Simvastatin Possesses Antitumor and Differentiation-Promoting Properties That Affect Stromal Cells in Giant Cell Tumor of Bone
ABSTRACT
Giant cell tumor of bone (GCTB) is a locally aggressive destructive bone lesion. The management of pulmonary metastasis and local recurrence after the surgical treatment of GCTB remains a challenge. Pathologically, stromal cells in GCTB are known as primary neoplastic cells and are recognized as incompletely differentiated preosteoblasts. Therefore, inducing GCTB stromal cells to differentiate into cells with a mature osteoblastic phenotype may stop tumor growth and recurrence. In this study, we aimed to investigate how simvastatin, a clinically approved and commonly used statin that has been known to promote the maturation of cells of the osteogenic lineage, affects GCTB stromal cells. We found that simvastatin effectively inhibited cell viability by suppressing proliferation and by inducing apoptosis in GCTB stromal cells. Moreover, simvastatin treatment upregulated the expression of genes related to osteogenic maturation, such as runt-related transcription factor 2, osteopontin, and osteocalcin, and increased the mineralization of the extracellular matrix in GCTB stromal cells. Ingenuity pathway analysis was used to discover that the vitamin D receptor pathway was involved in the simvastatin-induced osteogenic differentiation of GCTB stromal cells by upregulating the 1,25-dihydroxyvitamin D metabolism. Taken together, this in vitro study demonstrates the antitumor and differentiation-promoting effects of simvastatin on GCTB stromal cells and suggests the possibility of using simvastatin as an adjuvant therapy for GCTB. These findings support further clinical investigation of the efficacy of using simvastatin as an adjuvant therapy for GCTB to reduce recurrence and distant metastasis after surgical treatment. © 2019 Orthopedic Research Society. Published by Wiley Periodicals, Inc. J Orthop Res 38:297-310, 2020
Giant cell tumor of bone (GCTB) is a benign but locally aggressive bone destructive lesion that constitutes 16.7% of all primary bone tumors in China.1 The estimated annual GCTB incidence is approximately 1.49 per million people in China and is higher than that in Japan and the United States.2 GCTB primarily affects young adults, and these tumors typically grow near joints, leading to the loss of limb function if not properly treated. They can also arise in anatomically complex locations, such as the spine and pelvis, where it is difficult to completely surgically remove these tumors. Despite the different types of surgical treatment, recurrence occurs, and the recurrence rate can be as high as 65% according to the literature.3 Wide resection leads to reduced recurrence, but the removal of a larger segment of the bone requires obligatory reconstruction or prosthetic reconstruction and may compromise limb function. Patients may even die from the disease in cases of uncontrolled local tumor recurrence and the development of distant pulmonary metastases; these effects occur in approximately 3% of GCTB patients.4 Therefore, adjuvant treatments that can reduce the tumor aggressiveness of GCTB and improve oncological outcomes are required. However, there is a lack of clinical adjuvants.
Understanding the pathophysiology of GCTB provides insights into the possibility of developing new therapeutic agents. GCTB histologically consists of three major cell types: spindle-shaped stromal cells, mononuclear monocyte cells, and multinucleated osteoclast-like giant cells.5 The stromal cells of GCTB are considered to be the primary neoplastic cells, and they are the only proliferating cell component in long-term culture.6 GCTB stromal cells express high levels of receptor activator of nuclear factor kappa-B ligand (RANKL). RANKL is the osteoclastogenesis-inducing factor and drives the macrophage-like cells that express RANK, the receptor of RANKL, to undergo fusion to form multinucleated osteoclast-like giant cells. The osteoclast-like giant cells then resorb bone aggressively and cause skeletal destruction.7 Therefore, targeting stromal cells in GCTB in conjunction with surgical treatment may be a reasonable approach.
Bisphosphonates and denosumab are antiresorptive drugs with different mechanisms of action and are currently approved for osteoporosis treatment; these drugs are used for delaying the onset of skeletal-related events after surgical treatment in patients with GCTB8, 9 because of their potential to inhibit osteoclasts. However, there are concerns with the long-term use of bisphosphonates. In particular, the prolonged inhibition of bone remodeling may cause remodeling arrest in the skeleton and may lead to serious complications, such as osteonecrosis of the jaw bones and atypical fractures. However, the noninhibitory effect of denosumab on GCTB stromal cells10 raises the concern that tumor recurrence may occur after drug withdrawal. In fact, GCTB recurrence has been reported after stopping denosumab.11 Although the two drugs may control disease recurrence by reducing osteoclastic activities, they did not effectively target GCTB stromal cells, the neoplastic elements of the tumor. Once antiresorptive drugs are stopped due to side effects, GCTB might recur. Therefore, it is imperative to identify agents that can effectively target GCTB stromal cells instead of osteoclasts.
Simvastatin is a hypolipidemic drug that has anabolic effects on bone by inducing the differentiation of mesenchymal stem cells (MSCs) into osteoblasts.12 Simvastatin has also been reported to suppress hydrogen peroxide-induced signaling pathways in osteoclastogenesis13 and to inhibit tumor growth in different cancer types.14 However, the effects of simvastatin on GCTB are unknown. In this study, we aimed to investigate the antitumor and differentiation-promoting effects of simvastatin on GCTB stromal cells.
MATERIALS AND METHODS
Simvastatin (S6196 SIGMA) was Purchased From Sigma-Aldrich (St. Louis, MO)
Cultures of GCTB stromal cell were collected from the patients who underwent surgical excision of the tumor at the Prince of Wales Hospital. Written consent was acquired from the patients, and the study protocol was approved by the Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research Ethics Committee. Primary cultures of GCTB stromal cells were established as previously described.15 In brief, GCTB tissues from resections were rinsed and minced with scissors in Dulbecco's minimum essential medium (DMEM) containing 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 µg/ml streptomycin. The resulting suspension was transferred to 25-cm2 flasks for subsequent culturing at 37°C in 5% CO2 and 95% air. Culture medium was replaced every 2–3 days, and upon reaching confluence, cells were subcultured. Cultured GCTB stromal cells were obtained after the fourth passage, representing the proliferating homogenous tumor cell population, and were used for the following assays.
As GCTB stromal cells have been reported to be of MSC origin, bone marrow-derived mesenchymal stromal cells (BMSCs), a generous gift from Prof. Wayne Lee, were used as normal control cells for evaluation in this study. The isolation of BMSCs and culture expansion was performed as described previously.16 Briefly, the BMSCs were fractionated with Ficoll-Paque PREMIUM density gradient media (GE Healthcare Life Sciences, Buckinghamshire, UK) by centrifugation. The BMSC enriched fraction was washed, seeded into culture flasks, supplemented with α-minimum essential medium (α-MEM) containing 10% FBS and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin) and were maintained at 37°C in 5% CO2 and 95% air. BMSCs were incubated with fluorochrome-conjugated primary antibodies against CD34, CD44, CD45, CD73, CD90, and CD105 or the corresponding isotype control (BD Biosciences, Franklin Lakes, NJ) at 4°C for 30 min for phenotypic characterization. The stained cells were assessed with flow cytometry (BD Biosciences).
Cell Viability Assay
Three primary GCTB stromal cell lines, G33, G53 and G62, and a control MSC primary cell line were seeded into 96-well plates at a density of 7,000 cells/well for 24 h and were then exposed to different doses of simvastatin (0, 0.1, 1.0, and 10 μM) for 6 days. The three GCTB stromal cell lines were selected because they have consistent proliferation rates from the 4th to the 12th passage. The dose of simvastatin selected for the assays in this study was based on that of previous studies.17-19 At a low dose of 0.1–1 μM, simvastatin was capable of inducing osteogenic differentiation in mouse preosteoblast17 and mouse bone marrow MSCs,18 whereas at a high dose, 10 μM simvastatin was able to decrease the cell proliferation of UMR-106 rat osteosarcoma cells.19 The effect of simvastatin on cell viability was examined with cell counting kit-8 (CCK8) assays (Dojindo Molecular Technologies, Inc., Rockville, MD) by utilizing highly water-soluble tetrazolium salt, WST-8 (2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt). After drug administration, WST-8 solution was added to the treated cells, followed by incubation at 37°C for 2 h in a CO2 incubator. The absorbance was then measured at 450 nm using a microplate reader (3550; Bio-Rad, Laboratories, Irvine, CA).
Cell Proliferation Assay
In a parallel experiment for cell proliferation analysis, a 5-bromo-2′-deoxyuridine (BrdU) labeling solution was added to the treated cells, followed by incubation at 37°C for 16 h in the CO2 incubator. The cell proliferation rate was assessed with the Cell Proliferation ELISA, BrdU (colorimetric) Kit (Roche Diagnostics, GmbH, Mannheim, Germany) according to the manufacturer's instructions.
Cell Apoptosis Assay
The three primary cell lines of GCTB stromal cells were seeded at a density of 1 × 105 cells/well into six-well plates for 24 h. The GCTB stromal cells were treated with 0.1% dimethyl sulfoxide (DMSO) as a vehicle control, geranylgeraniol (GGOH) at 10 μM, simvastatin at 10 μM, or the combination of GGOH (10 μM) and simvastatin (10 μM) for 6 days. Cell apoptosis was assessed using the Annexin-V-FLUOS staining kit (Roche Diagnostics). The treated cells were resuspended in annexin-V-fluorescence and a propidium iodide (PI)-containing buffer solution according to the manufacturer's instructions. Labeled cells were analyzed on the BD LSR Fortessa cell analyzer with the BD FACSDiva v8.0.1 software (Becton Dickenson, Billerica, MA).
Osteogenic Maturation of GCTB Stromal Cells
GCTB stromal cells were seeded at a density of 2 × 104 cells/well in 24-well plates for 24 h. Cells were treated with the vehicle control (0.1% DMSO) or simvastatin (1 μM) in basal medium (BAS) containing α-MEM supplemented with 10% FBS and 1% penicillin-streptomycin (PS) or in osteogenic-stimulating medium (OS) containing α-MEM supplemented with 10% FBS, 1% PS, 10 mM β-glycerol phosphate, and 50 μM ascorbic acid for 7 days. The treatment was then stopped, and the treated cells were subjected to RNA extraction or continuously cultured in BAS or OS until day 14.
Detection and Quantification of Mineralization
Mineralization capacity is the terminal differentiation parameter for osteoblasts that was examined with an Alizarin Red S Staining Quantification Assay (ScienCell, Research Laboratories, Carlsbad, CA) according to the manufacturer's instructions. Mineral levels were normalized to the relative cell number, and monolayer cells were stained with crystal violet as described.20
Quantitative Real-Time Polymerase Chain Reaction (PCR) Analysis
RNA extraction from the treated cells was performed using the PureLink RNA Mini Kit (Invitrogen, Carlsbad, CA). Extracted RNA was reverse transcribed into first-strand complementary DNA using the QuantiTect Reverse Transcription Kit (Qiagen, Inc., Valencia, CA). Quantitative real-time PCR (qPCR) was performed in triplicate using PowerUp SYBR Green Master Mix in the QuantStudio 12 K Flex Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA). The housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used for normalization. Gene-specific primers for alkaline phosphatase (ALP), runt-related transcription factor 2 (RUNX2), osteopontin (OPN), and osteocalcin (OCN) were designed with Primer Express 3.0.1 Software (Thermo Fisher Scientific), and the primer sequence for 25-hydroxyvitamin D 1alpha-hydroxylase (CYP27B1) was obtained from Atkins et al.21 The primer sequences are shown in Table 1.
| Genes | Forward | Reverse |
|---|---|---|
| ALP | CTCCCAGTCTCATCTCCT | AAGACCTCAACTCCCCTGAA |
| RUNX2 | GGTTAATCTCCGCAGGTCACT | CACTGTGCTGAAGAGGCTGTT |
| OPN | ATCTCCTAGCCCCACAGAAT | CATCAGACTGGTGAGAATCATC |
| OCN | GCCTTTGTGTCCAAGC | GGACCCCACATCCATAG |
| GAPDH | GGAGTCAACGGATTTGGT | GTGATGGGATTTCCATTGAT |
RNA Sequencing
The GCTB stromal cells were treated with the vehicle control (0.1% DMSO) or simvastatin (1 μM) in BAS or in OS for 7 days as mentioned above. The treated cells were subjected to RNA extraction using the PureLink RNA Mini Kit (Thermo Fisher Scientific). Total RNA samples were treated with DNase I, and the integrity of the RNA samples was assessed using an Agilent Bioanalyzer. Libraries were generated using the TruSeq RNA Sample Preparation v2 Kit (Illumina, San Diego, CA) according to the user's manual. The samples were sequenced on the NovaSeq. 6000 System (Illumina) at Groken Bioscience Ltd.
Transcriptome Assembly and Differential Gene Expression Analysis
The RNA sequencing raw reads from were mapped by HISAT2 v 2.1.0 with the default parameters to the reference genome Ensembl GRCh38.95.22 Gene-level quantification and transcript annotation were performed by StringTie v1.3.5.23 Differential expression analysis between different libraries was then performed using Cuffdiff v2.2.1.24 To identify statistically significant differentially expressed genes, very stringent parameters were used: log2-fold change in expression ≥ 1 and a false discovery rate (FDR) adjusted p < 0.01. Further investigation of the differentially expressed gene (DEG) lists was performed using Ingenuity Pathway Analysis software (IPA; Qiagen Inc.) with Fisher's exact tests as previously described.25 Interactions among proteins encoded by the DEGs were identified by STRING 11.0.26 The overlapping DEGs among the simvastatin-treated GCTB stromal cell samples in BAS and OS was illustrated with a Venn diagram using publicly available software.27
Measurement of Vitamin D Levels in the Culture Supernatant
GCTB stromal cells were seeded at a density of 2 × 104 cells/well in 24-well plates for 24 h. They were treated with the vehicle control (0.1% DMSO) or simvastatin (1 μM) in BAS or OS for 7 days. Supernatants from the cell cultures were harvested and stored at −20°C prior to analysis for the vitamin D content. The quantitative determination of 1,25-dihydroxyvitamin D in the supernatants was performed with the Vitamin D ELISA kit (ab213966; Abcam, Cambridge, UK) according to the manufacturer's instructions. The results were normalized to the relative cell number with the crystal violet method mentioned above. The data were handled by an immunoassay software package using a 4 parameter logistic (4PL) curve fitting program.28
Statistical Analysis
At least three independent experiments were performed for all assays in this study, and the experimental results are presented as the means ± standard deviation. Statistical analyses were performed utilizing Prism 8 (GraphPad Software Inc., San Diego, CA). Statistical significance between groups was analyzed by one-way analysis of variance, and p < 0.05 were considered statistically significant.
RESULTS
Simvastatin Inhibited Cell Viability and Proliferation in GCTB Stromal Cells
Simvastatin significantly inhibited cell viability in a dose-dependent manner at concentrations of 0.1–10 μM in GCTB stromal cells (Fig. 1B–D). It also decreased the cell viability of the control MSCs at a higher dose from 1 μM or above (Fig. 1A). The mean cell viability of the three GCTB stromal cell lines under simvastatin treatment (1 μM) was 0.74 ± 0.05-fold that of the vehicle controls. Similarly, simvastatin effectively suppressed cell proliferation in GCTB stromal cells (Fig. 2B–D) and control MSCs (Fig. 2A). An inhibitory effect on cell proliferation by simvastatin was found in a dose-dependent manner in GCTB stromal cells (G33 and G62); the mean cell proliferation rate of GCTB stromal cells was 0.80 ± 0.13-fold that of the vehicle controls with a 1 μM simvastatin treatment.
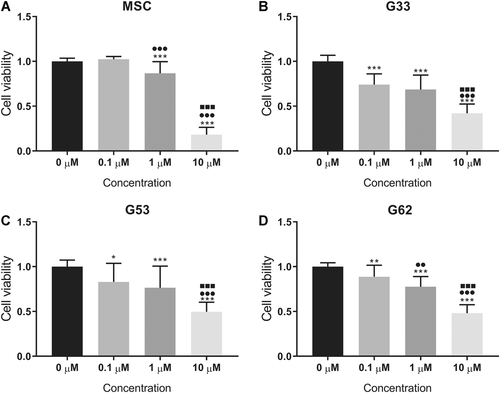
Cell viability inhibition caused by different concentrations of simvastatin in (A) the mesenchymal stem cell (MSC) control, and (B–D) the three giant cell tumor of bone (GCTB) stromal cell lines (B) G33, (C) G53, and (D) G62. The data represent the mean and standard deviation (SD) of three experiments. *p <0.05, **p <0.01, ***p <0.001 compared with 0 µM; ●●p <0.01, ●●●p <0.001 compared with 0.1 µM; ■■■p <0.001 compared with 1 µM, by one-way analysis of variance (ANOVA) and Tukey's multiple comparison test.
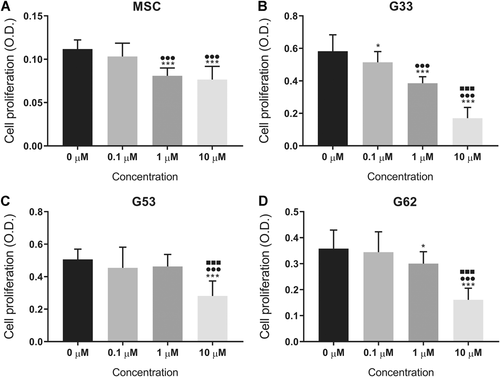
Cell proliferation inhibited by different concentrations of simvastatin in (A) the mesenchymal stem cell (MSC) control, and (B–D) the three giant cell tumor of bone (GCTB) stromal cell lines (B) G33, (C) G53, and (D) G62. The data represent the mean and standard deviation (SD) of three experiments. *p <0.05, ***p <0.001 compared with 0 µM; ●●●p <0.001 compared with 0.1 µM; ■■■p <0.001 compared with 1 µM, by one-way analysis of variance (ANOVA) and Tukey's multiple comparison test.
Simvastatin-Induced Cell Apoptosis in GCTB Stromal Cells
A 10 μM simvastatin treatment strongly induced cell apoptosis in the GCTB stromal cells G33, G53, and G63 with 76.78 ± 16.11%, 64.47 ± 14.91%, and 55.28 ± 6.74% (mean ± SD) apoptotic cells, respectively, compared with the levels of apoptosis in the corresponding vehicle controls, 12.33 ± 4.39%, 23.27 ± 10.51%, and 25.08 ± 12.28%, respectively (Fig. 3A–D). Interestingly, GGOH, an isoprenoid lipid substrate that is converted to geranylgeranyl pyrophosphate (GGPP) in the mevalonate pathway, hampered simvastatin-induced cell apoptosis in GCTB stromal cells and restored cell viability.
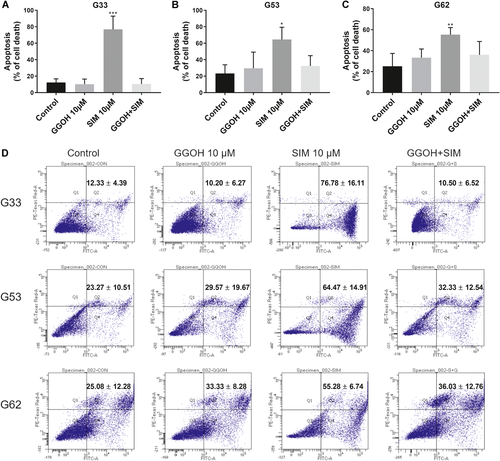
Cell apoptotic effect of simvastatin in the three giant cell tumor of bone (GCTB) stromal cell lines. The GCTB stromal cells were stained with annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) after they had been treated with dimethyl sulfoxide (DMSO) (0.1%, control), geranylgeraniol (GGOH) (10 µM), simvastatin (SIM, 10 µM), and the combination of GGOH and simvastatin (GGOH + SIM) for 6 days. (A–C) Significant cell apoptotic effects were observed in the three GCTB stromal cell lines (A) G33, (B) G53, and (C) G62 treated with simvastatin (10 µM). The bars represent the mean ± standard deviation (SD) of four independent experiments. *p <0.05, **p <0.01, ***p <0.001, compared with the control, by one-way analysis of variance (ANOVA) and Tukey's multiple comparison test. (D) Cell apoptosis was analyzed by flow cytometry. Lower-right and upper-right quadrants indicate the percentage of early and late apoptotic cells (annexin V-FITC and PI-stained cells). The figures represent the mean ± SD of four independent experiments. [Color figure can be viewed at wileyonlinelibrary.com]
Simvastatin Stimulated the Osteogenic Maturation of GCTB Stromal Cells
Simvastatin profoundly upregulated the messenger RNA (mRNA) expression of osteogenic markers, including RUNX2, OPN, and OCN, in GCTB stromal cells in BAS for 7 days, and these markers showed 1.68-, 3.16-, and 1.78-fold increases compared to their expression in the vehicle control, respectively (Fig. 4). Simvastatin also synergistically stimulated OPN mRNA expression in GCTB stromal cells in OS. Furthermore, simvastatin significantly increased the mineralization of the extracellular matrix (ECM) in two of the three GCTB stromal cell lines, G33 and G62, cultured in OS for 14 days (Fig. 5A and B). However, simvastatin did not enhance ECM mineralization in the GCTB stromal cells cultured in BAS.
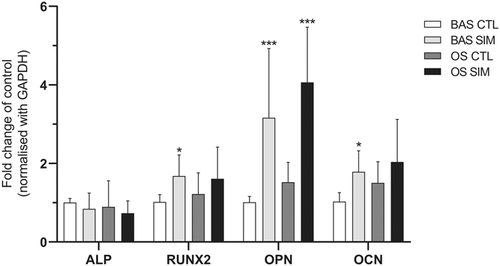
Giant cell tumor of bone (GCTB) stromal cells were treated with dimethyl sulfoxide (DMSO) (0.1%) in basal medium (BAS) (BAS CTL) or simvastatin (BAS SIM) in BAS; and GCTB stromal cells were treated with DMSO in osteogenic-stimulating medium (OS) (OS CTL) or simvastatin in OS (OS SIM) for 7 days. The messenger RNA (mRNA) expression of alkaline phosphatase (ALP), runt-related transcription factor 2 (RUNX2), osteopontin (OPN), and osteocalcin (OCN) was detected by real-time quantitative polymerase chain reaction (qPCR). The bars represent the mean ± standard deviation of three independent experiments. *p <0.05, **p <0.01, ***p <0.001 compared with the control, by one-way analysis of variance (ANOVA) and Bonferroni's multiple comparison test.
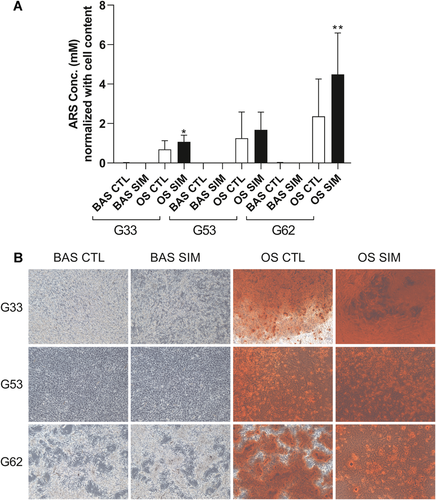
Giant cell tumor of bone (GCTB) stromal cells were treated with dimethyl sulfoxide (DMSO) (0.1%) in basal medium (BAS) (BAS CTL) or simvastatin in BAS (BAS SIM); and GCTB stromal cells were treated with DMSO in osteogenic-stimulating medium (OS) (OS CTL) or simvastatin in OS (OS SIM) for 14 days. Simvastatin significantly increased the mineralization of the extracellular matrix (ECM) in two of the three GCTB stromal cell lines, G33 and G62, cultured in OS for 14 days (A and B). Simvastatin did not enhance ECM mineralization in the GCTB stromal cells cultured in BAS. The bars represent the mean ± standard deviation of three independent experiments. *p <0.05, **p <0.01 compared with the control, by one-way analysis of variance (ANOVA) and Bonferroni's multiple comparison test. [Color figure can be viewed at wileyonlinelibrary.com]
Functional Annotation of the DEGs by IPA and STRING
RNA sequencing results showed that a total of 177 and 202 DEGs were discovered between simvastatin- and vehicle-treated GCTB stromal cells cultured in BAS or OS, respectively, using stringent criteria of a log2-fold change in expression ≥ 1 and a FDR adjusted p < 0.01. There were 50 overlapping DEGs among the two groups (Fig. 6A), which indicated that simvastatin exerted osteogenic effects on GCTB stromal cells concurrently under the influence of osteogenic-stimulating medium. IPA of the 177 and 202 DEGs in simvastatin-treated GCTB stromal cells in BAS or in OS revealed that significant alterations (p < 0.05) occurred in 34 and 97 pathways, respectively. The top 20 canonical ingenuity pathways regulated by simvastatin with BAS or OS are shown in Figure 6B and C. Simvastatin essentially regulated cholesterol biosynthesis, glucocorticoid receptor signaling, VDR/RXR activation pathways, zymosterol biosynthesis, eicosanoid signaling, hepatic fibrosis/hepatic stellate cell activation, and HMGB1 signaling in the GCTB stromal cells cultured in BAS. In addition, 14 overlapping ingenuity canonical pathways were altered in the simvastatin-treated GCTB stromal cells in BAS or OS, including the VDR/RXR activation and Wnt/β-catenin signaling pathways (Table 2). The top five upstream regulators of simvastatin-treated GCTB stromal cells in BAS were TGFB1, sterol regulatory element-binding proteins (SREBP) cleavage-activating protein (SCAP), β-estradiol, lovastatin, and SREBP2, which reflected the accuracy of the IPA.
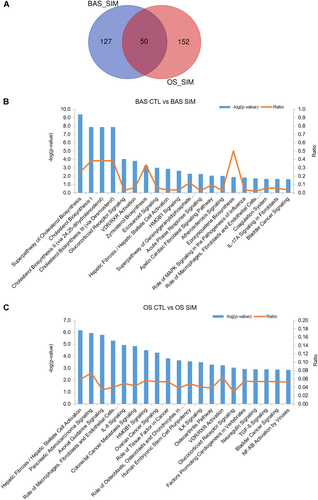
A Venn diagram showed that a total of 177 and 202 differentially expressed genes (DEGs) were discovered between simvastatin- and vehicle-treated giant cell tumor of bone (GCTB) stromal cells cultured in basal medium (BAS) or osteogenic-stimulating medium (OS), respectively, and there were 50 overlapping DEGs among the two groups (A). Ingenuity pathway analysis (IPA) of the 177 and 202 DEGs in simvastatin-treated GCTB stromal cells in BAS or OS revealed that significant alterations (p < 0.05) occurred in 34 and 97 pathways, respectively. The top 20 canonical ingenuity pathways regulated by simvastatin with BAS (B) or with OS (C) are shown in the graphs. [Color figure can be viewed at wileyonlinelibrary.com]
| Simvastatin in BAS | Simvastatin in OS | |||
|---|---|---|---|---|
| Ingenuity Canonical Pathways | p Value | Molecules | p Value | Molecules |
| Glucocorticoid receptor signaling | 8.71E−05 | KRT14, CCL2, POU2F2, TGFB2, HSPA6, CEBPB, SERPINE1, TSC22D3, KRT81, FGG | 8.91E−04 | KRT14, RALA, CCL2, TGFB2, FGFR2, PTGS2, SERPINE1, TSC22D3, KRT81, KRT31 |
| VDR/RXR activation | 1.51E−04 | IGFBP6, TGFB2, IGFBP1, CEBPB, KLF4 | 5.75E−04 | TGFB2, IGFBP5, PRKCH, IGFBP1, KLF4 |
| Hepatic fibrosis/hepatic stellate cell activation | 1.32E−03 | COL5A3, CCL2, TGFB2, SERPINE1, TNFRSF11B, PGF | 6.76E−07 | CCL2, TGFA, TGFB2, FGFR2, MMP13, VEGFC, ECE1, IGFBP5, SERPINE1, TNFRSF11B, PGF |
| HMGB1 signaling | 2.24E−03 | RHOB, CCL2, TGFB2, SERPINE1, TNFRSF11B | 3.16E−05 | RALA, CCL2, RHOB, TGFB2, FGFR2, SERPINE1, TNFRSF11B, PLAT |
| Apelin cardiac fibroblast signaling pathway | 8.71E−03 | TGFB2, SERPINE1 | 1.51E−02 | TGFB2, SERPINE1 |
| Role of mitogen-activated protein kinase (MAPK) signaling in the pathogenesis of influenza | 1.45E−02 | CCL2, ABHD3, PLA2G4C | 3.09E−02 | RALA, CCL2, PTGS2 |
| Coagulation system | 2.14E−02 | SERPINE1, FGG | 3.39E−03 | F13A1, SERPINE1, PLAT |
| IL-17A signaling in fibroblasts | 2.14E−02 | CCL2, CEBPB | 3.63E−02 | CCL2, CEBPD |
| Bladder cancer signaling | 2.29E−02 | DAPK1, THBS1, PGF | 1.35E−03 | RALA, MMP13, VEGFC, CCND1, PGF |
| Wnt/β-catenin signaling | 2.51E−02 | SFRP4, TGFB2, FZD7, SOX5 | 1.70E−02 | SFRP4, WNT7B, TGFB2, CCND1, WNT5A |
| Inhibition of matrix metalloproteases | 2.63E−02 | ADAM12, THBS2 | 4.57E−03 | THBS2, MMP13, TFPI2 |
| FAT10 cancer signaling pathway | 3.55E−02 | TGFB2, TNFRSF11B | 7.24E−03 | ACKR3, TGFB2, TNFRSF11B |
| Osteoarthritis pathway | 4.79E−02 | PRG4, CEBPB, FZD7, PGF | 5.13E−04 | PRG4, BMP2, SMAD6, MMP13, GDF5, VEGFC, PTGS2, PGF |
| Apelin endothelial signaling pathway | 4.90E−02 | CCL2, ANGPT1, KLF2 | 4.90E−03 | RALA, CCL2, FGFR2, PRKCH, KLF2 |
We also used STRING (version 11.0) to predict the protein–protein interactions of the DEGs of the GCTB stromal cells treated with simvastatin in BAS or in OS. The STRING diagram showed that there were three clusters of DEGs regulated by simvastatin in BAS (Fig. 7A), which were related to the VDR/RXR signaling pathway, cholesterol biosynthesis/activation of gene expression by SREBP, and keratinization. The genes associated with the VDR/RXR signaling pathway are highlighted. Furthermore, there was a large cluster of DEGs significantly enriched from the GCTB stromal cells treated with simvastatin in OS (Fig. 7B). The component genes of the large cluster were engaged in transforming growth factor-β (TGF-β) and bone morphogenetic protein (BMP) signaling in osteoblastic differentiation, pathways in cancer, and Hippo signaling pathways. Simvastatin synergistically enhanced the TGF-β and BMP signaling pathways when cells were cultured in OS.
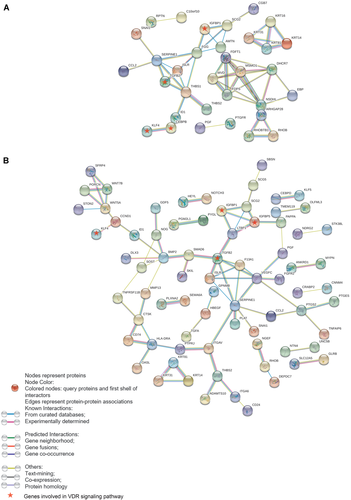
The STRING diagrams illustrate the predicted protein–protein interactions of the differentially expressed genes (DEGs) using high confidence (0.7). A total of 177 DEGs from simvastatin-treated giant cell tumor of bone (GCTB) stromal cells in basal medium (BAS) (A) and 202 DEGs from simvastatin-treated GCTB stromal cells in osteogenic stimulating medium (OS). (B) The disconnected nodes in the network were hidden. Colored nodes represent query proteins and the first shell of interactors, whereas white nodes represent the second shell of interactors. Edges represent protein–protein associations, and different colors of the edges represent different associations. The known interactions were found from curated databases (blue) or experimentally determined (purple); the predicted interactions were suggested by gene neighborhood (green), gene fusions (red), or gene cooccurrence (dark blue); and other protein–protein associations were suggested by text mining (yellow–green), coexpression (black), or protein homology (gray). [Color figure can be viewed at wileyonlinelibrary.com]
On the contrary, our RNA-seq results showed that simvastatin did not regulate mRNA expression of most of the osteoclastogenic factors, including CASR, CEBPB, CSF1, CXCL12, IGF1, IGF2, IL-6, IL-11, IL-34, c-JUN, MMP9, MMP13, PTHLH, RANKL, TGFB1, TNFα, and VEGFA (Supplementary Table S1), they involve in recruitment of monocytes, stimulation of RANKL expression, induction of osteoclast differentiation and activation, and promotion of bone resorption by giant cells. However, simvastatin significantly decreased mRNA expression of CCL2 which recruits monocytes through CCR1.
Upregulation of 1,25-Dihydroxyvitamin D by Simvastatin
The genes involved in the VDR/RXR activation pathway, including IGFBP6, TGFB2, IGFBP1, CEBPB, and KLF4, were all upregulated in the RNA sequencing results (data not shown); we speculated that 1,25-dihydroxyvitamin D secretion would be stimulated by simvastatin in GCTB stromal cells. Interestingly, the mRNA expression of CYP27B1 was upregulated (Fig. 8A) concurrently with the increase in 1,25-dihydroxyvitamin D synthesis in the simvastatin-treated GCTB stromal cells cultured in BAS (0.696 ± 0.432 ng/ml) or OS (0.694 ± 0.279 ng/ml) compared with those of the vehicle controls (Fig. 8B).
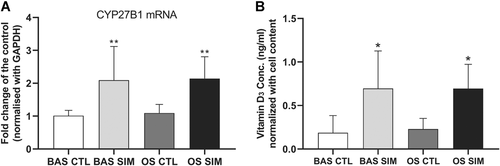
Messenger RNA (mRNA) expression of CYP27B1 in giant cell tumor of bone (GCTB) stromal cells was upregulated by simvastatin (1 µM) in basal medium (BAS) (BAS SIM) or in osteogenic-stimulating medium (OS) (OS SIM) (A). 1,25-Dihydroxyvitamin D synthesis was increased in simvastatin-treated GCTB stromal cells cultured in BAS (BAS SIM) or in OS (OS SIM) compared with their corresponding vehicle controls (BAS CTL and OS CTL) (B). *p <0.05, **p <0.01 compared with the vehicle control, by one-way analysis of variance (ANOVA) and Bonferroni's multiple comparison test.
DISCUSSION
In this study, we demonstrated that simvastatin significantly inhibited cell viability by inhibiting cell proliferation and inducing apoptosis in GCTB stromal cells at doses ranging from 0.1 to 10 μM. The apoptosis induced by simvastatin was rescued by GGOH, an isoprenoid lipid substrate, which is converted to GGPP in the mevalonate pathway. This indicates that the reduction in the bioavailability of GGPP caused tumor cell apoptosis. Simvastatin inhibits 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, which catalyzes the conversion of HMG-CoA to mevalonate, the rate-limiting step of the mevalonate pathway. This effect diminishes the downstream production of isoprenoids, farnesyl pyrophosphate (FPP) and GGPP, and subsequently inhibits protein prenylation. Prenylation of small guanosine triphosphatases (GTPases) is very important for maintaining their membrane localization and functions. For instance, prenylation is responsible for the correct localization of RhoA on the membrane and is vital for RhoA's function in inhibiting p21 activity. Statins reduced the level of membrane-bound RhoA in renal cancer and breast cancer cells and induced cell-cycle arrest in the G1 phase by upregulating p21 and p53; the upregulation was because p21 was no longer inhibited by membrane-bound RhoA.29, 30 Many studies have also demonstrated that statins impede cell proliferation by regulating the expression and activity of cell cycle progression-related proteins, such as cyclins, cyclin-dependent kinases, and inhibitors of CDK.31, 32 Furthermore, many in vitro experimental data have indicated that statins induce cell apoptosis through both intrinsic and extrinsic apoptotic pathways33-35 and through the inhibition of cholesterol synthesis.36
Our previous study15 has shown that either a single treatment with pamidronate (a nitrogen-containing bisphosphonate) or a combination treatment with GGTI-298 (a geranylgeranyl-transferase I (GGTase I) inhibitor) suppressed the downstream components of the mevalonate pathway, that is, farnesyl pyrophosphate synthase (FPPS) and GGTase I, respectively; these treatments strongly inhibited Rap1a prenylation. Consequently, inhibited prenylation reduced cell proliferation and induced cell death by promoting cell cycle arrest at the S phase and the caspase-3/7 activities, respectively. Therefore, we speculate that the simvastatin-induced inhibitory effects on the cell viability of GCTB stromal cells may have similar mechanisms to those of nitrogen-containing bisphosphonates, as they target the same mevalonate pathway. Detailed mechanisms of the inhibition of cell proliferation and the induction of apoptosis in GCTB stromal cells exposed to simvastatin should be investigated. Nevertheless, we also observed that simvastatin inhibited cell viability in the control MSCs and exerted its inhibitory effect on the control MSCs at doses of 1 µM and above, whereas simvastatin exerted effects on the GCTB stromal cells at doses of 0.1 µM and above. GCTB stromal cells were more sensitive to simvastatin treatment than the control cells; this effect may be due to the increased activity of the mevalonate pathway in tumor cells.37 Simvastatin is an oral lipid-lowering drug to treat hyperlipidemia and prevent cardiovascular disease. As orally taken simvastatin undergoes extensive first-pass metabolism in the liver, the availability of the drug in the systemic circulation is low. It may be challenging to achieve a potential therapeutic dose at the location of the GCTB in the clinical setting. Therefore, a direct local administration of the drug into the tumor site after an intralesional tumor curettage can be one viable approach. Given the nonspecific cellular inhibitory effect of simvastatin on MSCs, novel simvastatin-loaded nanoparticles based on poly(lactic-co-glycolic acid) (PLGA) were developed for sustained and controlled delivery of simvastatin for breast cancer treatment.38 A similar approach may be considered in GCTB for future research so that simvastatin can preferentially target the GCTB stromal cells.
Simvastatin at 1 μM effectively induced osteogenic differentiation of the GCTB stromal cells, as evidenced by the upregulation of the osteoblastic marker genes, including OPN and OCN, at day 7 in BAS and the increased mineralization of ECM at day 14 in OS. However, simvastatin could not induce the mineralization of GCTB stromal cells in BAS without an ascorbic acid and β-glycerophosphate supplement. Because of the lack of a phosphate source (β-glycerophosphate) for the formation of hydroxylapatite minerals in BAS, the GCTB stromal cells were unable to produce mineral nodules for terminal osteogenic differentiation. A similar phenomenon was observed in SAOS-2 cells, a human osteogenic sarcoma cell line expressing an osteoblast phenotype. In the presence of β-glycerophosphate, SAOS-2 cells produced an extensive collagenous matrix, and the matrix was able to mineralize; in contrast, in the absence of β-glycerophosphate, SAOS-2 cells did not form a mineral nodule.39
To investigate the underlying mechanism of simvastatin-induced osteogenic differentiation on GCTB stromal cells, we performed RNA-seq and bioinformatics analyses on the four samples. GCTB stromal cells were treated with the vehicle control or 1 µM simvastatin in BAS, and GCTB stromal cells were treated with the vehicle control or 1 µM simvastatin in OS. As mentioned above, simvastatin inhibits HMG-CoA reductase and causes the reduction in downstream vital isoprenoid precursor units, that is, FPP and GGPP, which hampers not only protein prenylation but also cholesterol and steroid synthesis and N-glycosylation. The majority of the top 10 canonical pathways regulated by simvastatin were cholesterol biosynthesis and related pathways. Importantly, VDR/RXR activation was also enriched in the top 10 ingenuity canonical pathways, and the genes under this pathway were all upregulated.
When the cellular cholesterol is depleted, the endoplasmic reticulum (ER) membrane proteins, SREBPs, bind to another ER membrane protein called SCAP; the binding of SREBP-SCAP facilitates the formation of coat protein complex II (COPII) membrane-coated vesicles, which transports the SCAP–SREBP complex from the ER to the Golgi apparatus.40 In the Golgi apparatus, SREBPs are cleaved by site 1 protease (S1P) and site 2 protease (S2P), which enables the N-terminal of SREBPs (n-SREBPs) to enter the nucleus and bind to the sterol regulatory element (SRE) on the promoter regions of target genes.41 Interestingly, SRE has been discovered in the human mitochondrial cytochrome P450 (CYP) enzyme 25-hydroxyvitamin D3-1αhydroxylase (CYP27B1) gene promoter region and is upregulated by SREBPs.42 The rate-limiting step in the bioactivation of 1,25-dihydroxyvitamin D is catalyzed by CYP27B1 from the main circulating form 25-hydroxyvitamin D in renal proximal tubular cells. In addition, our STRING analysis also showed that simvastatin significantly modulated the activation of gene expression pathways by SREBP. Therefore, we hypothesize that simvastatin inhibits HMG-CoA reductase and consequently reduces intracellular cholesterol levels in GCTB stromal cells. Upon deprivation of intracellular cholesterol, n-SREBPs are translocated into the nucleus and bind to the SRE on the promoter region of CYP27B1 and upregulate CYP27B1 gene expression. In turn, the upregulation of CYP27B1 stimulates the synthesis of 1,25-dihydroxyvitamin D in GCTB stromal cells and thus promotes the transcription of the 1,25-dihydroxyvitamin D-responsive genes, including OPN and OCN, and induces osteogenic differentiation (Fig. 9).
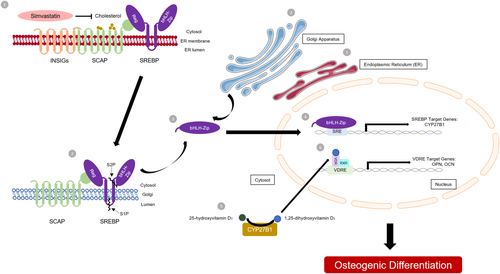
An illustration of the proposed model of simvastatin-induced osteogenic differentiation in giant cell tumor of bone (GCTB) stromal cells. (1) Simvastatin inhibits HMG-CoA reductase and consequently reduces intracellular cholesterol levels in GCTB stromal cells. (2) When the cellular cholesterol is depleted, the endoplasmic reticulum (ER) membrane proteins, sterol regulatory element-binding proteins (SREBPs), bind to another ER membrane protein called SCAP; the binding of SREBP-SCAP facilitates the formation of coat protein complex II (COPII) membrane-coated vesicles, which transports the SCAP–SREBP complex from the ER to the Golgi apparatus. In the Golgi apparatus, SREBPs are cleaved by site 1 protease (S1P) and site 2 protease (S2P), (3) which enables the N-terminal of SREBPs (n-SREBPs) to enter the nucleus. (4) The n-SREBP binds to the SRE on the promoter region of CYP27B1 and upregulate CYP27B1 gene expression. (5) In turn, the upregulation of CYP27B1 stimulates the synthesis of 1,25-dihydroxyvitamin D in GCTB stromal cells and (6) thus promotes the transcription of the 1,25-dihydroxyvitamin D-responsive genes, including osteopontin (OPN) and osteocalcin (OCN), and induces osteogenic differentiation. [Color figure can be viewed at wileyonlinelibrary.com]
We proved our hypothesis by assessing the CYP27B1 mRNA and 1,25-dihydroxyvitamin D protein levels in GCTB stromal cells treated with simvastatin. We found that CYP27B1 mRNA was expressed in GCTB stromal cells and that it was upregulated by simvastatin in BAS or OS. Notably, the synthesis of 1,25-dihydroxyvitamin D was also concomitantly increased by simvastatin in BAS or OS. In fact, extrarenal 1,25-dihydroxyvitamin D production in human osteoblasts and osteosarcoma cell lines has been investigated in previous studies,43-45 and these studies demonstrated that the mRNA expression of CYP27B1 was closely related to the synthesis of 1,25-dihydroxyvitamin D in osteoblasts. The knockdown of CYP27B1 by RNA interference (RNAi) resulted in the suppression of both 1,25-dihydroxyvitamin D secretion and the transcription of OCN.45 Our results suggest that the autocrine pathway of 1,25-dihydroxyvitamin D metabolism may regulate the osteogenic differentiation of GCTB stromal cells. Notably, recent studies have shown that the prevalence of vitamin D deficiency occurs in patients with primary GCTB46 and other bone tumors.47 Although the authors did not prove a causal relationship between vitamin D deficiency and the onset of GCTB in patients, it is worthwhile to investigate how the activation of the VDR signaling pathway modulates terminal osteogenic differentiation or the mineralization of GCTB stromal cells. Future research on the genome, transcriptome, and epigenome of the components of the VDR signaling pathway, including CYP27B1, cytochrome P450 family 24 subfamily A member 1 (CYP24A), VDR, vitamin D binding protein (VDBP), and megalin and cubilin (endocytic receptors for vitamin D), shall shed light on the association between vitamin D deficiency and the onset of GCTB and will lead to the development of potential treatments for GCTB.
On the contrary, our RNA-seq results showed that simvastatin significantly decreased mRNA expression of CCL2 which recruits monocytes through CCR1. Many studies have also shown that simvastatin exhibits inhibitory effects on osteoclastogenesis through different pathways. Simvastatin inhibits RANKL-induced NF-κB pathway activation through the suppression of IκBα phosphorylation, IκBα degradation, and IκBα kinase activity.48 Simvastatin prevents the production of reactive oxygen species, which hampers the H2O2-induced early signaling activities including AKT, JNK, p38, ERK, and NF-κB signaling pathways. This subsequently inhibits osteoclastogenesis.13 In addition, it suppresses osteoclastic differentiation in human osteoclasts induced by M-CSF and RANKL treatments through inhibiting Src and enhancing MAPK/AKT pathways.49 Those evidences indicate that simvastatin acts as an inhibitor in osteoclastogenesis.
To the best of our knowledge, this study is the first to investigate the effects of simvastatin in GCTB stromal cells. Current adjuvant therapies for GCTB do not effectively target stromal cells, the main neoplastic components of GCTB. Tumor recurrence after drug withdrawal is commonly reported. The potential beneficial effects of simvastatin on antitumor, osteogenic stimulation and possibly osteoclast inhibition make it a very attractive approach as an adjuvant therapy, as simvastatin has been used clinically for a long time to treat hyperlipidemia, and its safety has been demonstrated.50 Reduced tumor aggressiveness, less tumor recurrence, or even the biological elimination of the tumor may be made possible by simvastatin. Therefore, surgical margins during tumor removal may be reduced, and more normal tissues may be preserved, thus mitigating surgical morbidity and retaining better functions. GCTB metastases may also be controlled, which have previously had no effective treatment. Together, our study supports that simvastatin can be a potential adjuvant for treating GCTB. Further in vivo study using simvastatin to reduce GCTB xenograft development in nude mice is warranted.
AUTHORS' CONTRIBUTION
C.P.Y.L., PhD: Designed and performed experiments, analyzed data, drafted the manuscript. C.S.H.F.: Analyzed bioinformatics data. K.C.W., MD: Collected GCTB specimen. Y.H.W.: Performed experiments. L.H., PhD: Revise the manuscript. S.K.W.T., PhD: Designed the research and provided the equipment. O.K.L., MD, PhD: Interpreted the data and revised the manuscript. S.M.K., MD, PhD: Designed the research and approved the submitted version of the manuscript.
ACKNOWLEDGMENTS
This study was supported by CUHK Research Committee Funding (Direct Grants) to Professor Shekhar Madhukar Kumta (Reference no. 2014.1.046).




