Lidocaine induces ROCK-dependent membrane blebbing and subsequent cell death in rabbit articular chondrocytes
ABSTRACT
Local anesthetics are administered intraarticularly for pain control in orthopedic clinics and surgeries. Although previous studies have shown that local anesthetics can be toxic to chondrocytes, the underlying cellular mechanisms remain unclear. The present study investigates acute cellular responses associated with lidocaine-induced toxicity to articular chondrocytes. Rabbit articular chondrocytes were exposed to lidocaine and their morphological changes were monitored with live cell microscopy. The viability of chondrocytes was evaluated using a fluorescence based LIVE/DEAD assay. Acute treatment of chondrocytes with lidocaine (3–30 mM) induced spherical protrusions on the cell surface (so called “membrane blebbing”) in a time- and concentration-dependent manner. The concentration-response relationship for the lidocaine effect was shifted leftward by elevating extracellular pH, as expected for the non-ionized lidocaine being involved in the bleb formation. ROCK (Rho-kinase) inhibitors Y-27632 and fasudil completely prevented the lidocaine-induced membrane blebbing, suggesting that ROCK activation is required for bleb formation. Caspase-3 levels were unchanged by 10 mM lidocaine (p = 0.325) and a caspase inhibitor z-VAD-fmk did not affect the lidocaine-induced blebbing (p = 0.964). GTP-RhoA levels were significantly increased (p < 0.001), but Rho inhibitor-1 failed to suppress the membrane blebbing (p = 0.875). Lidocaine (30 mM) reduced the cell viability of isolated chondrocytes (p < 0.001) and in situ chondrocytes (p < 0.001). The chondrotoxicity was attenuated by pretreatment of cells with ROCK inhibitors or a myosin-II inhibitor blebbistatin (p < 0.001). These findings suggest that lidocaine induces ROCK-dependent membrane blebbing and thereby produces a cytotoxic effect on chondrocytes. © 2015 Orthopaedic Research Society. Published by Wiley Periodicals, Inc. J Orthop Res 34:754–762, 2016.
Local anesthetics (LAs) are widely used intraarticularly for many years, both in perioperative and ambulatory settings. Clinical use for intraarticular anesthesia requires the administration of high concentrations of LAs to achieve rapid and sufficient pain suppression. For example, clinically used doses of lidocaine are in the range 0.5–1.5%,1 which corresponds to 18–55 mM. Despite this clinical usage, high concentrations of LAs have been shown to be toxic to various types of cells, such as myocytes, neurons, corneal epithelial cells, synoviocytes, mesenchymal stem cells, tenofibroblasts, and articular chondrocytes.2-8 Previous studies demonstrated that LAs markedly reduce the viability of chondrocytes under in vitro and in vivo circumstances.9 Such deleterious effects of LAs on chondrocytes have been postulated to be a crucial event in the development of irreversible joint damage after intraarticular injection of these drugs,10 because chondrocytes are only cartilage cells responsible for maintenance of the extracellular matrix.
Cell death is generally classified into at least two forms, apoptosis and necrosis. Chondrocyte apoptosis is involved in not only physiological processes but also degenerative cartilage diseases. Increased chondrocyte apoptosis causes hypocellularity in the cartilage, which is believed to be associated with progression of osteoarthritis (OA).11 On the other hand, necrosis occurs as a result of cellular damage, hypoxia or exposure to toxins, which results in reduced matrix generation in impact damaged cartilage.12 Grishko et al. (13) reported that long-term treatment of chondrocytes with LAs causes mitochondrial dysfunction leading to apoptosis.13 Besides, chondrocyte death has also been reported to occur in the much earlier stages of LAs treatment than that expected for apoptosis,9, 13, 14 but the underlying mechanisms of this remain poorly understood.
Plasma membrane blebbing has been described as an early manifestation of LAs toxicity in previous reports.7, 15, 16 Membrane blebbing consists of a balloon-like cell protrusion; rapidly expanding and abruptly stopping, followed by slow shrinkage.17 Bleb formation is initiated by contraction of actin cortex, which causes the bilayer membrane to detach from the underlying actin cortex.18 Actomyosin contractility also increases intracellular hydrostatic pressure, which results in rapid bleb extrusion.17 Rho-associated coiled-coil forming protein serine/threonine kinase (ROCK), one of the effectors of the small GTPase Rho,19 is known to be a key regulator of actomyosin contraction through myosin light chain (MLC) phosphorylation.20 Previous studies showed that the ROCK inhibitor, Y-27632, inhibits blebbing and subsequent cell death caused by exposure to 1-butanol, arsenic trioxide and hydrogen sulfide.21-23 ROCK-mediated blebbing has not previously been reported to occur in chondrocytes.
The present study was undertaken to investigate the possible involvement of plasma membrane blebbing and ROCK activation in the lidocaine toxicity of isolated rabbit articular chondrocytes. We show that ROCK inhibitors prevent the lidocaine-induced membrane blebbing associated with chondrocyte death.
METHODS
Isolation of Rabbit Articular Chondrocytes
All animal care and experimental protocols were approved by the Institution's Animal Care and Use Committee (Shiga University of Medical Science, Permit Number: 2013-3-3HH). Adult male Japanese White rabbits (body weight 2.5 to 3.5 kg, N = 21) were housed 1 per cage (FRP bracket cages 350 × 527 × 350 mm3) under SPF condition at 23 ± 5°C and the humidity at 40–60%. A 12-h light/12-h dark cycle (lighting from 8:00 to 20:00) was used. Articular chondrocytes were isolated using the enzymatic dissociation procedure described previously.24-26 In brief, rabbits were deeply anaesthetized with an i.m. injection of ketamine (70 mg/kg) and xylazine (5 mg/kg) and then killed by an i.v. injection of sodium pentobarbital (70 mg/kg). Articular cartilage was obtained from bilateral knee, hip, and shoulder joints as previously described by our group.24-26 We carefully shaved off the cartilage to include full depth cartilage but not bone tissue. Cartilage was incubated in plastic culture dishes containing serum-free Dulbecco's modified Eagle's medium (DMEM; Gibco, Carlsbad, CA) supplemented with antibiotics in a humidified atmosphere of 95% air plus 5% CO2 at 37°C for up to a week. On the day of the experiments, cartilage was minced into small pieces (∼1 mm3) and digested with 0.5% collagenase (Type 2; Worthington Biochemical Corp, Lakewood, NJ) for 3.5 h. Dispersed chondrocytes were washed three times, resuspended in DMEM supplemented with 40 mM mannitol (∼360 mosmol/L; close to the physiological osmolality for chondrocytes27) and used for experiments within 6 h.
Solutions and Chemicals
All experiments were conducted in modified Tyrode's solution (in mM): 140 NaCl, 5.4 KCl, 1 NaH2PO4, 1 MgCl2, 1.8 CaCl2, 10 glucose and 5 HEPES (pH adjusted to 7.4). Lidocaine hydrochloride monohydrate (Sigma-Aldrich, St. Louis, MO) was added to the solution at concentrations of 1, 3, 10, 30 mM and the pH was readjusted to 6.8, 7.4, or 8.7 with HCl or NaOH. The osmolality of all solutions was adjusted to 360 mosmol/L by adding mannitol. Concentrated stock solution was made for Y-27632 (10 mM, Wako Pure Chemical, Osaka, Japan), fasudil hydrochloride (100 mM, TOCRIS, Bristol, UK), and Rho inhibitor-1 (100 μg/mL, Cytoskeleton, Inc. Denver, CO) in distilled water, and blebbistatin (10 mM, Sigma-Aldrich) and z-VAD-fmk (20 mM, Sigma-Aldrich) in dimethyl sulphoxide. Rho inhibitor-1 was stored in aliquots at −80°C and the others were stored at −20°C.
Microscopy and Image Analysis
To evaluate the lidocaine-induced blebbing, morphological changes of chondrocytes were monitored using time-lapse microscopy. Isolated chondrocytes were seeded onto a glass-bottom chamber (0.5 ml in volume) mounted on the stage of a light microscope (Diaphot300, Nikon) and were allowed to adhere lightly to the glass-bottom for a few minutes. The chamber was continuously perfused at a constant rate of 2 ml/min with a temperature-controlled experimental solution at 36°C. Chondrocytes were exposed to the control solution for 15 min, and then exposed to lidocaine solution. A small fraction (5.4 ± 1.8%) of cells displaying a bleb before application of the drug were excluded from the analysis. In experiments altering extracellular pH, chondrocytes were pretreated with a control solution with pH equal to that of the lidocaine solution for 30 min. Microscopy images were consecutively captured with a 3CCD camera at resolution of 1600 × 900. Blebbed cells were defined by the presence of dynamic hemispherical protrusions visible in the images. The percentage of blebbed cells was evaluated every minute.
RhoA Activity Measurement
GTP-bound RhoA levels were determined using a RhoA G-LISA assay (Cytoskeleton, Inc.). Approximately, 8 × 105 of isolated chondrocytes suspended in serum-free DMEM were seeded onto a 35 mm dish and incubated for 2 h to allow them to adhere to the bottom. After a 20-min treatment of the chondrocytes with 10 mM lidocaine, the chondrocytes were rinsed with ice-cold PBS and were lysed using the provided cell lysis buffer. The lysates were then clarified by centrifugation at 14,000 rpm at 4°C for 2 min. After equalizing protein concentrations in all lysed cell extracts, the luminescent signal was measured with a luminometer (Infinite M200, Tecan) according to the manufacturer's instructions. The results were expressed relative to the untreated controls.
Caspase-3 Activity Measurement
The levels of caspase-3 activity were measured in chondrocytes treated with 10 mM lidocaine for 20 min. Briefly, chondrocytes were lysed and the supernatant was collected for the measurement of caspase-3/7 activity using a Caspase-Glo 3/7 assay system (Promega, San Luis, CA) following the manufacturer's instructions. The luminescence signal was measured with a luminometer (Infinite M200, Tecan). The results were expressed relative to the untreated controls.
Cell Viability Measurement in Isolated Chondrocytes
The viability of chondrocytes treated with lidocaine was determined using a LIVE/DEAD® viability/cytotoxicity assay kit (Life Technologies, San Diego, CA). The viability is based on the simultaneous determination of live and dead cells with two probes that recognize the parameters of cell viability, namely, intracellular esterase activity (calcein-AM) and plasma membrane integrity (ethidium homodimer-1). After an hour pretreatment without or with various test compounds, chondrocytes were exposed to 30 mM lidocaine (pH 7.4, 360 mosmol/L) at 37°C for an hour in collagen-coated flat-bottomed 96-well plates. Five microliters of 2 mM ethidium homodimer-1 and 1.25 μl of 4 mM calcein-AM were added to 2.5 ml of PBS. This working solution was then added directly to each well. Cells were stained at room temperature for 15 min in the dark. Images were taken immediately using a LSM 510 META (Carl Zeiss) fluorescent microscope at an excitation wavelength of 488 nm and emission wavelengths of 517.5 ± 12.5 nm for calcein and 560 nm for ethidium homodimer-1. To evaluate the percentage of living cells, the number of living cells (green) and dead cells (red) of each image were counted using an ImageJ software (NIH).
Cell Viability Measurement in In Situ Chondrocytes in Cartilage Explants
Humeral heads, femoral heads, femoral condyles, and tibial plateaus were obtained from rabbit bilateral shoulder, hip and knee joints, and were immediately cultivated in DMEM. Each joint was then immersed into 25 ml modified Tyrode's solution (pH 7.4, 360 mosmol/L) or lidocaine solution (30 mM, pH 7.4, 360 mosmol/L) at 37°C for 1 and 4 h. Explants were then cut through its full thickness with a no. 11 scalpel blade just before the staining. A LIVE/DEAD® viability/cytotoxicity assay (Life Technologies) was carried out by staining the chondrocytes of an unfixed tissue section (0.1 mm thickness) with calcein-AM and ethidium homodimer-1 for 30 min. After washing in PBS, the tissue sections were microscopically analyzed with the fluorescence microscope. The assay was performed before and 1 and 4 h after the exposure to the solutions.
Statistical Analysis
Data values are expressed as means ± SEM, with the number of experiments (cell preparation) and animals were indicated by n and N, respectively. Statistical comparisons were evaluated using either Student's t-test or ANOVA followed by a post hoc Newman–Keuls test, and differences were considered significant at p < 0.05.
RESULTS
Lidocaine Caused Membrane Blebbing in Isolated Chondrocytes
Figure 1A shows microscopic images of chondrocytes exposed to various concentrations of lidocaine. At the beginning of the experiment, all of the chondrocytes exhibited spherical shapes and contained vacuolar structures in their cytoplasm, which are typical features of freshly isolated chondrocytes.28 These morphological characteristics were maintained in control conditions throughout the 30-min observation. Exposure of chondrocytes to a lidocaine solution readily caused membrane blebbing, which was characterized by the formation of balloon-like protrusions on the cell surface. As shown in Figure 1B, the effect of lidocaine increased in a time- and concentration-dependent manner. The mean percentages of blebbing cells after 30-min exposure of lidocaine (pH 7.4), obtained from three independent experiments (n = 3, N = 3), at the concentrations of 1, 3, 10, and 30 mM were 9.6 ± 1.1% (p = 0.22), 35.5 ± 11.6% (p < 0.001), 84.1 ± 2.2% (p < 0.001) and 100 ± 0% (p < 0.001) respectively, compared to the controls (1.2 ± 2.1%).
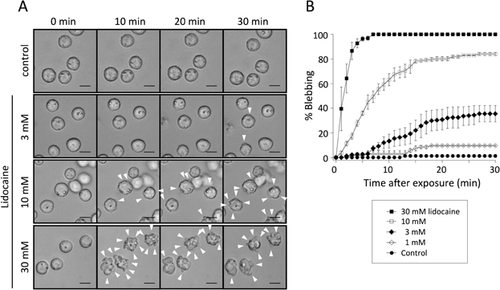
The Non-Ionized Lidocaine Induces the Blebbing
Lidocaine exists in non-ionized or ionized forms in an aqueous environment. The lipophilic non-ionized form can penetrate into membrane lipid bilayers and its proportion is determined by the environmental pH.29 We therefore investigated lidocaine-induced membrane blebbing under different pH conditions. In experiments altering extracellular pH, cells were pretreated with experimental solutions of different pH for at least 30 min before lidocaine application. No cell blebbing was occurred during this period, suggesting that the altered pH itself has little effects on membrane blebbing. As shown in Figure 2A, the concentration-response relationships were apparently shifted by the changes in pH of the solution. Each relationship was well fitted with the Hill equation, giving the EC50 of 11.72 ± 1.29, 4.13 ± 0.18, and 1.37 ± 0.03 mM at pH 6.8, 7.4, and 8.7, respectively, in three experiments (n = 3, N = 3).
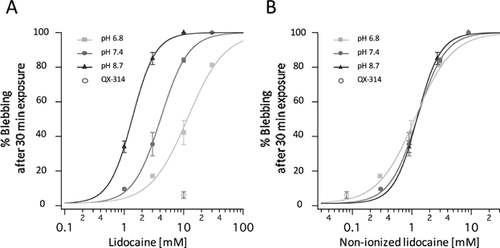
When re-plotting as a function of predicted concentrations of non-ionized lidocaine (assuming pKa = 7.77),30 the Hill curves obtained at three different pH conditions were superimposed (Fig. 2B). The EC50 values for non-ionized lidocaine were 1.14 ± 0.13, 1.24 ± 0.05 and 1.23 ± 0.03 mM at pH 6.8, 7.4, and 8.7, respectively.
We also examined the effect of QX-314, a positively charged derivative of lidocaine with a pKa of 9.5: the percentage of the non-ionized form is calculated to be only 0.8% at pH 7.4. QX-314 (10 mM, pH 7.4) caused membrane blebbing only in 6.1 ± 3.3% of cells (n = 3, N = 3, p = 0.663), which was not significantly different from the controls. Taken together, these observations suggest that the lidocaine-induced membrane blebbing is mainly dependent on the concentration of membrane-permeable non-ionized forms.
ROCK Inhibitors and Blebbistatin Inhibited Lidocaine-Induced Blebbing
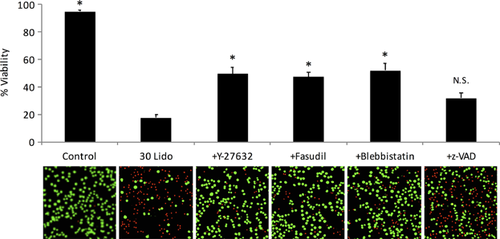
It is generally known that ROCK and MLC phosphorylation are key determinants of bleb formation in various cell types.19, 20 To investigate whether these intracellular processes are involved in the lidocaine-induced blebbing, ROCK inhibitors, Y-27632 and fasudil hydrochloride, or a myosin-II inhibitor, blebbistatin, were tested for their ability to avoid the effect of lidocaine. After a 30-min pretreatment of these inhibitors, chondrocytes were then exposed to 10 mM lidocaine at pH 7.4 for 30 min. As shown in Figure 3A–C, lidocaine-induced blebbing was almost completely prevented by 10 μM Y-27632, 100 μM fasudil hydrochloride, or 30 μM blebbistatin (p < 0.001, n = 3, N = 3, Fig. 3F).

Lidocaine-Induced Blebbing is Caspase-3 Independent
Membrane blebbing is considered as one of the most common morphological features of apoptosis, along with chromatin condensation and DNA laddering. Apoptotic blebbing is typically caused by the caspase-3-induced cleavage of ROCK-I, which results in a constitutive activation of the kinase.31 Since it was reported that lidocaine caused apoptosis in cultured chondrocytes,13, 14 it would be expected that caspase-3 activation would mediate lidocaine-induced ROCK activation and subsequent membrane blebbing. z-VAD-fmk (20 μM, a pan-inhibitor of caspases), however, failed to prevent the lidocaine-induced blebbing (p = 0.964, n = 3, N = 3, Fig. 3D). Furthermore, the level of caspase-3 activity was not appreciably influenced by 20-min exposure of chondrocytes to 10 mM lidocaine (p = 0.325, n = 8, N = 2, Fig. 3G). These results indicate that the lidocaine-induced blebbing is independent of caspase-3 activity.
Lidocaine Induced RhoA Activation
Next, RhoA activation was evaluated to investigate whether canonical Rho/ROCK signaling is involved in the lidocaine-induced bleb formation. Figure 3H shows that GTP-RhoA levels were significantly increased by 20-min exposure to 10 mM lidocaine (3.01 ± 0.76 folds, n = 5, N = 5, p < 0.001). On the other hand, pretreatment of chondrocytes with Rho inhibitor-1 (2.0 μg/ml, purified C3 Transferase) failed to reduce the lidocaine-induced blebbing (Fig. 3E).
ROCK Inhibitors and Blebbistatin Attenuated the Lidocaine-Induced Cytotoxicity
The involvement of membrane blebbing and ROCK activation in the lidocaine-induced chondrotoxicity was investigated by cell viability measurement under the confocal microscope (Fig. 4). Chondrocyte viability significantly decreased to 17.6 ± 2.1% after 1-h exposure to 30 mM lidocaine, compared to the control viability of 94.8 ± 0.9% (p < 0.001). The lidocaine-induced chondrotoxicity was significantly attenuated by pretreatment with 10 µM Y-27632, 100 µM fasudil or 30 μM blebbistatin (49.4 ± 4.7%, 47.2 ± 3.4%, and 52.1 ± 5.4% viability respectively, n = 7, N = 7, p < 0.001).
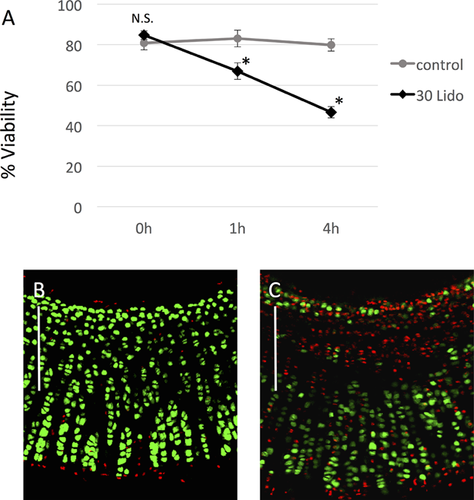
Lidocaine Penetrates Intact Cartilage and Reduces In Situ Chondrocyte Viability
To address the question whether lidocaine penetrates the cartilage matrix and indeed affects the viability of chondrocytes in situ, the drug was applied to rabbit cartilage explants (Fig. 5). In the absence of lidocaine in medium (control), the cell viability remained approximately 80% throughout the 4-h experiments (Fig. 5A and B). On the other hand, the tissue section obtained from cartilage explants exposed to lidocaine (30 mM, pH 7.4, 360 mosmol/L) displayed considerable cell death primarily in the area close to the surface. The viability measured at 1 and 4 h after exposure to lidocaine were 66.9 ± 4.1% and 46.6 ± 2.8%, respectively, which were significantly lower than those from their respective time-matched control (n = 9, N = 2, p < 0.001) (Fig. 5A and C).
DISCUSSION
Because of the great utility of lidocaine in orthopedic surgery, it is important to understand its action on chondrocytes. In the present study, we examined the effect of lidocaine on chondrocyte viability and its underlying cellular mechanisms. Our novel findings include that: (i) lidocaine acutely causes membrane blebbing in articular chondrocytes and subsequent cell death at clinically relevant concentrations, (ii) ROCK activation mediates this lidocaine-induced membrane blebbing, and (iii) inhibition of bleb formation attenuates the lidocaine chondrotoxicity. Taken together, these data suggest that ROCK-mediated membrane blebbing is an important cellular process associated with lidocaine-induced chondrocyte death.
There have been previous investigations of LAs-induced chondrotoxicity, however, there are some disagreement in the literature over the levels of the toxicity.13, 14, 32 These inconsistencies are at least partly attributable to the difference in experimental conditions, including the pH environment used for drug testing. The proportion of non-ionized (lipophilic)/ionized (lipophobic) forms of LAs is determined by lidocaine's pKa and the pH of the aqueous solution. Thus, the extracellular pH greatly affects the permeability of the drug across the cell membrane. As judged from the results of our pH-shifting experiments, it is most likely that the non-ionized membrane-permeable lidocaine, rather than cationic membrane-impermeable lidocaine, is responsible for causing the membrane blebbing and subsequent chondrocyte death. This parallels the action potential inhibiting properties of LAs that also depend on the non-ionized species of these “anesthetics.”33 Assuming the pKa value to be 7.77 for lidocaine,30 the concentration of the non-ionized form is steeply increased with pH elevation within the physiological dynamic range (pH 6.9–7.3 in cartilage27). Since injectable preparations of lidocaine hydrochloride are typically acidic, lidocaine is present in non-membrane permeable (ionized) form before administration. However it is important to note that membrane-permeable (non-ionized) form of lidocaine will be increased after intraarticular injection by buffering effect of synovial fluid (pH 7.4).34 Therefore, our findings provide a better understanding of lidocaine chondrotoxicity in physiological conditions, and suggest that a young patient who has cartilage of neutral pH condition will have higher susceptibility to LAs toxicity than older OA patient (with lower pH cartilage).35
It has long been known that high concentrations of LAs change cell membrane properties,15 however, the cellular signaling pathway remains largely unknown. The present study found that ROCK activation was an essential molecular event responsible for the LAs-induced membrane blebbing (Fig. 3). In support of our findings, chondrocytes reportedly express ROCK proteins.36 In addition, it has been demonstrated that the ROCK-mediated myosin hyperactivation leads to membrane blebbing and subsequent cell death during exposure to other drugs such as 1-butanol, arsenic trioxide, and hydrogen sulfide.21-23
In recent years, there is growing interest in the physiological and pathophysiological roles of ROCK in chondrocytes, mostly focusing on the involvement of Rho/ROCK signaling in chondrogenic differentiation and metabolism.37 Considerable experimental evidence has suggested that ROCK modulates the activity of Sox9, a transcription factor regulating chondrogenic gene expression.38-40 It is thus an intriguing question as to whether lidocaine affects chondrogenesis by turning on the ROCK/Sox9 pathway. Previous studies have reported that chronic treatment of chondrocytes with lidocaine decreases cell viability but increases glycosaminoglycan production per living cell.41 Therefore we suggest that lidocaine may promote chondrogenesis, even though this effect will be largely masked by the acute chondrotoxicity.
To the best of our knowledge, the present study is the first to show that ROCK is activated by clinically used concentrations of lidocaine. Other LAs such as bupivacaine and ropivacaine appear to have similar activating effects on ROCK, since these compounds are also known to cause the membrane blebbing.7 In general, three mechanisms have been proposed for ROCK activation; (i) canonical Rho/ROCK signaling42; (ii) caspase-mediated cleavage of ROCK31; and (iii) direct activation of ROCK by unsaturated fatty acids such as arachidonic acid.43 Our data showed that RhoA-GTP levels were markedly increased by lidocaine treatment (Fig. 3H). Nevertheless, Rho inhibitor-1 did not suppress the bleb formation of cells exposed to lidocaine, suggesting a missing link between Rho and ROCK activation (Fig. 3E). In addition, our results also excluded the involvement of caspase-3 (Fig. 3D, G). We failed to examine possibility of the direct activation of ROCK by lidocaine because of technical difficulties. Further investigation would be required to clarify how lidocaine activates ROCK proteins.
It is generally accepted that voltage-gated Na+ channel inhibition is the main action of LAs, but the blocking dose (IC50 of 50–200 μM) is much lower than the concentration inducing chondrocyte blebbing in our study. It is thus unlikely that the Na+ channel block itself causes the lidocaine-induced chondrocyte death. On the other hand, there is evidence that millimolar concentrations of LAs can rather activate other ion channels such as TRPV144 and TRPA1.45 Consistently, in our preliminary patch-clamp study, 30 mM lidocaine readily activated the non-selective cationic current in chondrocytes. Although its relation to chondrotoxic effects of lidocaine remains unclear, the enhanced ion permeability can disrupt the electrical gradient across the plasma membrane. In chondrocytes, the membrane potential is fundamental for cellular processes including metabolism and cell volume regulation.46 We have recently reported that impaired cell volume regulation by the aberrant activation of Cl- channels results in chondrocyte apoptosis.24
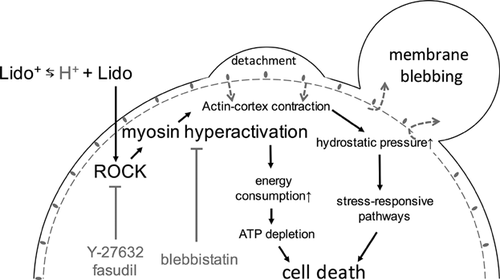
Our results showed that lidocaine chondrotoxity was significantly attenuated not only by ROCK inhibitors but also by the myosin-II inhibitor, blebbistatin. This suggests that the myosin hyperactivation contributes to the lidocaine cytotoxicity. There are at least two possible pathways downstream of the myosin hyperactivation (see Fig. 6). During membrane blebbing, myosin hyperactivation causes excessive energy consumption and thereby ATP depletion, leading to failure of various cellular functions and subsequent cell death. Consistent with this hypothesis, Grishko et al. (13) reported that ATP is largely depleted in chondrocytes treated with high concentration of lidocaine.13 Another possibility is that the myosin hyperactivation nonspecifically triggers the activation of stress-responsive pathways including mitogen-activated protein kinases (MAPKs). Supporting this possibility, it is reported that LAs exposure causes p38-MAPK activation.47
Proper regulation of ROCK activity may be required for maintaining the healthy condition of cartilage. Pharmacological control of ROCK activity with fasudil hydrochloride has been tested, clinically as an anti-spasmotic in the treatment of brain arterial spasms.48 Thus the present study provides a rationale for the clinical application of ROCK inhibitors in cartilage disorders too. Further support for this idea comes from a previous report that has demonstrated potential efficacy of ROCK inhibitors for the treatment of OA in animal models.49
Consistent with previous studies in human and bovine cartilages,9 our experiments demonstrate that lidocaine caused cell death in in situ chondrocytes within cartilage explants. However, the cytotoxic effect was much lower than the observation in isolated chondrocytes, suggesting that the extracellular matrix acts as a barrier to lidocaine penetration. In this context, our results obtained from rabbit models should not be simply extrapolated to humans, because there is a marked difference in the cartilage thickness between rabbits (0.3 mm) and humans (2.2–2.5 mm).50 In addition, altered physical/chemical properties of the cartilage (partial thickness defect, acidification with arthritis, etc.) would further influence the diffusion and accessibility of lidocaine into the cartilage, thereby the drug toxicity. Considering the safe clinical use of intraarticular lidocaine injection, it would be important to elucidate pharmacokinetic properties of lidocaine in cartilage.
AUTHORS' CONTRIBUTIONS
T.M., F.T., S.I. and H.M. designed the experiments. T.M. performed the experiments and analyzed the data. All authors participated in data interpretation. T.M., F.T. and H.M. wrote the manuscript. All authors revised the manuscript and approved the final manuscript. All experiments were performed at Department of Orthopedic Surgery or Department of Physiology, Shiga University of Medical Science, Japan.
ACKNOWLEDGMENTS
This work was supported by a Grant-in-Aid for Young Scientists (B) from the Japan Society for the Promotion of Science (no. 26861185) to K.K. The funding source had no role in the study design, collection, analysis and interpretation of the data or the decision to submit the manuscript for publication.




