies have demonstrated that bone VCAs may be maintained in small animal models without the need of long-term IS by switching the circulation of the transplant from allogenic to autogenous vessels, enabled by implantation of an arteriovenous bundle (AV bundle) elevated from adjacent soft tissue and placed within the allotransplant. The allogenic nutrient vessels are repaired microsurgically at the same time, but only short-term immunosuppression is used.6-11 The immunosuppressive period allows nutrient pedicle to maintain transplant blood flow and cell viability. During this short period, angiogenesis from the AV bundle develops to provide long-term bone perfusion. Because the anatomy and physiology of small animals differ from patients, we cannot necessarily extrapolate these data to humans. Our porcine tibial defect model more closely approximates clinical use, due to similarities with human physiology as well as transplanted bone size and shape.8, 12 Additionally, VCA research should permit microsurgical transplantation of the VCA components of interest, take place across a major MHC mismatch, result in minimal morbidity of the animal and allow long-term monitoring of the transplanted.13 Miniature swine have distinct advantages for allogeneic tissue research, particularly as a bridge to clinical application. Size, anatomy, physiology, and immunology are well known and comparable to man. Most importantly, both blood type and the major histocompatibility haplotypes (swine leukocyte antigen complex) have been well defined, the latter determined by routine DNA sequencing.14, 15 This study reports transplant viability, the formation of neo-angiogenic circulation, bone remodeling, and biologic activity after transplantation in a series of bone VCAs using this methodology avoiding the need for long-term IS.
METHODS
Experimental Design
The Institutional Animal Care and Use Committee approved this study and all experiments were performed according to the established National Institutes of Health guidelines. Fourteen Yucatan miniature swine underwent orthotopic tibial bone VCA reconstruction in combination with surgical induced neo-angiogenesis and short-term immunosuppression. Seven living male Yucatan swine provided 14 vascularized tibia segments (VCAs). The allotransplant harvest, creation of the defect, transplantation, and fixation were performed largely as previously described, modified with proximal dissection of the vascular pedicle to include the superficial femoral artery and vein12 (Fig. 1). One donor provided a pair of vascularized tibial segments for transplantation: one each from the left and right hindlimb. The ipsilateral hindlimb was used in each of two recipient swine. Donor and recipient were matched by age (mean 5.8 months), size (15–35 kg) and blood type (type A). The animals were mismatched by pre-operative DNA sequence haplotyping to ensure five to ten class I and II swine leukocyte antigen (SLA) mismatches.
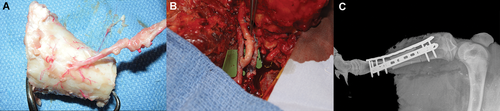
(A) 3.5 cm tibia segment with vascular pedicle showing the nutrient artery entering the graft by the nutrient foramen, (B) microsurgical anastomosis of the vascular pedicle to the femoral artery in a side-to-end fashion and the vein in an end-to-end fashion, (C) radiographic evaluation postoperative showing the reconstruction with internal fixation. [Color figure can be viewed at wileyonlinelibrary.com]
Donor VCA Harvest
A single nutrient artery supplying the proximal tibial diaphysis is consistently found on its posterior surface 1–2 cm distal to the tibial tubercle (Fig. 1A). The nutrient pedicle is a branch of the caudal interosseous artery and vein. Briefly, the tibial VCA segment is harvested through an anterolateral incision, exposing the interosseous membrane and both cranial and caudal interosseous vessels. The proximal cut is made distal to the tubercle but proximal to the nutrient foramen. The distal cut is next made with a cutting jig to ensure donor bone matching with recipient tibial defect length. Proximal dissection of the pedicle to include the superficial femoral artery and vein completed the VCA harvest. The larger diameter of the femoral pedicle simplified the arterial anastomosis and enabled an end-to-end venous anastomosis not possible with the previously described method (Fig. 1B).
VCA Transplantation
The proximal tibia was exposed through an identical anterolateral hindleg incision. A segment of the tibia was removed with the same cutting jig, from the same location as the donor VCA. A second incision in the medial thigh was used to expose the superficial femoral artery and vein, tunneling between the two incisions subcutaneously for pedicle passage. The VCA segment was placed into the defect and stabilized with dual locked compression plates (Fig. 1C), followed by end-to-side arterial and end-to-end venous anastomoses to the superficial femoral vessels. Surgical induced neo-angiogenesis was achieved by implanting an autogenous cranial tibia arteriovenous bundle (AV bundle) into the medullary canal. Group 1 had a patent AV bundle and in group 2 a ligated AV bundle as a control. All animals were randomly divided into groups and received 2 weeks of immunosuppression postoperatively. This consisted of Tacrolimus 0.6–1.5 mg/kg (Sandoz Inc., Princeton, NJ, orally), Mycophenolate Mofetil 30–60 mg/kg (Mylan Institutional Inc., Rockford, IL, orally) and Methylprednisolone sodium succinate (Pfizer Inc., New York, NY) intravenous for 2 weeks. Methylprednisolone was tapered over the immunosuppressive period. Immunosuppression levels were monitored by blood draws taken every other day from the central venous catheter. Dose adjustments were made to maintain a therapeutic level of Tacrolimus (between 5.0 and 15.0 ng/ml) and Mycophenolate (between 1.0 and 3.5 mcg/ml). Only short-term immunosuppression was used, to test the ability of autogenous angiogenesis to maintain allotransplant viability long-term. All animals received prophylactic antibiotic therapy, appropriate analgesics and were monitored by staff veterinarians. The animals were individually housed, for a planned 20-week survival period. Unrestricted weight-bearing was allowed directly after the procedure.
Sacrifice Procedure
A 20-week survival period was used in this study, chosen based upon our previous experience as sufficient to demonstrate substantial healing of the VCA segment as well as angiogenesis and resulting bone remodeling from a patent autogenous neo-angiogenic blood supply.8 Practical considerations do not permit the many months or even years likely required for complete remodeling in a large animal model, nor multiple time points with large numbers of animals at each survival period. After the 20-week survival period all animals were anesthetized with Telazol + Xylazine IM, and euthanized with intravenous administration of Pentobarbital Sodium 0.22 ml/kg (Vortech, Dearborn, MI) as recommended by the Panel on Euthanasia of the American Veterinary Medical Association and performed according to NIH guidelines under the direction of the Institutional Animal Care and Use Committee. Both femoral arteries of the animal were dissected proximately, cannulated and flushed with heparin and saline. Later micro-angiographic analysis of neo-angiogenesis was enabled by injection with a contrast agent (Microfil, MV-122; Flow Tech, Carver, MA). After 45 min of curing, both the experimental and contralateral normal tibiae were harvested with sterile technique. A 5 mm proximal segment was used for histology and a more distal 2 mm segment for gene analyses. Micro-CT angiography was performed on a 20 mm mid-VCA section. The contralateral tibia was harvested, scanned, and analyzed in the same manner for control purposes; we will refer to this as normal bone.
Histology
The selected bone segment was fixed in 10% buffered formalin for 48 h, embedded in methyl methacrylate, sectioned using a diamond band saw, ground to 15-µm-thick sections (Exact technologies Inc., Oklahoma City, OK) and stained with a Sanderson rapid bone stain (SRBS). The SRBS stained slides were used to analyze transplant viability by quantifying osteocytes, osteoblasts, and empty lacunae using light microscopy (×20, Olympus BX51) on the endosteal surface and periosteal surface (six random fields on each surface). Quantifications and calculations were done with the semi-automatic bone image analysis software (Osteomeasure; Osteometrics, Atlanta, GA). Bone viability was measured by calculating the percentage of either vacant or osteocyte-occupied lacunae using the Osteomeasure system. The percentage of occupied lacunae was calculated by dividing the number of occupied lacunae by the total (occupied and empty) lacunae X 100.
Micro CT Angiography
The 20 mm mid-VCA segment was fixed in 10% buffered formalin (48 h) and decalcified over a 7-week period in Richard-Allan Scientific™ Decalcifying Solution (Thermo Fisher Scientific, Waltham, MA). Micro-CT scanning was performed using an Inveon PET CT scanner (Siemens Medical Solutions USA, Inc., Malvern, PA) using settings of 80 kV and 500 μA and imaging software (PMOD Technologies, Zurich, Switzerland) at a medium-high magnification resolution. We used the bone microarchitecture application of AnalyzePro software (Analyze; Mayo Clinics, Rochester, MN) to measure total transplant volume, cortex volume, and medullary canal volumes. The segmentation portion of the application was used to separate the contrast-filled cortical and medullary vessels from surrounding bone. Capillary density within the allotransplants and normal tibiae were then calculated, reported as a percentage of total bone, medullary space, and cortical bone volumes occupied by vessels.
RNA Extraction, Complementary DNA (cDNA) Synthesis, and Real-Time Quantitative polymerase chain reaction (RT-qPCR)
Another 2 mm section of the allotransplant was cut with a cooled sterile oscillating bone saw. The section was cleared of excess soft tissue, snap frozen in liquid nitrogen, and stored at −80°C for later analysis. Bone sections were then individually pulverized in liquid nitrogen using the A11 basic analytical mill (IKA-Werke GmbH & Co. KG, Staufen, Germany). RNA was extracted from the pulverized bone with PureLink RNA mini kit, TRIzol reagent and an on-column Pure link DNase treatment (Thermo Fisher Scientific, Cat no. 12813018 A, 12034977, 12185-010, Carlsbad, CA). Quantification and determination of RNA purity were performed with a Nano-drop Spectrometer (ThermoScientific Nano-drop Technologies, Wilmington, DE) and absence of RNA degradation confirmed by gel electrophoreses of the total RNA before conversion to cDNA. An iScript cDNA synthesis kit (Bio-Rad Laboratories Inc., Hercules, CA) was used for the reverse transcription reaction. Total RNA (200 ng) was mixed with nuclease-free water, iScript reverse transcriptase, and 5× reverse transcription reaction mix and converted to cDNA following the iScript protocol. RT-qPCR was performed to quantify the expression of target genes with iQ SYBR green Supermix using the CFX384 Real-Time detection system (Bio-Rad, Hercules, CA). Transcript quantity measurements were normalized to GAPDH, and gene expression levels were quantified using the 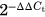 method.16 Primer sequences and genes of interest are given in Table 1 (ThermoScientific, Invitrogen, Wilmington, DE).
method.16 Primer sequences and genes of interest are given in Table 1 (ThermoScientific, Invitrogen, Wilmington, DE).
| Genes | Full name | Sequence |
|---|---|---|
| Neo-angiogenesis | ||
| VEGF-A | Vascular endothelial growth factor | 5′–3′: CTACCTCCACCATGCCAAGT 3′–5′: ACACTCCAGACCTTCGTCGT |
| EGFL-6 | Epidermal growth factor-like 6 | 5′–3′: AGATGAACGGTGGAAGATGG 3′–5′: CAGATAAAGGGCCATCTGGA |
| HIF-1A | Hypoxia-inducible factor-1α | 5′–3′: TTACAGCAGCCAGATGATCG 3′–5′: TGGTCAGCTGTGGTAATCCA |
| CD-34 | Cluster differntiation-34 | 5′-3′: GGAAACCACACCAGATGCTT 3′-5′: AGGTCTGAGGCTGGACAGAA |
| Bone formation | ||
| CTSK | Cathepsin K | 5′-3′: CGTGGCATTGACTCAGAAGA 3′-5′: CCACAGAGACAGGTCCCACT |
| RANKL | Receptor activator NF-kB ligand | 5′-3′: TCACCAAAACCAGCATCAAA 3′-5′: AAGTACGTGGCGTCTTGGTC |
| OPG | The tumor necrosis factor superfamily-11B (Osteoprotegerin) | 5′-3′: ATATCGGGCACATGAACCTC 3′-5′: GGGGAAGTGGTACGTCTTGA |
| BGLAP | Bone gamma-carboxyglutamate protein (Osteocalcin) | 5′-3′:TCACACTGCTTGCCCTACTG 3′-5′:GGGTTGAGCTCACACACCTC |
| Housekeeper | ||
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase | 5′-3′: ACACTCACTCTTCTACCTTTG 3′-5′: CAAATTCATTGTCGTACCAG |
Statistics
Since the data were collected from a low sample size (N = 14), a non-parametrical test (Wilcoxon rank sum test) was used to detect a difference between the two groups (allotransplants with Patent AV bundle versus allotransplants with ligated AV bundle). For the same reasons, a non-parametric (Wilcoxon signed-rank test) test was used to detect a difference between the operated and contra-lateral tibia. All statistical tests were two-sided and differences were considered significant for p < 0.05. Statistical analyses were performed using the statistical program JMP Pro 13.0.0 (SAS Institute Inc.) and GraphPad Prism 5.03 for illustrations (GraphPad Software, La Jolla, CA). Power calculations were made by the division of biostatistics at Mayo Clinic using nQuery Advisor for outcomes of interest, including capillary density, and osteocyte viability. This study was powered for an estimate of 80%, with the significance level set at 0.05, to detect a minimal difference between the groups, based upon our previous studies of structural orthotopic bone allotransplants in rats and rabbits.6, 7, 9 Due to complications during allotransplant harvest, only five animals in each group were included for gene analyses. Statistical analysis was supported by the Center for Translational Sciences Activities (CTSA) at Mayo Clinic.
RESULTS
Surgical Outcome
All animals were able to ambulate with partial weight bearing immediately postoperatively, with full weight bearing by the fourth postoperative day in both groups. One abscess occurred at 6 weeks with a deep infection as a result. Six weeks after transplantation, another animal developed uncontrollable seizures. Extensive treatment was without success, requiring its sacrifice. Due to these complications, the two animals were excluded from this study for analyses. Thus, a final cohort of 12 pigs remained for analysis (six in each group). No fractures were observed in these twelve animals and all united at the proximal host-allotransplant interface. Nine of the 12 VCAs united at the distal interface.
Neo-Angiogenesis
To evaluate neo-angiogenesis we performed micro-angiography to visualize the vascular pattern within the allotransplant. The micro-angiography showed that all AV bundles (N = 6) in group 1 were patent at 20 weeks. These implanted AV bundles sprouted numerous neo-angiogenic vessels within the medullary space and endosteal surface of the allotransplanted tibial segment (Fig. 2A and B). The control group (group 2) had no significant medullary vasculature. In all animals, the VCA tibial cortical bone was also supplied from its periosteal surface (Fig. 2B–D). The medullary vessel volume and calculated capillary density in the medullary proportion of the allotransplant were statistically significantly higher in Group 1 than Group 2 (p = 0.04) (Table 2). Micro-CT scanning did not have sufficient resolution to determine the relative contributions of the AV bundle, periosteal vessel ingrowth, or inosculation of vessels at the bone contact points to the significant cortical bone capillary density. Observed differences in cortical and total bone vessel density were not significant.
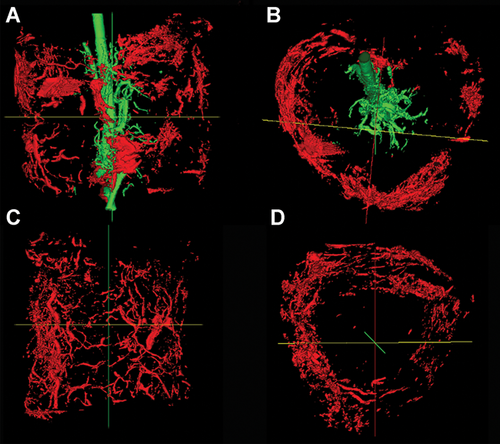
Three-dimensional (3D) reconstruction of the micro-angiography of the allotransplant showing the patent arteriovenous (AV) bundle (green) in the medullary canal with neo-angiogenesis (A + B) in the longitudinal (A) and transverse view (B), in the ligated AV-bundle group showing only cortical vascularity (C+D) in red. [Color figure can be viewed at wileyonlinelibrary.com]
| Group I (AV+) (n = 6) | Group II (AV−) (n = 6) | p value I-II | |
|---|---|---|---|
| Total vessel volume (%) | 1.50 (0.45–2.18) | 1.10 (0.87–1.72) | 0.75 |
| Medullary vessel volume (%) | 1.18 (0.49–3.84) | 0.19 (0.05–0.61) | 0.04* |
| Cortical vessel volume (%) | 1.45 (0.45–1.72) | 1.31 (0.95–2.27) | 0.63 |
- Values are given in median and interquartile range.
- * Significant.
Transplant Viability
The osteocyte count expressed as a ratio or percentage is defined as the number of lacunae occupied by an osteocyte divided by the sum of empty and occupied lacunae in a microscopic field, and is a standard means to assess bone viability. In this study, we measured the osteocyte count on the endosteal cortical surface in both experimental groups and also in the contra-lateral normal tibia. Bone viability was higher with a patent AV bundle (p = 0.04) (Table 3). No statistically significant differences were found between the two intervention groups in the number of osteoblasts. However, we did observe an increase in osteoblasts filling the endosteal surface of allotransplants in the patent AV-bundle group (Fig. 3B). No statistically significant differences were found on the periosteal surface of the bone.
| Contralateral (n = 12) | Group I (AV+) (n = 6) | Group II (AV−) (n = 6) | p value cl-I | p value cl-II | p value I-II | |
|---|---|---|---|---|---|---|
| N.Ob/B.pm (/mm2) | 10.68 (7.58–16.19) | 19.67 (15.21–22.50) | 13.62 (7.43–28.09) | 0.005* | 0.56 | 0.52 |
| Occupied Lacunae (%) | 83.98 (81.61–88.63) | 88.24 (84.98–90.03) | 78.35 (70.35–86.90) | 0.15 | 0.22 | 0.04* |
- Values are given in median and interquartile range. Osteoblasts (N.Ob), Bone parameter (B.pm).
- * Significant.
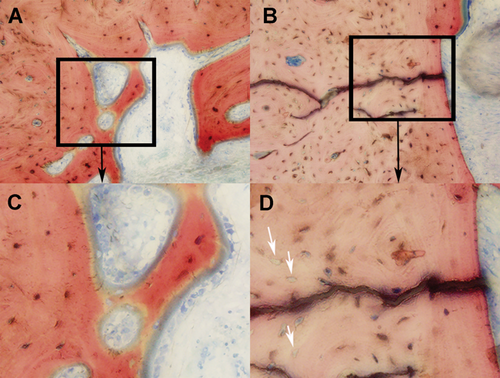
Representative histologic Sanderson rapid bone stain (SRBS)-images of the endosteal surface of the allografts with respect to the arteriovenous (AV)-bundle patency at ×20 magnification. (A + C) allotransplant showing good osteocyte viability and osteoblast lining in the patent AV-bundle group, (B + D) Allotransplant with visible empty lacunae (white arrows) and a reduced amount of bone surface filled with osteoblasts in the control group (group 2). [Color figure can be viewed at wileyonlinelibrary.com]
GENE EXPRESSION ANALYSES
Bone Homeostasis and Remodeling Markers
To examine the biological effect of vascularized allotransplantation on the bone homeostasis, we compared the expression of important osteoblast and osteoclast genes in normal bone and in our allotransplant groups. The Tumor Necrosis Factor superfamily of ligands (TNFSF11/osteoclast marker) and receptors (TNFRSF11B/osteoblast marker) provide key paracrine communication signals for the differentiation of osteoclasts.17 These genes are also known as Receptor Activator of Nuclear factor Kappa-B Ligand and Osteoprotegerin (RANKL/OPG).18 This interplay mirrors the important role of osteoblasts on osteoclast differentiation.19 Cathepsin K (CTSK), which encodes a lysosomal cysteine protease involved in bone remodeling and resorption, is predominantly expressed by osteoclasts. Bone Gamma-Carboxyglutamate Protein (BGLAP), also known as osteocalcin, is a protein secreted by osteoblasts that regulate bone remodeling and energy metabolism.17 Although we did find numerical differences between the groups, differences in important osteoclast (CTSK) and osteoblast (BGLAP) markers were not statistically significant. There were neither statistically significant nor biologically relevant differences in biomarkers for bone homeostasis (RANKL, OPG) and bone remodeling (CTSK, BGLAP) between the groups or compared to normal bone. Thus, bone homeostasis and remodeling appear to be comparable between treatment groups as measured by gene expression analyses (Fig. 4).
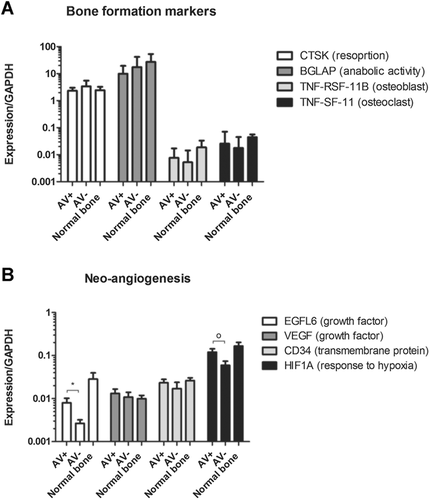
(A) Expression of bone formation markers, (B) expression of neo-angiogenesis markers with significantly (*p = 0.03) higher expression of EFGL-6 in the patent AV-bundle group (AV+) and a trend O (p = 0.07) toward higher HIF-1A expression in the AV+ group. Values are given in median and interquartile range.
Neo-Angiogenesis Markers
We examined the biological effect of different genes associated with neo-angiogenesis. Endothelial growth factor-like 6 (EGFL6), which is a member of the epidermal growth factor (EGF) repeat superfamily, promotes endothelial cell migration and angiogenesis by activation of extracellular signal-regulated kinase. In bone, EGFL6 mediates cross-talk between endothelial cells and osteoblasts by this mechanism.17, 20 Hypoxia-inducible factor 1 alpha (HIF1A) is a master regulator of cellular response to hypoxia in tissues. Vascular endothelial growth factor A (VEGFA) is one of the most important growth factors for regulation neo-angiogenesis. Cluster differentiation-34 (CD34) is a transmembrane protein on the vascular associated tissue.
A biologically and statistically significant (p = 0.03) increase in the expression of EFGL6 was found for the patent AV-bundle group (Fig. 4). In addition, a trend toward higher expression of HIF1A was found for the implementation of an AV bundle (p = 0.07) (Fig. 4). VEGFA and CD34 were expressed in all groups with no median significant difference between the groups. EGFL-6 and VEGFA expression in allotransplants are correlated to the intramedullary and total vessel volumes measured with micro-angiography (VEGFA correlation coefficient: 0.80, p ≤ 0.001, EGFL-6 correlation coefficient: 0.70, p = 0.03) (Fig. 5).
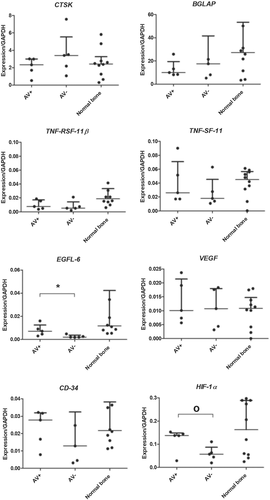
Supplemental polymerase chain reaction (PCR) data, with individually plotted relative expression ratios for each animal. *statistically significant (p < 0.05), O = trend toward difference between groups (p = 0.07).
DISCUSSION
The ideal reconstructive method for large bone defects is one that functions immediately and is biologically equivalent to the missing bone segment. It should provide immediate strength, permit stable internal fixation, heal promptly, and remain viable to resist infection while resisting late structural failure by active bone remodeling. Transplantation of living allogenic bone is a potential candidate for such a “perfect” solution—but only if the appreciable risks of long-term immunosuppression can be avoided. We have sought to replace the allogenic bone nutrient blood supply with autogenous neo-angiogenic blood supply to allow survival with only short-term immunosuppression. This has shown promise in prior small animal studies from our laboratory but required a large animal study before consideration of this methodology in clinical practice. This porcine orthotopic tibial defect model was developed for this purpose.8, 21 Our study which reports results at 20 weeks after transplantation surgery provides further insights into the potential future clinical utility of our method for bone vascularization.
In long bones, approximately 70% of the bone is vascularized by longitudinal endosteal blood vessels, and the remaining 30% is provided by periosteal blood supply.3, 22 A vascularized bone autograft, such as the fibular free bone flap will maintain blood flow long-term, while a bone VCA pedicle will thrombose without sustained immunosuppression.6-8, 23 A means to maintain VCA bone blood flow and viability without the risks and expense of long-term immunotherapy is desirable for such non-life-critical VCA reconstructions. In small animal models, we have shown surgically induced autogenous neo-angiogenesis to maintain bone VCA viability in rats with a competent immune system, without induction of donor-specific tolerance.6-8, 21 Maintenance of viability and perfusion in VCA segments placed across a swine tibial defect provides further encouragement for possible translation to a clinical setting.
The implantation of a patent AV bundle within the bone is hypothesized to develop a neo-angiogenic autologous circulation in the allotransplant during the immunosuppressive period, as we have seen in rat and rabbit bone VCA allotransplants.6, 7, 24, 25 This is expected to maintain bone viability despite the expected thrombosis of the allogenic nutrient pedicle following stoppage of drug immunosuppression. In our initial studies of bone VCA survival, rat femoral allotransplants were placed heterotopically into an abdominal pocket and enveloped in silicone sheeting to block the effect of soft-tissue ingrowth. This was done primarily to avoid masking the effect of medullary neo-angiogenesis by inosculation or ingrowth of periosteal new vessels from surrounding soft tissues.6, 7 The effect on capillary density and bone blood flow was significant. The effect is likely obscured without blocking ingrowth of vessels from non-AV bundle sources. In this study, neo-angiogenesis in the medullary canal was only seen in Group 1, due to sprouting of vessels from the implanted cranial interosseous vascular pedicle as in small animal studies. No significant medullary vessels were seen in Group 2 vessels with a ligated AV bundle. These observed differences were significant. Evaluation of total and cortical bone vessel volume must include the substantial effect of well-vascularized tissue surrounding the VCA segment on the periosteal surface. The effect of the AV bundle in Group 1, placed within the medullary canal, will be seen primarily in the medullary space and its major effect expected to be on endosteal bone surfaces, at least in the first few weeks or months following transplantation. This is in fact what we found; the increased medullary vessel volume in Group 1 had a significant positive effect on bone vitality on the endosteal surface of the allotransplant. Additionally, The implantation of an AV bundle seems to have a positive biological effect on neo-angiogenesis (EGFL-6, HIF-1A, VEGFA) markers measured with gene analyses. Gene expression values (EGFL-6, VEGFA) correlated with the measured vessel volumes in the allotransplant. The effect becomes less obvious when summed with the total bone and cortical bone volume. Contact of the periosteal surface with well-vascularized soft tissue and/or inosculation at the VCA contact points with normal bone are the likely explanations for this observation.
While heterotopic bone VCA implantation was useful to measure angiogenesis, orthotopic placement across a weight-bearing hindlimb segmental defect is a more clinically relevant experimental setting. In prior studies of rabbit femora and a feasibility study in swine tibiae, immediate weight-bearing and unrestricted motion have been allowed for practical considerations.8, 26 Rigid internal fixation has been used to minimize their effect on bone healing. It is likely, however, that the three distal nonunions observed were the result of the postoperative activity. Proximally, the larger amount of cancellous bone, thinner cortical bone, and a more stable broad contact area allowed rapid healing. The thick, less well-vascularized cortical bone at the distal junction site would not be expected to heal at the same rate nor with the same success. Survival times, although longer than many VCA studies, do not approach the many months or even years potentially required to evaluate the final results in large structural bone allotransplants. Both survival time and postoperative limb use are likely significant factors in the outcomes we have reported for not only bone healing, but also bone viability. Osteocyte counts demonstrated greater endosteal bone viability in the patent AV-bundle VCAs.
Angiogenesis is required for bone development, growth, and repair. It is influenced by the local bone environment that involves cross-talk between endothelial cells and adjacent bone cells.20 The role of EGFL6 has been described in calvarial osteoblastic cell culture (ex vivo).20 EGFL-6 plays an important role in this cross-talk and promotes endothelial cell migration and angiogenesis. Although gene expression literature on angiogenesis markers after allotransplantation is scarce, we have shown significantly higher expression of EGFL-6 in our patent AV-bundle group. The higher EGFL-6 expression may have contributed to a higher medullary vessel volume since we found a significant association between neo-angiogenesis and EGFL-6. CD34 is a transmembrane protein in vascular associated tissue, therefore, it is a parameter of the amount of vascular tissue when quantified by RT-qPCR. Quantification of total vessel volume showed a slightly higher volume of vessels (0.40%) in the patent AV-bundle group (Group 1). CD34 levels were not significantly greater in Group 1, although it would be expected to correlate with the amount of vascular tissue present. This is likely due to the amount of variation found in CD34 expression not only in the VCAs but also the normal contralateral bone. The insignificant differences in the expressions found between the two intervention groups for all genes studied other than EGFL-6 is a limitation of the study.
The discovery of the RANKL/OPG system and its role in the regulation of bone resorption has been relatively well-described in literature since the 1990s.18, 19, 27 In our results, the implantation of an AV bundle does not seem to have a positive biological effect on bone resorption when we look at the RANKL/OPG interplay and the excretion of CTSK by osteoclasts when we compare them between the two intervention groups. We monitored putative bone anabolic activity using BGLAP gene expression as a biomarker. Overall, Group 1 and 2 molecular biomarkers for bone homeostasis were not statistically different. It is possible that technical issues may be part of the explanation, including the difficulty of acquisition of RNA samples, and the complexities of RT-qPCR. Further research should analyze the bone homeostasis, bone remodeling, and neo-angiogenesis by gene expression using endosteal and periosteal surfaces separately.28
Our previous results in small animals have shown neo-angiogenesis from the AV bundle reached the outer cortex in bone clearing studies.6, 7, 26 Bone clearing protocols to visualize the contrast agent macroscopically have generally failed in larger animal models due to the thickness of the bone. Micro-angiographic quantification of the vasculature with micro-CT provides a good alternative, although it is a demanding and slow process. Microfil contrast fills the microvasculature but unfortunately has the same Hounsfield unit density as a mineralized bone. Micro-CT after decalcification provides reasonably good imaging of microvasculature in bone but proved unable to image the contrast agent from the AV bundle into the cortical bone to its periosteal surface (Fig. 2). Microfil contrast agent was found in endosteal and periosteal bone surfaces in Group 1, but not on the endosteal surface of Group 2.
CONCLUSION
Large bone-only VCAs were used successfully to reconstruct segmental tibial defects in a miniature swine model. Instead of long-term immunosuppression, an autogenous neo-angiogenic circulation was developed by placing an AV bundle within the medullary canal with only 2 weeks' immunosuppression. In this experimental study, we found significantly higher amounts of medullary vessels in the patent AV-bundle group at 20 weeks post-transplantation. Endosteal osteocyte counts were highest in this group. A significant increase in the expression of endothelial growth factor-like 6 (EGFL-6) was also observed, with a positive correlation with measured vessel volumes. Further study is needed to fully understand the long-term behavior of VCA, using larger sample size and longer survival periods.
AUTHORS' CONTRIBUTION
A.T.B. was the principal investigator with overall responsibility for planning, funding, and supervising the study, with significant contribution from D.K., R.H.H., and P.F.F. A.T.B., A.Y.S., R.H.H., D.K., and P.F.F. participated in the surgical transplantation procedures. R.H.H., P.F.F., and R.T. did the experimental follow-up and collection of the samples. R.H.H. and R.T. performed all the analyses of the data. R.H.H., A.T.B. and wrote the manuscript. A.Y.S., A.v.W., D.K., and P.F.F. revised the manuscript. All authors have read and approved the final submitted manuscript.
ACKNOWLEDGMENTS
These studies were supported by the National Institute of Health (NIH grant: 5R01AR049718). Additional support was provided by NIH grants AR049069 (to AJvW). The authors would like to thank Mrs. T. Decklever from the Mayo Clinic Nuclear Medicine Research Core for her contribution to all the micro-CT imaging. We would like to thank Mr. R.A. Brown of the Mayo Clinic Bone Histomorphometry Core Laboratory for his dedicated contribution to the histological processing and staining of our bone samples. We thank Mr. T.J. Dockter from the Center for Clinical and Translational Science (CCATS) at Mayo Clinic for his statistical help.




