Vancomycin displays time-dependent eradication of mature Staphylococcus aureus biofilms
ABSTRACT
This study was carried out to determine the time and concentration profile required to achieve vancomycin-mediated eradication of Staphylococcus aureus biofilm. This information is critical for the identification of performance targets for local antibiotic delivery vehicles that target biofilm infections. S. aureus UAMS-1 biofilms were grown for 7 days on titanium–aluminium–niobium discs in Mueller Hinton broth. After 7 days, the discs were then incubated in Mueller Hinton broth containing vancomycin at concentrations of 100, 200, 500, 1,000, and 2,000 mg/L. Biofilm eradication was assessed under both static and shaking conditions. Samples were retrieved at regular intervals for up to 28 days for quantification of residual biofilm. One additional disc was processed per time point for scanning electron microscopy. Progressive and significant reduction of viable bacteria was observed over time at all concentrations compared to unexposed controls. After 28 days under static conditions, the S. aureus biofilm was completely eradicated at 200 mg/L vancomycin and higher concentrations, but not at 100 mg/L. In contrast, bacterial biofilm could not be eradicated under shaking conditions at any concentration.
Clinical significance: The present study shows that it is possible to eradicate mature S. aureus biofilm from metal implants by vancomycin alone although the time concentration profile required cannot be achieved by systemic administration or any of the local delivery vehicles currently available. Identifying targets for antibiotic delivery is the first step in developing fit for purpose local antibiotic delivery vehicles that will successfully and predictably treat established biofilm infection. © 2016 Orthopaedic Research Society. Published by Wiley Periodicals, Inc. J Orthop Res 35:381–388, 2017.
The glycopeptide vancomycin is one of the most frequently used antibiotics in local delivery vehicles in orthopedic surgery.1-3 This is largely due to familiarity built up through years of clinical use,2 the spectrum of pathogens covered,4-6 and due to the fact that it is successfully released from polymethylmethacrylate (PMMA) bone cement,1, 7 the carrier material used most often in orthopedic and musculoskeletal trauma-related infections.2
Despite many improvements in the treatment of implant-associated infections, treatment failure remains a major clinical issue.8-10 Biofilm formation is one of the key elements explaining why implant-associated infections are able to resist antibiotic treatment.11-14 Bacteria within a biofilm are very resistant to antibiotics, resisting concentrations up to 1,000-fold higher than minimum inhibitory concentration (MIC) of the planktonic form, which has been attributed to metabolic dormancy, altered microenvironments within the biofilm, persister cells, and active resistance mechanisms.13, 15, 16
Vancomycin disrupts the synthesis of the peptidoglycan cell wall of Gram-positive bacteria, including staphylococci.14, 17-19 In vitro experiments have shown that vancomycin does penetrate into biofilm and is able to kill a large proportion (but not all) of biofilm-growing staphylococci within 24 h.14, 18 However, the concentration of antibiotic tested in these in vitro experiments (between 50 and 1,000 mg/L) are not achievable in patients via parenteral administration due to toxicity issues.20, 21 The current guidelines of vancomycin are 1 g every 12 h for 2 weeks22 that target trough serum concentrations of 10–15 mg/L and peak serum concentrations of 25–40 mg/L.21, 23 However, it remains unknown if elevated concentrations achieved by local antibiotic delivery can ever totally eradicate the biofilm, rather than merely achieve a reduction in viability.
The aim of this study was to identify the minimum concentration and duration required for vancomycin-mediated eradication of in vitro Staphylococcus aureus biofilm from titanium-7% aluminium-6% niobium (TAN) discs, a common implant metal. This information should prove a useful target for the many antibiotic releasing materials being developed for the treatment of biofilm-related infection.
MATERIALS AND METHODS
Bacterial Strain
S. aureus strain UAMS-1 (ATCC 49230) was used in this study (vancomycin MIC < 5 mg/L). For storage, a single colony was taken and resuspended in 1 ml Tryptic Soy broth (TSB, Sigma–Aldrich, Buchs, Switzerland) containing 20% glycerol and stored at −80 °C.
Discs
The discs used were manufactured from medical grade titanium alloy TAN (ISO 5832/11) and were 13 mm in diameter and 1 mm thick (surface area 1.33 cm2). All discs were anodized in the final processing step (KKS Ultraschall AG, Steinen, Switzerland). Before use, all discs were sonicated three times for 15 min, each time in 70% (v/v) ethanol, followed by one time for 15 min in sterile water using an ultrasound water bath (Bandelin Sonorex Super 10P, Bandelin, Berlin, Germany) operating at a frequency of 40 kHz with a peak ultrasonic output of 640 W. As a final step, the discs were air-dried, packed, and sterilized in an autoclave at 121 °C for 20 min.
Biofilm Formation on TAN Discs
S. aureus biofilms were grown on TAN discs in Mueller Hinton broth (MHB, Oxoid, Pratteln, Switzerland). Starting inocula were standardized to ∼3 × 107 CFU/ml, which was confirmed for each experiment by 10-fold serial dilution and plating on Mueller Hinton agar (MHA, Oxoid, Pratteln, Switzerland). One milliliter of inoculum was then added to each disc in a 24-well plate and incubated at 100 rpm at 37 °C for 7 days, with culture media exchange every 48 h.
After 7 days, MHB was aspirated, followed by adding and removing 1 ml of sterile phosphate-buffered saline (PBS, Sigma–Aldrich Chemie, Buchs, Switzerland) to remove loosely adherent bacteria. Thereafter, 1 ml of MHB, free of antibiotics or containing vancomycin (Sigma–Aldrich Chemie) at concentrations of 100, 200, 500, 1,000, or 2,000 mg/L, was added to the well. The mature biofilms were then incubated at 37 °C either statically or with shaking at 100 rpm. Antibiotic-free and antibiotic-containing MHB was refreshed every 48 h to ensure active and intact vancomycin was always present, and nutrients were always available for surviving bacteria. After 7, 14, 21, and 28 days, separate triplicate sets of discs were retrieved for quantification of viable biofilm. The fluid was removed from the wells before the discs were transferred into 10 ml of PBS solution in a sterile container. Biofilm was quantified by sonication of the disc for 5 min in an ultrasound water bath (Bandelin Sonorex Super 10P, Bandelin, operating as mentioned above) followed by 30 s of vortexing, and subsequent serial 10-fold dilution onto MHA plates. Plates were incubated at 37 °C, and colony forming units (CFU) were counted after 24 h.
Scanning Electron Microscopy
At each time-point, one additional sample disc was taken from each test series for scanning electron microscopy (SEM). Discs with biofilm were dehydrated through a series of ethanol steps (50%, 60%, 70%, 80%, 90%, 96%, and 100% ethanol for 5 min each) and then coated with 10 nm Gold/Palladium (Au/Pd). Images were taken in the secondary electron mode with an accelerating voltage of 3 kV and an emission current of 40 μA using a Hitachi S4700 scanning electron microscope (Hitachi, Tokyo, Japan).
Statistical Analysis
All experiments were performed independently at least two times and performed in triplicate. Data are presented as mean values ± standard deviations. Two-way analysis of variance (ANOVA) and Tukey's multiple comparisons test were applied comparing CFU counts using GraphPad Prism 5 software (GraphPad Software, La Jola, CA). Correlation of CFU counts with time was analyzed by logistic regression using SPSS version 22 (IBM Corp., Armonk, NY). p-values < 0.05 were considered statistically significant.
RESULTS
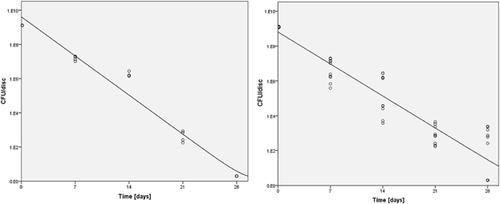
The average starting inoculum of S. aureus was consistent throughout the groups with a mean of 2.92 × 107 CFU/ml (range 1.36 × 107 to 5.42 × 107 CFU/ml). After 7 days of incubation at 37 °C, a biofilm developed and completely covered the discs (Fig. 1). The total viable bacterial count at this time averaged 1.27 × 109 CFU/disc (range 8.00 × 107 to 4.6 × 109 CFU/disc). In the absence of vancomycin, the bacterial amount within the biofilm increased further after the additional 7 days growth (d7), to a maximum of 2.91 × 1010 CFU/disc, thereafter reaching an apparent plateau (Fig. 2A).
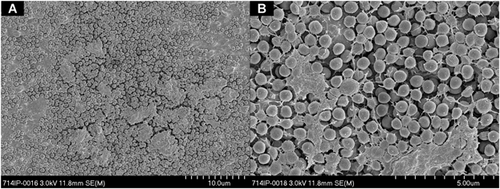
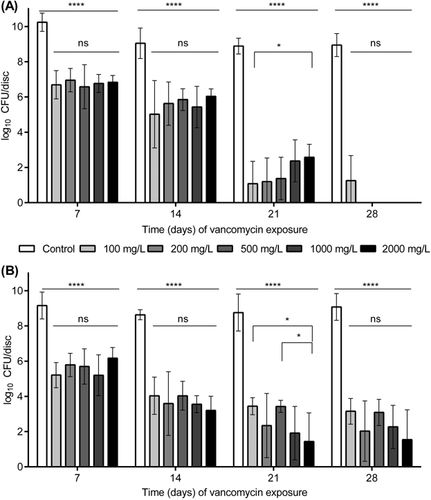
Exposing the 7-day-old mature biofilm to vancomycin for 7 and 14 days under static conditions resulted in a progressive decrease in viable bacteria cultured from the discs at all concentrations, and this reduction was statistically significant (p < 0.0001) compared to the unexposed control discs (Fig. 2). There was no statistically significant increase in bacterial killing with increased antibiotic concentration. By 21 days of vancomycin exposure, bacterial numbers decreased further, although at this time point there appeared to be an inverse relationship between concentration and anti-biofilm effect, with 100 mg/L unexpectedly displaying greater activity than 2,000 mg/L (p < 0.05). At day 28, all concentrations except 100 mg/L achieved complete bacterial eradication (Fig. 2A).
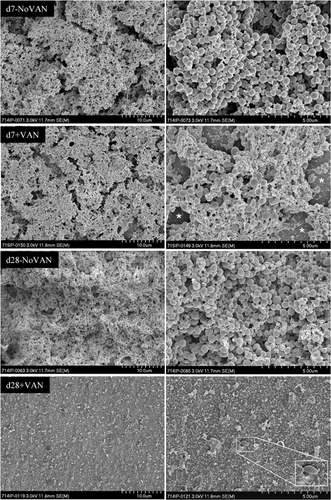
Qualitative SEM observations confirmed quantitative bacteriological counts. The control discs without vancomycin treatment revealed a thick bacterial layer after 7 and 28 days (Fig. 3). In contrast, after 7 days of vancomycin exposure with 1,000 mg/L, the bacterial layer appeared less densely packed with bacterial cells and the metal substrate became visible between the bacterial layers (Fig. 3). After 28 days of vancomycin exposure, only a few scattered and misshapen bacteria remained (Fig. 3). These remaining bacteria are most likely dead as no viable bacteria were retrieved from the discs at this concentration.
In the experiment with incubation under shaking conditions, the viable bacterial count from the 7-day-old biofilm (T0) retrieved from the TAN discs averaged 9.38 × 108 CFU/disc (range 5.5 × 107 to 1.82 × 109 CFU/disc), which was similar to the value for static conditions. In the absence of vancomycin, the bacterial amount within the biofilm increased further after an additional 7 days (d7) to a maximum of 3.67 × 109 CFU/disc (Fig. 2B), which was also similar to the value for static conditions. After 7 and 14 days of exposure to vancomycin, the total viable count of retrieved bacteria was reduced for all concentrations (Fig. 2B). Once again, there was no statistically significant increase in bacterial killing with an increase in antibiotic concentration at these time points. Exposing the biofilm to vancomycin for 21 days resulted in further reduction of viable bacteria, although at this time point, there was a concentration effect, with greater activity at 2,000 mg/L versus 100 and 500 mg/L (p < 0.05) (d21, Fig. 2B). At the final time point, bacterial biofilm could not be eradicated with any of the vancomycin concentrations (d28, Fig. 2B). The lowest average viable bacterial count at this time was 6.00 × 102 CFU/disc for the highest vancomycin concentration 2,000 mg/L (d28, Fig. 2B).
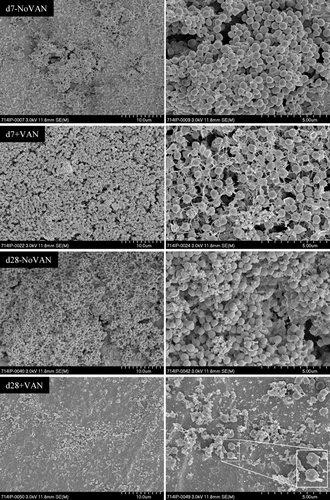
The pattern of reduction of bacterial numbers in the experiment under shaking conditions was also confirmed by SEM. The control discs without vancomycin treatment show a thick bacterial layer after 7 and 28 days (Fig. 4). Visually, there was no difference observed between the biofilm under static or shaking condition at these time points. After 7 days of vancomycin exposure with 2,000 mg/L, the bacterial layer appeared less dense with gaps appearing between bacteria (Fig. 4). However, bacteria were still detected after 28 days of vancomycin treatment (2,000 mg/L, Fig. 4). In comparison to the static condition where only scattered and misshapen, most likely dead bacteria were observed, these bacteria were normal-shaped and clustered. Since bacterial growth from the disc was seen, this indicates that these bacteria were likely still viable.
Comparing the results between the static and the shaking conditions, there was no statistically significant difference observed in anti-biofilm activity at d7 (Fig. 2A and B). Greater anti-biofilm activity was observed under shaking conditions at day 14 for all concentrations except for 100 mg/L (p < 0.05). At d21, the situation was somewhat reversed and greater anti-biofilm activity was observed under static conditions (only for 100 and 500 mg/L). At day 28, anti-biofilm activity was greater under static conditions. The decrease in CFU count due to vancomycin exposure over time is shown in Figure 5.
DISCUSSION
The ability of bacterial biofilms to resist antibiotic therapy is one of the key challenges in treating implant-associated infections.11-14 Prior in vitro experiments have shown that vancomycin is able to penetrate Staphylococcus biofilms14, 18, 24 and can achieve significant reduction in viable bacterial counts within 24 h (e.g., 96% reduction at 160 mg/L vancomycin).14 However, any biofilm remnants are likely critical in failed treatment and so complete eradication should be the target of antimicrobial therapy. However, the time:concentration profile required for complete eradication of staphylococcal biofilms by vancomycin remains unknown. Thus, the present study aimed to identify basic parameters required for vancomycin-mediated eradication of in vitro S. aureus biofilm from metal discs, simulating a colonized orthopedic implant.
Two observations were seen in this experiment. Firstly, under static conditions, complete eradication of S. aureus biofilm can be achieved by vancomycin alone. Secondly, biofilm eradication was not possible under conditions of fluid flow. The first observation is of utmost importance as it justifies further efforts to optimize antibiotic delivery for the treatment of implant-associated infections.
After growing the biofilm for 7 days under shaking conditions to a mature biofilm, the vancomycin eradication phase followed either under static or shaking conditions. Under static conditions, complete vancomycin-mediated biofilm eradication could be achieved with a concentration of 200 mg/L or higher for a minimum of 28 days. Studies in human patients have shown that vancomycin released from PMMA spacers achieve a peak concentration up to 1,500 mg/L in joint fluid within the first 24 h. However, this concentration rapidly drops to levels under 100 mg/L.25 Interestingly, vancomycin activity against S. aureus biofilms is not a concentration-dependent phenomenon: Increasing the concentration of the antibiotic did not have any significant effect in bacterial killing at most time points. This reflects the partially time-dependent (AUC24/MIC, T > MIC) activity of vancomycin against planktonic staphylococci,26, 27 but to the best of our knowledge this has not been previously assessed for biofilms. This result has implications for the ideal delivery parameters to be achieved by vancomycin-loaded drug delivery vehicles. Such concentrations remain, however, approximately 10 times higher than those achievable via systemic administration with vancomycin trough concentrations between 10 and 15 mg/L20, 21 and prolonged systemic exposure would also have significant patient toxicity concerns.23 Local administration of vancomycin might, however, be capable of approaching these concentrations while maintaining a low systemic exposure. PMMA is the carrier material currently most often used in orthopedic and trauma surgery.2 Within the context of our data, it now appears that the concentration of vancomycin eluted from PMMA in human patients is only high enough for the first few days with a peak concentration above 1,500 mg/L in joint fluid within the first 24 h, and rapidly dropping to insufficient levels.1, 25 As such current vehicles are unlikely to independently treat an established biofilm infection. There are a number of practical techniques to improve vancomycin delivery from PMMA. Increasing the surface area,1 applying antibiotic solutions, rather than powder,28 or poragens such as xylitol29 may all improve release kinetics. However, constant high levels of vancomycin elution from these modified or standard carrier material has not been achieved.1, 7 More continuous and prolonged release kinetics would be desirable.
Of course, if more advanced local delivery vehicles finally do achieve the targets identified in this study, local cytotoxicity should be considered. Vancomycin has been shown in vitro to have no effect on osteoblast replication or proliferation for at least 14 days at concentrations up to 2,000 mg/L,30-32 indicating biofilm eradication might be achievable without local toxicity. Further extended observation times may be required to confirm this for antibiotic release extending up to 30 days and beyond.
With regard to testing anti-biofilm efficacy, the maturity of the biofilm needs to be considered. Most in vitro studies examine immature biofilms of less than 24 h, and killing of bacteria by antibiotics is then still possible.14, 33, 34 For example, S. aureus growing and attaching for 2 h to fibronectin-coated coverslips were still optimally killed by antimicrobial agents such as oxacillin (4 mg/L) and vancomycin (8 mg/L).33 In contrast, a thick, multi-layered, mature biofilm that is comparatively metabolically inactive, allows bacteria to tolerate extremely high antibiotic concentrations and therefore eradication may be significantly more challenging.13, 33-35 Stewart recently summarized the investigation of several studies testing the efficacy of antibiotics against biofilms of different ages.34 From this comparison, it was found that biofilms develop tolerance of antibiotics within a few days.34 This is especially critical in orthopedic and trauma implant-associated infections as these infections might be undiscovered for a number of weeks, at which stage the bacterial biofilm has become tolerant of antibiotics. In our study, we examined a 7-day-old mature biofilm, reflective of a mature biofilm.35 While differences can be observed between S. aureus strains regarding their ability to form biofilm36, 37 the selected reference strain UAMS-1 is a robust biofilm forming strain in vitro38 and thus a representative of the worst case scenario with regard to eradication of biofilm.
We also examined whether vancomycin treatment under shaking conditions influences biofilm eradication. Although horizontal shaking cannot be regarded as a controlled flow, it does provide some indication of the possible influence of wound fluid flow. A significant difference between static and shaking condition was seen after 28 days, whereby the biofilm could not be completely eradicated under shaking conditions, contrary to static conditions. Although to our knowledge, no mechanism has been described for an increase in antibiotic resistance secondary to fluid flow, a stress-type response may in theory induce antibiotic resistance within the bacterial population. Changes in the biofilm structure may significantly influence antibiotic tolerance within biofilms39-42 and bacteria in biofilm react to shear by changing their morphology, size, density, growth rate, and metabolism.39, 42 These differences most likely also might have an influence on the susceptibility to antimicrobial treatments and may at least partially explain our findings.
The exact conditions, with respect to fluid flow, around an infected orthopedic or trauma implant are to the best of our knowledge unknown, and thus how the results under shaking conditions relate to treatment outcome remains unclear. Should fluid exchange and motion be present at sufficient levels adjacent to orthopedic implants, then significantly longer exposure periods might be necessary in vivo, and thus set local delivery performance targets at an even more challenging level.
Some limitations to the present study are noteworthy. For example, S. aureus biofilm eradication was tested only with one clinical S. aureus strain. The eradication of biofilm from other strains, for example, some with confirmed vancomycin resistance remains to be evaluated. Furthermore, the effect of bacterial biofilm eradication using different antibiotics alone and in combination would be of interest considering the standard treatment of infection involves combinations of two or more antibiotics. In this study, vancomycin was the antibiotic of choice as it has a high rate of clinical utilization, particularly in local application. However, other antibiotics commonly used in local delivery vehicles such as the aminoglycosides gentamicin and tobramycin, or linezolid and aztreonam have been shown in vitro as well as in vivo to be effective in killing of bacteria within a biofilm.14, 25, 32 Rifampicin alone or in combination with other antibiotics such as vancomycin or teicoplanin has also been shown to be effective in eradication of biofilm.43-45 However, the use of rifampicin in local delivery should be carefully managed due to toxicity46 and the ease of emergence of resistance.47, 48 Furthermore, rifampicin in local carriers such as PMMA might not be suitable as curing of PMMA is delayed or incomplete.45
CONCLUSION
The present study shows that S. aureus biofilm eradication from TAN implants was possible by vancomycin alone. However, this was only achieved by concentrations higher than 100 mg/L for extended periods. However, the required concentrations are not achievable by systemic therapy, and the duration required is not achieved by currently available local antibiotic delivery vehicles.
AUTHORS’ CONTRIBUTIONS
VP conducted and designed the research. PW, GR, and FM designed the study. All authors have read and approved the final manuscript.
ACKNOWLEDGMENT
The authors would like to thank P. Furlong, AO Research Institute Davos, for taking the SEM pictures.




