Analgesic Effect of Duloxetine on an Animal Model of Monosodium Iodoacetate-Induced Hip Osteoarthritis
Conflicts of interest: None.
ABSTRACT
We investigated the efficacy of duloxetine on hyperalgesia, histopathological and radiographic findings, pain-related sensory innervation of dorsal-root ganglia (DRG), and spinal changes in a rat model of induced hip osteoarthritis (OA). The right hip joints of male Sprague–Dawley rats (n = 6 rats/group) in the Sham group were injected with 25 μl of sterile saline and 25 μl of sterile saline with 2 mg of monosodium iodoacetate (MIA) were injected to the MIA + Vehicle and MIA + Duloxetine groups. We injected duloxetine 20 mg/kg intraperitoneally in the MIA + Duloxetine group 28 days after injection, whereas rats in the MIA + Vehicle group were injected with 0.5 ml of 20% dimethyl sulfoxide. We assessed hyperalgesia, histopathological changes, immunoreactive (-ir) neurons for calcitonin gene-related peptide and activating transcription factor 3 in DRG, and immunoreactive neurons for ionized-calcium-binding adaptor molecule 1 (Iba1) in the dorsal horn of the spinal cord. MIA administration into the hip joint let to mechanical hyperalgesia of the ipsilateral hind paw (p < 0.05). A single injection of duloxetine significantly attenuated it in induced hip OA (p < 0.05) and suppressed the number of Iba1-ir microglia of the ipsilateral dorsal horn (p < 0.05). These results suggest that a single injection of duloxetine suppressed mechanical hyperalgesia and may influence the expression of Iba1 in the microglia of the ipsilateral dorsal horn in the MIA-induced hip OA. This finding implies the inhibitory effects of duloxetine against neuropathic pain, which may lead to a change of microglial activities. © 2019 Orthopaedic Research Society. Published by Wiley Periodicals, Inc. J Orthop Res 38:422-430, 2020
Osteoarthritis (OA) is one of the major musculoskeletal disorders that affects at least 50% of the elderly population,1 with hip and knee OA ranked as considerable contributors to global disability.2 The main symptom in OA pathologies is pain, which occurs more commonly than stiffness or disability.3 In many countries, the increasing prevalence of OA is causing an increasing number of individuals with chronic pain, which creates a huge burden. Nevertheless, the pathophysiology of joint pain received little attention for many years, compared with extensive research on inflammation and immunity in joint diseases,4 and many important research questions remain unresolved. Many patients suffer from pain at rest or at night despite lower mechanical stress. Some patients complain about referred pain, which is perceived at a location other than the site of the painful stimulus. Referred pain is difficult to be treated clinically. Several studies report advanced OA pain in end-stage OA, which is characterized as ongoing pain that persists during rest and is resistant to nonsteroidal anti-inflammatory drugs (NSAIDs).5-7 In patients with OA pain, joint pathology does not necessarily correspond to the degree of pain reported. It is not surprising that up to 40% of individuals with radiographic damage have no pain and patients with minimal and even non-radiographically detectable cartilage abnormality exhibit significant, debilitating pain.8 The mechanisms of OA pain are not still fully understood. Because chronic OA pain is often inadequately treated, many patients are determined to undergo joint replacement surgeries to relieve pain.5-7 Therefore, the improved understanding of the mechanisms of chronic OA pain is needed for the development of new effective therapies.
Recently, many studies on neuropathic pain in OA have been reported.7, 9, 10 Patients who regularly reported moderate to severe pain present signs of central sensitization, such as referred pain and allodynia.11 The pain relief effect of NSAIDs for refractory pain gradually attenuates with long-term use.12 A recent study supports possible neuropathic pain occurs in a notable proportion of patients with end-stage hip and knee OA and is more strongly associated with pain at rest than pain on activity. Clinical presentation of pain at rest may warrant more thorough evaluation for potential neuropathic pain and may have implications for appropriate pain management.13 With regard to the pain mechanism for hip OA, we have reported an animal model of monosodium iodoacetate (MIA)-induced hip OA and evaluated the expression of calcitonin gene-related peptide (CGRP) and activating transcription factor 3 (ATF3) in the dorsal-root ganglia (DRG) and the expression of the ionized-calcium-binding adapter molecule 1 (Iba1) in the spinal cord.14, 15 In MIA-induced knee OA model in rats, some studies reported duloxetine attenuated the neuropathic pain.16, 17 To the best of our knowledge, no report investigated the effect of duloxetine on neuropathic pain and its mechanism in hip OA animal model to date.
In the clinical situation, duloxetine attracts attention because of its analgesic effect on chronic musculoskeletal pain including OA in the United States and other countries. Duloxetine is classified as a serotonin and norepinephrine reuptake inhibitor (SNRI) with antidepressant, central pain inhibitory, and anxiolytic activities. The Osteoarthritis Research Society International (OARSI) guidelines recommend duloxetine as treatment for multiple-joint OA with or without comorbidities.18 Some RCTs and meta-analyses revealed the efficacy and safety of duloxetine for OA pain.19-21 Moreover, the SNRIs, including duloxetine, are regarded as the first therapeutic agents for neuropathic pain.22 Considering these facts, duloxetine is reported to affect not only the descending pain modulatory system but also the microglia directly in the dorsal horn of the spinal cord in recent years.23
This study aimed to investigate the efficacy of duloxetine on hyperalgesia, histopathological and radiographic findings, and pain-related sensory innervation of the DRG and spinal changes by means of immunohistochemistry in the MIA-induced hip OA model. This study examined for the first time the effect of duloxetine on the spinal expression of microglia in an animal hip OA model as a possible mechanism involved in its curative effect.
METHODS
All research protocols in this study were reviewed and approved by the ethics committee of our institution and were performed in compliance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (2010 revision).
Intra-Articular Injection of MIA and Retrograde Neurotracing
Male, 6-week-old, Sprague–Dawley rats, weighing 200–300 g, were prepared (CLEA, Tokyo, Japan). The rats were housed in a semi-barrier system with a controlled environment (12 h/12 h light/dark cycle; temperature: 21–23°C; humidity: 45–65%). Animals were given free access to food and water upon arrival to the facility. All rats were fed a diet of standard rodent chow (CRF-1; Oriental Yeast Co., Ltd., Tokyo, Japan). On the basis of methods previously published,24 all rats were anesthetized with an intraperitoneal injection of 0.3 mg/kg of medetomidine, 4.0 mg/kg of midazolam, and 5.0 mg/kg of butorphanol and treated aseptically throughout the experiments. Using a 27-gauge needle, the following solutions were injected into the right hip joint of each rat using the posterior approach25, 26: 25 µl of sterile saline with 2 mg of MIA (Sigma-Aldrich, St. Louis, MO) and 1% of retrograde neurotracer fluorogold (FG; Fluorochrome, Denver, CO) in the 12 rats, and 25 µl of sterile saline with 1% FG in the six rats (Sham group). The injected agent was confirmed to be restricted to the joint cavity. The MIA dose was chosen based on previously published protocols.14, 25, 27 On day 28 post-induction, the 12 MIA-induced rats were divided into the MIA + Duloxetine group, wherein the rats were injected intraperitoneally with duloxetine (20 mg/kg/day, n = 6); and the MIA + Vehicle group, with vehicle (0.5 ml 20% dimethyl sulfoxide, n = 6). The rats in the Sham group were administered with vehicle alone (n = 6). Duloxetine was dissolved in 0.5 ml 20% dimethyl sulfoxide and its dose was determined based on previous studies. Hence, 20 mg/kg has been shown to result in 80% serotonin receptor occupancy, which is comparable to clinically used doses in humans.28, 29
Behavioral Tests
Six rats from each group were used for behavioral testing. The rats were randomized and acclimated to the test chamber for 1 h before performing the behavioral tests. Baseline thresholds were obtained before MIA induction (day 0). On day 28 post-induction, behavioral testing was performed before and 1 and 3 h after intraperitoneal administration of duloxetine or vehicle. Calibrated von Frey Filaments (Mono-filament Kit; Smith & Nephew, Germantown, WI) were applied for 4 s or until withdrawal (whichever occurred first), and the 50% paw withdrawal threshold (PWT, g) was calculated.30 The stimulus intensity ranged from 1 to 60 g, corresponding to filament numbers (4.08, 4.17, 4.31, 4.56, 4.74, 4.93, 5.07, 5.18, 5.46, and 5.88). For each animal, the actual filaments used within the aforementioned series were determined on the basis of the lowest filament to evoke a positive response followed by five consecutive stimulations using the up–down method.30, 31 The filament range and mean interval were incorporated with the response pattern into each individual threshold calculation. Mechanical hyperalgesia was assessed on the plantar surface of the right (ipsilateral) hind paw through wire-mesh observation cages. Data were presented as 50% PWT for each group ± standard error of the mean.
Tissue Preparation and X-Ray Imaging of the Hip Joint
Tissue preparations and X-ray imaging of the hip joint were performed as previously described by Miyamoto et al.25 After intraperitoneal anesthesia (0.3 mg/kg of medetomidine, 4.0 mg/kg of midazolam, and 5.0 mg/kg of butorphanol), the rats were laid in the supine position with 0° of hip flexion, abduction, and internal and external rotation. Anteroposterior bilateral X-rays of the hips were taken with an in vivo imager (Xtreme, Bruker, WI). Lateral images were taken at 45° of flexion and abduction and 0° of internal and external rotation. For histological evaluation, the samples were obtained after intraperitoneal administration of duloxetine or vehicle on day 28. The rats were intraperitoneally anesthetized by means of 0.3 mg/kg of medetomidine, 4.0 mg/kg of midazolam, and 5.0 mg/kg of butorphanol, and were perfused transcardially with 0.9% saline, followed by 500 ml of 4% paraformaldehyde in phosphate buffer fixative (0.1 M, pH 7.4). The soft tissues around the right hip joint including cartilage, synovium and capsule were resected. The resected limbs were cut at midfemur and the center of the femoral head immersed in 10% neutral buffered formalin for 3 days. The specimens were continuously demineralized in reagent K-CX (FALMA, Tokyo, Japan) for 30 h and 5% sodium sulfate for 16 h, followed by paraffin embedding for subsequent coronal sectioning. The samples were serially sectioned in steps of 8 µm, and stained using hematoxylin and eosin, Safranin-O, and Toluidine blue, respectively. Osteoarthritic changes were evaluated using the OARSI histopathology score.32 For each joint, 10 slices centered on the maximum diameter of the femoral head were scored. Each sample was assessed by the depth (Grading) and width (Staging) of the osteoarthritic changes, respectively, and the score was finally expressed by multiplication of the depth and width. The average scores of each group were compared among the groups.
Immunohistochemistry of the DRG and Spinal Cord Specimens
The samples were prepared after the behavior test on day 28 in six rats of each group. After the transcardiac perfusion with 0.9% saline, followed by 500 mL of 4% paraformaldehyde in phosphate buffer (0.1 M, pH 7.4), the right DRGs of L4 and spinal cord at the level of the lumbar enlargement were resected and prepared for immunohistochemistry, which was considered as the ipsilateral side. The DRG and spinal cord specimens were immersed in phosphate-buffered paraformaldehyde overnight at 4°C. They were frozen in liquid nitrogen after storage in 0.01 M phosphate-buffered saline (PBS) containing 20% sucrose for 20 h at 4°C. Using a cryostat, the DRG and spinal cord were sectioned at 10 and 30 µm, respectively (CM3050S; Leica Microsystems, Wetzlar, Germany). Subsequently, the sections were mounted on poly-l-lysine-coated slides. The specimens were then treated at room temperature with a blocking solution for nonspecific binding sites, consisting of PBS containing 0.3% Triton X-100 and 3% skim milk for 90 min. They were processed for CGRP immunohistochemistry using rabbit antibody against CGRP (1:1,000, Cat# PC205L; Chemicon, Temecula, CA) or for ATF3 immunohistochemistry using a mouse antibody against ATF3 (1:50, Cat# sc-81189; Santa Cruz, Delaware, CA). The spinal cord specimens were processed by using a rabbit antibody against Iba1 (1:1,000, Cat# 019-19741; Wako, Osaka, Japan). After incubation with the diluted antibodies for 20 h at 4°C, DRG sections were incubated with Alexa 488-conjugated goat anti-rabbit IgG (for CGRP immunoreactivity, 1:1,000; Molecular Probes, Eugenem, OR) and Alexa 594-conjugated goat anti-mouse IgG (for ATF3 immunoreactivity, 1:1,000; Molecular Probes). The spinal cord specimens were incubated with Alexa 488-conjugated goat anti-rabbit IgG (for Iba1 immunoreactivity, 1:1,000). After each step, the sections were rinsed thrice in PBS. The immunostained sections were observed using a fluorescence microscope (Olympus, Tokyo, Japan), in a treatment-blinded manner. The numbers of FG-labeled CGRP-ir DRG neurons and FG-labeled ATF3-ir DRG neurons were counted by blinded observers, and their proportion to the total number of FG-labeled DRG neurons was calculated for each DRG sample. The number of Iba1-ir dorsal horn neurons per 0.1 mm2 was counted using a counting grid. Three skilled observers evaluated positive staining. Results were only accepted if the counts of two of the three observers were within the agreement of each other (i.e., 95% of each other).
Statistical Analysis
Statistical analysis was performed using JMP® Pro 13 (SAS Institute Inc., Cary, NC). The PWT, OARSI scores, proportions of CGRP-ir, and ATF3-ir FG-labeled neurons in the DRGs and the number of Iba1-ir neurons among groups were compared by using a Steel–Dwass test. A p-value < 0.05 was considered significant.
RESULTS
Behavioral Tests
Compared with the Sham group, the pain behavioral tests showed that rats in the MIA + Vehicle and MIA + Duloxetine groups showed significant mechanical hyperalgesia on day 28 post-induction (Fig. 1, Pre, p < 0.05). Compared with the MIA + Vehicle group, significant increases in the PWT on the ipsilateral side in the MIA + Duloxetine group were observed at 1 and 3 h after the intraperitoneal administration of duloxetine (post 1 h, post 3 h, p < 0.05).
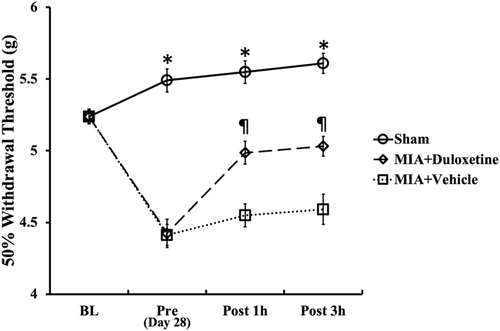
Effect of intraperitoneal administration of duloxetine on the withdrawal threshold of the pressure applied to the hind paw on the ipsilateral side. Changes in hyperalgesia determined using von Frey filaments and expressed as the 50% withdrawal threshold in grams (g). Behavioral testing was performed before the monosodium iodoacetate (MIA) administration (baseline, BL); then, the animals were injected with 2.0 mg of MIA (MIA + Vehicle and MIA + Duloxetine groups) or saline (Sham group) and tested before and 1 and 3 h after intraperitoneal administration of duloxetine or vehicle (pre, post 1 h, and post 3 h) on day 28. Lower thresholds indicate increased hyperalgesia. Data are expressed as the mean ± standard error of the mean for six rats in each group. *p < 0.05 compared with the MIA + Vehicle and MIA + Duloxetine groups, p < 0.05 compared with the MIA + Vehicle group.
Histopathological and Local X-Ray Findings
The MIA + Vehicle and MIA + Duloxetine groups presented progressive OA changes, whereas the Sham group maintained a normal appearance (Fig. 2). The MIA + Vehicle and MIA + Duloxetine groups showed flattening of the femoral head, extensive area of cartilage loss, degeneration and fibrosis, subchondral bone collapse, and decreased cartilage matrix (Fig. 2E–L). Significant differences in the OARSI score were observed on day 28 post-induction (Fig. 3; Sham: 2.8 ± 1.6; MIA + Vehicle: 22.7 ± 1.9; MIA + Duloxetine: 23 ± 1.7). Compared with the Sham group, OA changes were significantly apparent in the MIA + Vehicle and MIA + Duloxetine groups (p < 0.01). No significant difference was found between the MIA + Vehicle and MIA + Duloxetine groups.
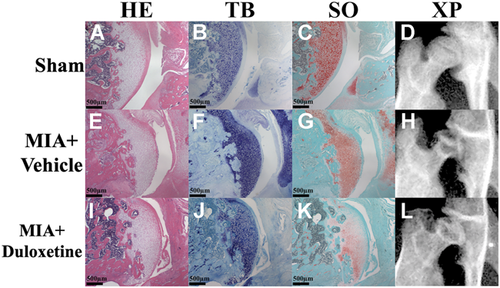
Histopathology and radiograph of the hip in the Sham (A–D), monosodium iodoacetate (MIA) + Vehicle (E–H), and MIA + Duloxetine (I–L) groups after intraperitoneal administration of duloxetine or vehicle on day 28. Hematoxylin and eosin (HE; A, E, and I), Toluidine blue (TB; B, F, and J), and Safranin-O (SO; C, G, and K) staining, anteroposterior radiographs of the right hip (XP; D, H, and L). The MIA + Vehicle and MIA + Duloxetine groups show flattening of the femoral head, extensive area of cartilage loss, degeneration and fibrosis, subchondral bone collapse, and decreased cartilage matrix in (E–L). [Color figure can be viewed at wileyonlinelibrary.com]
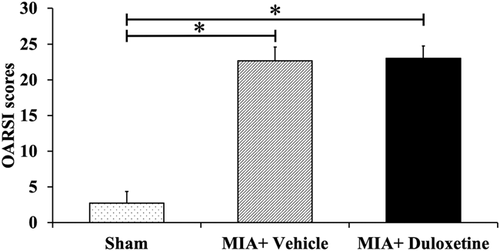
Graphs showing Osteoarthritis Research Society International (OARSI) scores in each group. Osteoarthritic changes are apparent in the monosodium iodoacetate (MIA) + Vehicle and MIA + Duloxetine groups compared with the Sham group. Data are expressed as the mean ± standard deviation for six rats in each group. *p < 0.01.
Immunohistochemistry
Expression of CGRP and ATF3-ir Neurons
On day 28, the MIA + Vehicle and MIA + Duloxetine groups presented significantly higher expressions of FG-labeled CGRP-ir and FG-labeled ATF3-ir DRG neurons in L4 than the Sham group (Fig. 4C and F, p < 0.05). No significant difference was found between the MIA + Vehicle and MIA + Duloxetine groups.
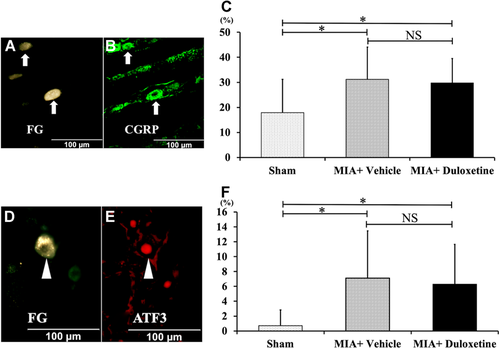
Representative fluorescence photomicrographs of L4 dorsal-root ganglia (DRG) neurons after intraperitoneal administration of duloxetine or vehicle on day 28. Photomicrographs of (A and B) and (D and E) are from the same section each. (A and D) fluorochrome (FG)-labeled DRG neurons, (B) CGRP-ir DRG neurons, (E) ATF3-ir DRG neurons. Arrows indicate FG-labeled CGRP-ir DRG neurons and arrowheads show FG-labeled ATF3-ir DRG neurons. (C) The proportions of FG-labeled CGRP-ir neurons of L4 in the MIA + Vehicle and MIA + Duloxetine groups are significantly higher than those in the Sham group (n = 6, respectively). (F) The proportions of FG-labeled ATF3-ir neurons of L4 in the MIA + Vehicle and MIA + Duloxetine groups are significantly higher than those in the Sham group (n = 6). Data are shown as mean ± standard deviation. ATF3, activating transcription factor 3; CGRP, calcitonin gene-related peptide; NS, not significant. *p < 0.05. [Color figure can be viewed at wileyonlinelibrary.com]
Expression of Iba1-ir Microglia in the Dorsal Horn of the Spinal Cord
Iba1-ir microglia were observed in the dorsal horns of each group bilaterally (Fig. 5A–C). Iba1-ir microglia in the ipsilateral dorsal horns of the MIA + Vehicle and MIA + Duloxetine groups showed a more apparent hypertrophy, compared with those on the contralateral side (Fig. 5A–F). Compared with the contralateral side, the number of Iba1-ir microglia on the ipsilateral side significantly increased in the MIA + Vehicle and MIA + Duloxetine groups (Fig. 5G) (p < 0.01). The numbers of microglia in the ipsilateral dorsal horns of the MIA + Vehicle and MIA + Duloxetine groups were significantly higher than those of the Sham group on day 28 post-induction (p < 0.01). In the MIA + Duloxetine group, the number of Iba1-ir microglia of the ipsilateral dorsal horn was significantly less in the MIA + Vehicle group (p < 0.05).
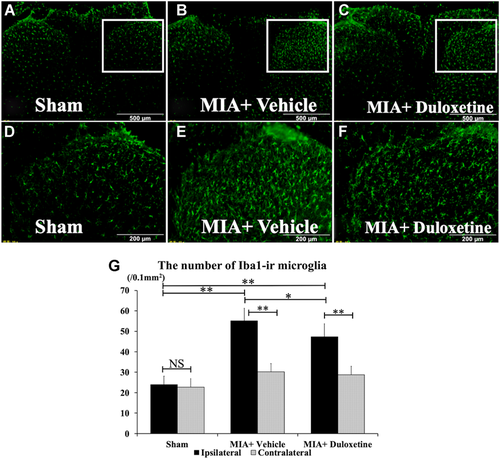
Fluorescent photomicrographs of the dorsal horn of the spinal cord immunostained for ionized-calcium-binding adaptor molecule 1 (Iba1). (A–F) are magnifications after intraperitoneal administration of duloxetine or vehicle on day 28 in each group. Iba1-ir microglia are observed in bilateral dorsal horns (A–C), and those in the ipsilateral dorsal horn are apparent relative to those in the contralateral side (D–F). (G) Number of Iba1-ir microglia in the ipsilateral dorsal horn and the contralateral dorsal horn on day 28. Compared with the contralateral side, the number of Iba1-ir microglia on the ipsilateral side significantly increased in the monosodium iodoacetate (MIA) + Vehicle and MIA + Duloxetine groups. The numbers of Iba1-ir microglia on the ipsilateral side in the MIA + Vehicle and MIA + Duloxetine groups are significantly greater than in the Sham group. The number of Iba1-ir microglia on the ipsilateral side in the MIA + Duloxetine group is significantly lower than that in the MIA + Vehicle group (n = 6, respectively). Data are shown as mean ± standard deviation. NS, not significant. **p < 0.01, *p < 0.05. [Color figure can be viewed at wileyonlinelibrary.com]
DISCUSSION
In the present study, increased mechanical hyperalgesia in neuropathic pain was induced by the injection of MIA into the hip joint in the MIA + Vehicle and MIA + Duloxetine groups. This result suggests that the hip pain in severe hip OA includes neuropathic pain. We previously reported on the aspects of neuropathic pain in the MIA-induced knee and hip OA model.14, 15, 33 Havelin et al.34 showed that the development of persistent joint pain in the MIA-induced knee OA model is associated with the development of central sensitization and responsive to therapies used to treat neuropathic pain in patients. In the clinical practice, some studies reported on the prevalence of neuropathic pain in hip and knee OA. Hochman et al.35 and King et al.36 stated that sensory abnormalities, such as mechanical hyperalgesia and allodynia, have also been identified in individuals with knee OA by using quantitative sensory testing. Furthermore, the effect sizes for first-line analgesics that target nociceptive pain, such as NSAIDs, are small to moderate and decrease with long-term use.12, 37 A recent meta-analysis, including nine studies, eight focusing on knee OA and one on hip OA, has identified an estimate of the prevalence of neuropathic pain in knee or hip OA populations as 23% based on self-report scales, such as the pain DETECT.38 As basic researches have been demonstrating, patients with OA reported to include severe OA pain have neuropathic pain-like symptoms and develop central sensitization.7, 9, 11 Thus, firm evidence shows that central sensitization is present in patients with painful OA. Clinically, pain at rest is a significant symptom that affects the decision of surgeons in terms of surgical indication. The latest study supports that possible neuropathic pain is experienced by a notable proportion of patients with end-stage hip and knee OA and is more strongly associated with pain at rest than pain on activity. The clinical presentation of pain at rest may warrant a more thorough evaluation for potential neuropathic pain and have implications for appropriate pain management.13 The current study also supports these results that neuropathic pain exists in severe OA.
This study clarified that the systematic administration of duloxetine improved the mechanical hyperalgesia in the severe hip OA model. Compared between MIA + Vehicle and MIA + Duloxetine groups, the systematic administration of duloxetine improved the mechanical hyperalgesia, which has never been reported by using the animal hip OA models. Regarding MIA-induced knee OA models in rats, several reports have indicated that MIA induces ongoing neuropathic pain by central sensitization, which is considered to be blocked by duloxetine. Havelin et al.34 showed that advanced OA pain is associated with central sensitization, and two aspects of central sensitization, spinal sensitization and descending facilitation, are observed in rats with persistent ongoing pain. Brederson et al.16 stated that p38 and phosphorylated extracellular signal-regulated kinase (pERK) were increased in the spinal cord, and that phosphorylated cAMP response element binding protein (pCREB) was enhanced in the prefrontal cortex in the MIA-induced knee OA model. MIA-induced knee OA is characterized by central sensitization and neuropathic pain. Chronic OA pain, including neuropathic pain accompanied by central sensitization in MIA-induced knee OA was treated with duloxetine.17, 34 Duloxetine also rectified the weight asymmetry in MIA-induced knee OA.39 In another study evaluating duloxetine's effects on chronic pain in MIA-induced knee OA, duloxetine was moderately efficacious in reversing OA-induced reduction in grip force.40 The authors emphatically indicated that duloxetine might be effective in patients with persistent, NSAID-resistant neuropathic OA pain, and it might diminish the number of patients requiring joint replacement surgery in the future.34, 41, 42 In addition, duloxetine improved the sensitivity to non-noxious mechanical stimuli measured by a pain behavioral test in the intervertebral disc-related radiculopathy model in rats, which is truly regarded as a model for neuropathic pain.43 With regard to clinical results, several studies conducted in the United States, Europe, and Asia have shown significant improvements in pain, joint junction and function, and health-related quality of life for patients with hip or knee OA who were treated with duloxetine compared with placebo without serious adverse events.19-21, 44 Because duloxetine has been already recommended as the first-line drug for neuropathic pain based on strong GRADE recommendations for use,22 our hypothesis that duloxetine is effective for neuropathic pain in OA can be considered reasonable.
In the present study, apparent OA changes were detected post-induction day 28 in the MIA + Vehicle and MIA + Duloxetine groups. A single dose of duloxetine did not influence the histological and radiographic findings during a short observation period. We previously examined cartilage degeneration by using the Mankin score in the same model in the past and confirmed that severe cartilage degeneration occurred 4 weeks after induction.25, 45 Moreover, we also reported on the possibility of neuropathic pain due to elongated nerves from the subchondral bone 4 weeks after the induction.14, 15 Duloxetine is one of the SNRIs that acts on the descending pain modulatory system and theoretically does not influence the histological and pathological findings. It is reasonable that the results of this study are similar to those of previous studies, because the effect of duloxetine was assessed after a single administration, and its effects were observed at 3 h after the administration.14, 25 A future study on the daily administration of duloxetine with a long observation period is needed because if OA patients do not feel pain while using strong analgesics, progressive changes of OA might occur in these patients. A past prospective randomized study reported that the daily administration of strong analgesic agents such as opioids induced progressive radiographic changes in the knee or hip OA patients.46 Pain is an important and useful signal for human lives. Duloxetine can suppress the physiological pain from OA in some patients; however, as a result, progressive joint changes, such as Charcot neuro-osteoarthropathy joint, may occur. Long-term prospective research is necessary for evaluating whether the administration of duloxetine is involved in the progressive histopathological or radiographic changes in joint degeneration.
The expressions of FG-labeled CGRP-ir and ATF3-ir DRG neurons in the MIA + Vehicle and MIA + Duloxetine groups were significantly higher than those in the Sham group on day 28 post-induction. ATF3 is dramatically induced in DRG neurons following a cellular response to nociceptive stimuli of the peripheral nerves.47 This implies that MIA-induced severe OA, which is the end-stage OA characterized by loss of the cartilage layer, exposure of subchondral bone, and elongated nerve endings resulting from the loss of cartilage, is associated not only with inflammatory pain but also neuropathic pain. In a past study on MIA-induced hip OA, Miyamoto et al.15 revealed that ATF3 was not observed during the acute phase but was expressed in the late phase after MIA injection, because of a time lag between the injury of the nerve ending and the neuronal damage. This finding supports our results. MIA seems to reproduce a nerve-injury-like response with some axonal damage as time progresses. These phenomena were similar to those seen in a rat model of knee OA induced by MIA.33, 48 In the current study, no significant difference was found in these expressions of FG-labeled CGRP-ir and ATF3-ir DRG neurons between the MIA + Vehicle and MIA + Duloxetine groups. This may imply that single administration of duloxetine attenuated OA pain with less acting on neuropeptides in the DRG. Considering that the present study is the first to evaluate how duloxetine affects the expression of neuropeptide in the DRG, this is notable.
The numbers of microglia in the ipsilateral dorsal horns of the MIA + Vehicle and MIA + Duloxetine groups were significantly higher than those of the Sham group on day 28 post-induction and the number of Iba1-ir microglia on the ipsilateral side in the MIA + Duloxetine group was significantly lower than that in the MIA + Vehicle group. This result indicates that the analgesic effect caused by duloxetine might potentially be related to an effect on microglial activity. In a mouse model of diabetic neuropathy, the peripheral and central neuroprotective effects of duloxetine in neuropathic pain were, at least in part, related to its downregulation in the spinal astrocytes and microglia.49 In another study, the occurrence of activated microglia labeled with Iba1 was tested in a disc-related radiculopathy model in rats and duloxetine-inhibited microglia activation.43 Duloxetine is classified as an SNRI that influences the descending pain modulatory system; therefore, it is assumed that duloxetine dampens pain via the descending pain modulatory system, and this may affect the expression of either Iba1 or microglial chemotaxis from different areas. Moreover, a recent study showed that duloxetine also militated microglia directly by blocking the P2X4 receptor, which was expressed on microglia and considered to play the principal role in the occurrence of allodynia after nerve injury.23, 50 Another study revealed that pharmacological blockade of the P2X4 receptor significantly inhibited the microglial chemotaxis. Knockdown of the P2X4 receptor in microglia by RNA interference through the lentivirus vector system also suppressed microglial chemotaxis. These results indicate that P2X4 receptor is involved in ATP-induced microglial chemotaxis.51 Thus, in addition to the primary action of duloxetine on monoamine transporters, the inhibition of the P2X4 receptor may be involved in the antiallodynic effect of duloxetine in a neuropathic pain model.
The present study has some limitations. First, the expression of P2X4 receptors was not examined. Examination of the expression of the P2X4 and other receptors in further studies is necessary to clarify the pharmacological mechanism of duloxetine for OA pain via the suppression of microglia. Second, we have not considered changes in astrocytes closely related to microglia and changes in chemokines, such as CX3CL1 and CCL2. We need to investigate the neuron–glia interaction in the spinal cord to elucidate neuropathic pain in the future. Third, this current study indirectly evaluated the pain-related sensory innervation with immunohistochemistry staining. Forth, the present study examined only a single administration of duloxetine with a short observation period. Given a previous study reported decreased tumor necrosis factor expression in DRG after administration of duloxetine for consecutive 10 days, downregulation of inflammatory mediators may require longer exposure times.43 Therefore the further study about daily administration of duloxetine with long observation period is needed to verify the influence on sensory innervation in DRG.
In conclusion, duloxetine suppressed mechanical hyperalgesia and expression of Iba1 in microglia in the dorsal horn ipsilateral to MIA-induced severe hip OA in rats. Duloxetine may therefore be effective for the treatment of NSAID-resistant pain at rest, which is thought to be driven by neuropathic mechanisms. These findings suggest a novel conservative treatment for patients with severe OA who are neither eager to nor eligible for surgery because of severe general condition, such as the elderly and presence of complications, and eventually may contribute to decrease the cases of the joint replacement surgery.
AUTHORS’ CONTRIBUTION
Y.K. contributed to the original idea, literature review, data collection, statistical analysis, data interpretation, manuscript writing, preparation of figures, revision, and approval of the final work. S.Or., J.N., K.I., and H.S. contributed to the original idea, data interpretation, manuscript revision. S.M. and M.S. participated in the design of the study and helped to create this study model. T.S., T.N., and T.A. contributed to literature review and data interpretation. S.Oh. contributed to data interpretation and approval of the final work. All authors have read and approved the final submitted manuscript.




