Use of novel chitosan hydrogels for chemical tissue bonding of autologous chondral transplants
ABSTRACT
The objective of this study was to evaluate the effect of chemical tissue bonding (CTB) on adhesion strength, fluid permeability, and cell viability across a cartilaginous graft-host interface in an in vitro autologous chondral transplant (ACT) model. Chitosan-based cross-linkers; Chitosan-Rose Bengal [Chi-RB (Ch-ABC)], Chitosan-Genipin [Chi-GP (Ch-ABC)], and Chitosan-Rose Bengal-Genipin [Chi-RB-GP (Ch-ABC)] were applied to bovine immature cartilage explants after pre-treatment with surface degrading enzyme, Chondroitinase-ABC (Ch-ABC). Adhesion strength, fluid permeability and cell viability were assessed via mechanical push-out shear testing, fluid transport and live/dead cell staining, respectively. All three chitosan-based cross-linkers significantly increased the adhesion strength at the graft-host interface, however, only a statistically significant decrease in fluid permeability was noted in Chi-GP (Ch-ABC) specimen compared to untreated controls. Cell viability was maintained for 7 days of culture across all three treatment groups. These results show the potential clinical relevance of novel chitosan-based hydrogels in enhancing tissue integration and reducing synovial fluid penetration after ACT procedures in diarthoidal joints such as the knee and ankle. © 2015 Orthopaedic Research Society. Published by Wiley Periodicals, Inc. J Orthop Res 34:1139–1146, 2016.
Cartilage reconstruction surgery, such as autologous chondral/osteochondral transplantation (ACT), aims at restoring the contour of the articular cartilage surface by replacing a chondral/osteochondral lesion with an autologous transplant. Although such a technique had good-to-excellent early clinical outcomes, recent studies have expressed concern over the transplants’ long term durability.1-5 Major concerns include the formation of fibrocartilage, which is biomechanically inferior to hyaline cartilage, at the graft-host interface. This has been implicated as a possible focal point that compromises the integrity of the graft.2-4, 6 Several factors have been identified to be involved in the pathophysiology of this process. The integrative repair of the graft-host interface may be inhibited by synovial fluid components by binding to the boundaries between the chondral/osteochondral graft and the native cartilage.7, 8 Synovial fluid may also intrude along the graft-host interface to produce hydrostatic pressure mediated subchondral cysts.9, 10 Therefore, the early integration of the graft-host interface is a unique challenge that, if not addressed, may lead to subchondral cyst formation, poor graft-host integration, and graft destruction.
Chemical and photochemical tissue bonding (CTB/PCTB) has been used in the repair of various collagen-based tissues such as skin, vocal cords, cornea, intestine, blood vessels, and cartilage.11-13 Unlike laser tissue ablation, CTB induces extracellular matrix (ECM) bonding across a repair interface without producing a barrier to tissue integration and with no collagen denaturation (32°C).14 This is achieved with the aid of agents that are activated by either chemical (CTB) or photosensitive (PCTB) reactions to establish a bond, such as through collagen cross-linking to itself or another biomaterial.
Chitosan (Chi) is a natural and biodegradable polysaccharide that has been widely used in various biomedical applications, including cartilage regeneration.15 Due to its abundant amino groups in its main chains, it promotes the formation of stable collagen-chitosan complexes.16, 17 In addition, chitosan has some of the same structural characteristics as glycosaminoglycans (GAGs), the second major constituent of articular cartilage after collagen. This makes chitosan an appealing GAG analog for cartilage repair.18 Rose Bengal (RB) is a biocompatible photosensitive cross-linking agent that absorbs light at much lower wavelengths (431–532 nm) than other photosensitive agents and has been used clinically with safety in corneal healing.19-21 A further favorable chemical cross-linker is the naturally occurring reagent, genipin (GP), which is biocompatible, significantly less cytotoxic than other cross-linking agents and used in cartilage tissue engineering applications.22-24
The aim of this study was to investigate the ability of three different cross-linkers combined with a chitosan-based hydrogel carrier to bond the articular cartilage at the graft-host interface in an in vitro ACT-like model: Chitosan-Rose Bengal (Chi-RB), Chitosan-Genipin (Chi-GP), and Chitosan-Rose Bengal-Genipin (Chi-RB-GP), applied together with a surface-degrading agent Chondroitinase-ABC (Ch-ABC). Treated graft-host interfaces were evaluated for adhesion strength, fluid permeability and cell viability. We hypothesized that CTB would increase adhesion strength and decrease permeability at the interface without noticeable cytotoxic effects on chondrocytes.
MATERIALS AND METHODS
Tissue Preparation
Five to seven full-depth, 14.5 mm diameter cylindrical plugs of articular cartilage, without subchondral bone, were harvested from the trochlea and femoral condyles of six immature bovine stifle joints (4–8 weeks old, male and female) acquired from a local abattoir within 24 h of the animals death. From these plugs, 2–3 mm of cartilage was removed from the top and bottom to obtain two flat and parallel surfaces. Then, ten 2 mm thick discs were made from each, stored in protease inhibitor cocktail (cOmplete Protease Inhibitor tablets, Roche) at 4°C for no more than 48–72 h and randomly distributed based on location and knee for each mechanical push-out shear test. The removed articular cartilage sections were maintained in serum-rich culture media [Dulbecco's modified Eagle medium (DMEM, 4.5g/L D-Glucose, L-Glutamine, no Sodium pyruvate), 10% Fetal Bovine Serum (FBS), 100U/ml antibiotic-antimycotic (GIBCO, Gaithersburg, MD)] for cell viability testing or stored in protease inhibitor for permeability testing, as explained above. On CTB treatment day, 5 mm diameter defect was then created in the center using a biopsy punch, establishing an outer annulus and inner core pair. Inner cores and outer annulus were not mismatched to ensure a good fit by minimizing the interface gap, and to prevent the inner core from dislodging while treatments were applied. Prior to testing each disc was measured for thickness.
Enzymatic Degradation and Photo/Chemical Tissue Bonding Treatments
In order to enhance tissue bonding, an enzymatic treatment using Ch-ABC was performed on specimens to remove proteoglycans at the interfacial surface. For specimens intended for tissue bonding treatments, 1 U/ml of Ch-ABC (Sigma, St. Louis, MO) was brushed onto the inner surface of the annulus and outer surface of the core and incubated for 15 min at room temperature (RT).12, 25 To end the enzymatic degradation process, specimen were washed in PBS (Phosphate-Buffered Saline) containing protease inhibitors for 10 min at room temperature (RT). The chitosan solution was prepared in 1% acetic acid and neutralized to pH 7 using β-glycerophosphate (Sigma).21, 26, 27 A 100 μl solution of chitosan (2.0% w/v; Sigma) with a cross-linker/tissue bonding reagent (Chi-RB, Chi-GP, or Chi-RB-GP) was then deposited into the cavity of the annulus, and the core was inserted into the annulus. Specimens treated with GP (WAKO Chemicals, Germany) were incubated for 15 min at RT to allow diffusion of the GP into the tissue and initiation of the chemical gelation process. Samples treated with RB (Sigma) were also incubated for 15 min, and then exposed to visible light filtered with a band pass filter (431 nm ± 2) for another 15 min using an 8 mm diameter endoscopic light source (Concept Inc., Intravision 7400 Light Source, 150W) located 25 mm above the specimen.28 These settings were calculated to allow for an optical penetration of depth of 1.47 mm with a power density of 7.5 mW/cm2. For the control group used in mechanical testing and tissue culture, the cores were inserted into their respective annuli and incubated in PBS at RT without any CTB treatments. Preliminary testing using a range of concentrations led to the final tests being performed with 2.87 mg/ml solution of deacetylated chitosan (85%), 1 mg/ml of GP, and 0.14 mg/ml RB (data not shown). The following experiments were, as a result, performed on Chi-RB (Ch-ABC), Chi-GP (Ch-ABC), and Chi-RB-GP (Ch-ABC).
Adhesion Strength Mechanical Testing
Post tissue bonding treatments, push-out shear tests were performed to determine the adhesion strength at the interface. The thickness of the specimens used for adhesion strength were 2.43 ± 0.24 mm. Treated and control cartilage explants (n = 5/group) were firmly mounted into a stainless steel loading fixture that included a ring to prevent the specimen from buckling during push-out testing (Fig. 1A). A 4.5 mm diameter solid platen was used to load the specimen's inner core. The platen was displaced at a rate of 2 mm/s and the force was recorded using a 22.24 N load cell (Sensotec, Columbus, Ohio). The adhesion shear strength was defined as the maximum force required to break the bond divided by the interfacial surface area. The treatment combination that resulted in the highest adhesion strength were chosen for further testing, looking at permeability before and after treatments and cell viability post treatment.
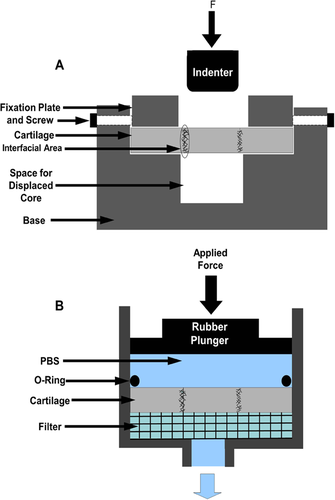
Permeability Testing
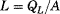 (1)
(1)Cell Viability
Following Ch-ABC and CTB treatments, treated and control specimens were maintained incubated in serum-free culture media [Advanced DMEM/F12 (GIBCO, Gaithersburg, MD) supplemented with 0.1 g/ml dexamethasone (Sigma), 50 mg/ml ascorbate-2-phosphate (Sigma), 100U/ml antibiotics-antimycotic, and 0.05 mg/ml L-Glutamine (Invitrogen, Eugene, OR)] for up to 7 days, with media changed every 2 days (n = 3/ group). After 0, 3, and 7 days, cell viability was assessed using the LIVE/DEAD Cell Viability/Cytotoxicity Kit (Invitrogen). A thin (∼1 mm thick) cross-section through the center of each cartilage explant was stained according to the manufacturer's instructions. Briefly, the cross-sections were washed three times in PBS (10 min each), incubated in a solution containing 2 µM Calcein AM and 2 µM EthD-1 for 40 min followed by three PBS washes (10 min each). Images were captured at several penetration depths, approximately 7 μm apart, using a confocal microscope (Leica TC SSP5). The images were then overlaid to produce the final composite images. Three independent experiments were performed in duplicates for each time point and CTB condition. The zone of chondrocyte death was determined by measuring the distance from the edge of the cartilage at the interface to the first live chondrocyte. Ten measurements were made along the edge of the cartilage for each treatment and each time point using Image J software.
Histology and Immunohistochemistry
After 0, 3, and 7 days in culture, specimens were fixed in 10% formalin at 4°C for 2 days, dehydrated and embedded in paraffin for histological analysis. Six-micrometer-thick sections were routinely dewaxed and rehydrated. Slides were then stained with either Safranin O (Saf-O) and counterstained with Fast Green, or antibodies for collagen type I (Col I, 1:60; CalBioChem, San Diego, CA) and collagen type II (Col II, 50 ng/μl; Developmental Studied Hybridoma Bank at the University of Iowa, Iowa City, IA). Saf-O staining was used to visualize proteoglycan content and the presence of chitosan. Col I slides were blocked using H2O2 for 30 min followed by three 5 min washes with PBS. They were then protein blocked for 20 min. The primary antibody was incubated overnight at room temperature. DAB was used for detection and incubated for 20 min. Col II slides were processed the same way except with an additional hyaluronidase treatments before the protein block. Washing between steps were 5 min with PBS. Col II immunohistochemistry was performed to detect the presence of Col II in the extracellular matrix (ECM) and assess the effect of Ch-ACB pretreatment in exposing Col II fibers. Col I immunohistochemistry was used as a negative control and to detect the presence of fibrocartilage formation at the interface. Both Col I and II immunostaining were performed without the use of antigen retrieval in order to get a better representation of the collagen fibers exposed by the Ch-ABC pretreatment at the cut edges of the annulus and core.
Statistical Analysis
For mechanical push-out shear testing, five independent experiments were performed for each treatment group (n = 5) and one-way repeated measures ANOVA with Tukey post-tests was used to compare treatments (α = 0.05). For permeability testing, nine independent experiments (n = 9) were performed and ANOVA with Tukey post-tests was used to compare the conditions and treatment groups (α = 0.05). For the zone of chondrocyte death a two-way repeat measures ANOVA with Tukey post-tests was used to compare all treatments and time points (α = 0.05). All parameters are given as mean ± standard error of the mean (SEM). All statistical tests were performed using Graph Pad Prism software (GraphPad 6 Software Inc., San Diego, CA).
RESULTS
Adhesion Strength
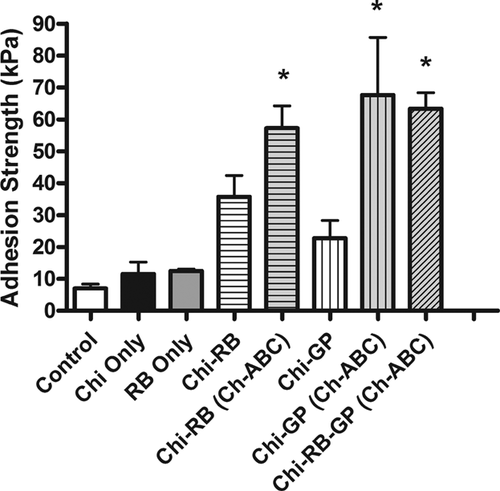
Results from testing various hydrogels, tissue bonding reagents, and pre-treatments on our in vitro model determined that the best results were achieved using combinations of chitosan, Ch-ABC, and tissue bonding reagents (Fig. 2). The use of chitosan in combination with GP (Chi-GP) and/or RB (Chi-RB and/or Chi-RB-GP) with the Ch-ABC pretreatment had a significant effect on the interfacial adhesion strength between the annulus and the inner core. Treating cartilage explants with Chi-RB (Ch-ABC), Chi-GP (Ch-ABC), and Chi-RB-GP (Ch-ABC) each significantly increased the adhesion strength at the cartilage-to-cartilage interface when compared to the control (Fig. 2). Chi-RB (Ch-ABC) (p = 0.0092), Chi-GP (Ch-ABC) (p = 0.021), and Chi-RB-GP (Ch-ABC) (p = 0.0026) treatments resulted in respective mean adhesion strengths of 57.27 ± 7.01 kPa, 67.61 ± 18.10 kPa, and 63.32 ± 5.02 kPa, while controls, Chi Only, Chi-GP, Chi-RB, and RB treated specimen resulted in lower mean adhesion strengths of 7.01 ± 1.36 kPa, 11.53 ± 6.51 kPa, 22.78 ± 12.38 kPa, 35.76 ± 13.30 kPa, and 12.49 ± 0.87 kPa, respectively. Interestingly, there was no statistical significance found between Chi-RB (Ch-ABC), Chi-GP (Ch-ABC), and Chi-RB-GP (Ch-ABC) treatment groups.
Permeability
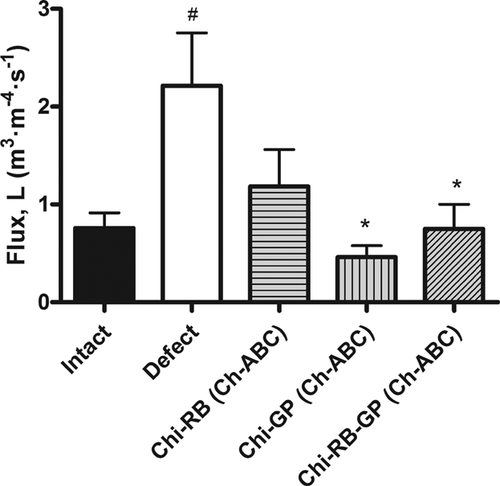
Fluid flux testing of intact, defect and Chi-GP (Ch-ABC), Chi-RB (Ch-ABC), and Chi-RB-GP (Ch-ABC) treated cartilage explants found that CTB treatments were able to prevent fluid infiltration into the graft-host interface. Although minimal, intact cartilage explants allowed some fluid to pass through the extracellular matrix due to the permeable nature of immature cartilage (Fig. 3). Creating a defect into the intact specimen and inserting the core into its annulus without any Ch-ABC or CTB treatment resulted in a significant increase in fluid flux from 0.76 ± 0.15 m3 · m−4 · s−1 to 2.213 ± 0.54 m3 · m−4 · s−1, measured as an increase in the fluid flux (p = 0.0004). A statistically significant decrease in fluid flux was only seen in both Chi-GP (Ch-ABC) and Chi-RB-GP (Ch-ABC) treated specimens with average fluid fluxes of 0.46 ± 0.12 m3 · m−4 · s−1 (p = 0.0015), and 0.75 ± 0.25 12 m3 · m−4 · s−1 (p = 0.0435), respectively. However, for all treatment groups, no statistically significant difference was observed between the intact and treated states.
Cell Viability
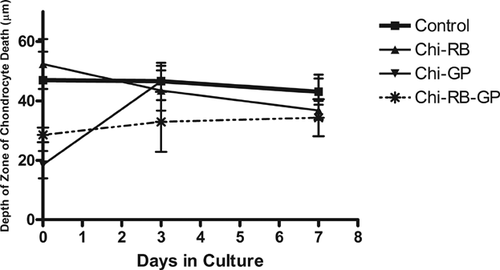
Chondrocyte viability was evaluated to assess the cytotoxicity of the three CTB treatments after 0, 3, and 7 days in culture. Shown in Figure 4 are confocal images of untreated control and treated cartilage explants, with the annulus-inner core interface located in the middle and the articular surface at the top of each image. Untreated control cartilage explants, had viable cells independent of time in culture. However, at the peripheral areas of the explants there was some cell death which corresponded to the areas where cuts were made to section the samples.30 The size of the zcd of the CTB treated explants was not statistically significant compared to the untreated control explants regardless of treatment type and culture time (p > 0.05). Therefore, prolonged time in culture did not affect chondrocyte viability in treated or control specimens.
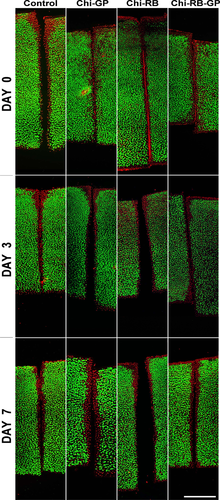
Histochemical and Immunohistochemical Staining
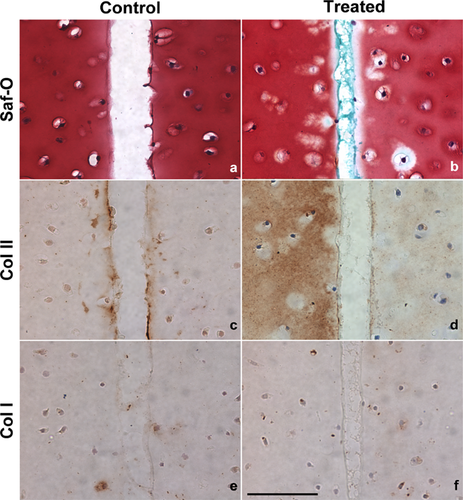
Histochemical and immunohistochemical staining was performed on CTB treated with Ch-ABC pretreatment and control (without any treatments) cartilage explants to determine the effects of the enzymatic degradation and PCTB at the graft-host interface. Representative images of Control-Day 3 and Chi-GP (Ch-ABC)-Day 3 treated cartilage explants are shown in Figure 5, with the annulus-inner core interface located in the middle of each image. The interface of the Chi-RB (Ch-ABC) and Chi-RB-GP (Ch-ABC) treated specimen produced similar results to the Chi-GP (Ch-ABC) treated specimen (images not shown). Saf-O staining of all treated specimens revealed proteoglycan depletion at the edges as a consequence of the Ch-ABC pretreatment (Fig. 6a), and chitosan can be visualized in blue/green at the interface due to the Fast Green strong affinity for positively charged chitosan (Fig. 6b).31 Chitosan also appeared as translucent in the Col I and II immunostaining images (Fig. 6d and f). Due to the Ch-ABC pretreatment at the edges, Col II fibers were exposed as evidenced by a more intense brown staining (Fig. 6d) compared to untreated specimens (Fig. 6c). No Col II staining was detected at the interface of Ch-ABC and CTB treated specimens (Fig. 6c and d). Col I was not detected within any samples or at the interfaces for both control and PCTB treated specimens (Fig. 6e and f).
DISCUSSION
Although cartilage replacement procedures, such as chondral/osteochondral transplantation, have resulted in promising outcomes by replacing degenerative cartilage with a hyaline cartilage graft, the chondral portion of the transplant does not integrate well with the host cartilage. The gaps at the graft-host interface are potentially spaces that are filled with a non-homogenous repair tissue, often fibrocartilage. This facilitates intrusion of synovial fluid along the interface and may produce subsequent subchondral edema and cyst formation.2, 7 Strategies to improve the integrity of the graft-host interface may prevent this ingress of synovial fluid and prevent long-term tissue degeneration. The results of this study show that the use of Chi-RB, Chi-GP, and Chi-RB-GP combined with pre-treating the surface with Ch-ABC had a significant effect on increasing adhesion strength at the graft-host interface compared to untreated groups, while also substantially decreasing fluid flux at the graft-host interface to levels approximating that of intact explants (except in the Chi-RB (Ch-ABC) group). The significant difference in the fluid flux of Chi-GP and Chi-RBGP treated specimen compared to the defect specimen suggests that GP is the more efficient cross-linker than RB, under these circumstances. These results also represent the lack of effect the addition of RB to the Chi-GP combination has on both adhesion strength and fluid flux.
In similar in vitro models, adhesion strengths of approximately 155 kPa for 1% trypsin-treated explants have been reported, while a mean value of 104 kPa was reported for explants treated with Ch-ABC and a phthalocyanine photosensitive dye.12, 25, 32 Adhesion strengths up to 65 kPa, achieved by guanidine or pepsin pre-treatment with various cross-linking agents, have also been reported.12, 25, 32 This is comparable to an overall mean adhesive shear strength of 62.7 kPa for our chitosan-based tissue bonders. Differences in adhesive shear strengths between previous studies are probably due to differences in testing methods as values in the current study were still significantly higher than untreated explants. This increase in adhesion strength at the graft-host interface in conjunction with decreased permeability at this interface suggest that CTB has a potential role in preventing the aforementioned drawbacks of chondral/osteochondral transplantation that may lead to disintegration of the graft-host interface, compromising the long term results of such procedures.
Harvesting of chondral/osteochondral grafts usually results in a zone of chondrocyte death, which creates a barrier that chondrocytes have to migrate through in order to populate the graft-host interface.33 Major concerns with early conventional cross-linkers such as glutaraldehyde included their high cytotoxicity.34, 35 GP has less cytotoxic effects on cells, from 5,000 to 10,000 times less toxicity than grutaraldehyde.36 Chi-GP scaffolds for cartilage tissue engineering were found to be non-cytotoxic to chondrocytes as well as stem cells in vitro, which maintained morphology, surface adherence and cell-to-cell interaction.26, 27 An in vitro calf intestine repair model with Chi-RB adhesive films demonstrated confluent fibroblastic growth across the repair site without any cellular morphological changes with temperatures not exceeding 32°C.21 The current study demonstrates that all three chitosan-based CTB cross-linkers had no effect on chondrocyte viability at the graft-host interface, which is similar to the PCTB findings reported by other in vitro ACT-like models.12
The proteoglycan rich-matrix of articular cartilage has anti-adhesive properties that inhibit cell adhesion and collagen cross-linking.37, 38 The effect of pretreatment with surface degrading agents that remove proteoglycans at the graft-host interface has been well established in enhancing integration of chondral surfaces. Enzymes employed for articular cartilage degradation include trypsin, Ch-ABC and hyaluronidase.32, 39, 40 While articular cartilage has not been shown to bond when treated only with cross-linkers, pre-treatment alone with Ch-ABC has been found to enhance reparative cells migration across the graft-host interface by 53% as well as chondrocyte adhesion force across all layers of hyaline cartilage explants.12, 25, 39 Moreover, aggressive Ch-ABC pre-treatment at the interface resulted in increased bond strengths after cross-linking.12 McGregor et al. reported an 80% reduction in the zone of chondrocyte death in an in vitro ACT-like model after pre-treatment with surface degrading enzymes followed by various chemotactic agents.30 Results from this study has also found that the Ch-ABC treatment before the cross-linking agents were applied have a beneficial effect of increasing adhesion strengths when compared to specimens not pretreated with Ch-ABC. For these reasons, in the current study all experimental groups were pre-treated with Ch-ABC prior to CTB treatment.
There are a number of limitations to this study. Firstly, the effect of graft-host interface biological healing was not investigated in this study due to its in vitro nature. However, the current study exhibits a stable front of viable chondrocytes throughout the graft-host interface. This would theoretically support the potential for gap filling and cartilage-to-cartilage healing instead of an area of potential cell death and disintegration. Secondly, we did not investigate differential adhesion shear strengths among the different zones of articular cartilage. It has been demonstrated that little strength develops between opposed superficial layers of cartilage after pre-treatment, and that adhesion strength increases towards the middle and deep zones.7, 10 While, this might have implications at the microscopic level and thus on the long term results of PCTB, our aim was to test changes in global adhesion and permeability throughout the entire depth of the cartilaginous graft-host interface. This may be more clinically relevant in order to prevent the events after ACT that lead to disintegration of the graft-host interface. Thirdly, our model was designed of immature bovine cartilage explants for feasibility of acquisition and well established models in the literature. Immature cartilage, however, differs in extracellular matrix content, structure, and mechanical properties than mature cartilage, which should be considered when constructing future in vivo animal experimental models.41, 42 Fourthly, cell viability was not assessed in long-term cultures after CTB, which should be a point of further investigation. Finally, we did not investigate on a molecular level what effects the interaction between native cartilage collagen fibers and the various components of the CTB treatment had on the graft-host interface that might have resulted in such observations reported in our study. A potential explanation is that chitosan provided a polymerizable hydrogel with sufficient improved mechanical properties that enhanced the tissue bonding effect of the various cross linkers. Another explanation could be the presence of a potential synergistic effect in adhesion strength when combing both chemical (GP) and photochemical (RB) tissue bonders. These molecular studies could also provide information on why the Chi-RB (Ch-ABC) treatment has similar adhesion strengths to the other treatments but does not result in a statistically significant fluid flux compared to the defect specimen. This requires further studies on a molecular level for further investigation and validation, which was beyond the scope of the current study.
In conclusion, this study has demonstrated the potential of CTB in an ACT-like model to increase adhesion shear strength and decrease fluid permeability without affecting cellular viability at the graft-host interface. Further in vivo studies are required to assess reproducibility of such effects in animal models. Our results indicate that CTB may have clinical relevance in potentially enhancing tissue integration and preventing fibrocartilage as well as subchondral cyst formation, all of which may aid in improving long-term survivorship, and clinical outcomes of chondral and osteochondral transplants in weight-bearing joints such as the knee and ankle.
AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to the research design, or the acquisition, analysis or interpretation of data; participated in drafting the paper or revising it critically; and have read and approved of the final version of this manuscript.
ACKNOWLEDGMENTS
Support for this study was made possible by Grant Number R21-AR059203 (PAT) from the National Institutes of Health—National Institute of Arthritis and Musculoskeletal and Skin Diseases. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH-NIAMS. This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program Grant Number C06-RR12538-01 from the National Center for Research Resources, National Institutes of Health. Additional funding for this study included educational grants from the Ohnell Family Foundation and Mr. and Mrs. Micahel J. Levitt, given directly to Hospital for Special Surgery.




