Advanced glycation end-products induced VEGF production and inflammatory responses in human synoviocytes via RAGE-NF-κB pathway activation
ABSTRACT
Aging and diabetes are known to be the major cause to affect the progression of osteoarthritis (OA). Advanced glycation end products (AGEs) have been observed to accumulate in various organs especially in joint tissue and do damage to the joint tissue during aging and diabetes. Synovial angiogenesis and inflammation are observed across the full range of OA severity. The signaling pathway of AGEs on vascular endothelial growth factor (VEGF) production and inflammatory responses in synoviocytes are still unclear. Here, we investigated the role of receptor for AGEs (RAGE) and the signaling pathway involved in AGEs-induced VEGF production and inflammatory responses in human synoviocytes. Human synoviocytes were cultured and treated with AGEs (25–100 µg/ml). AGEs significantly induced the protein expressions of cyclooxygenase-2 (COX-2) and VEGF and the productions of prostaglandin-E2 (PGE2), VEGF, interleukin-6 (IL-6), and metalloproteinase-13 (MMP-13) in human synoviocytes in a dose-dependent manner. Moreover, AGEs markedly activated the phosphorylations of IκB kinase (IKK)α/β, IκBα, and nuclear factor (NF)-κB-p65 proteins in human synoviocytes in a time-dependent manner. Treatment with neutralizing antibody for RAGE statistically significantly decreased the AGEs-induced increase in COX-2, VEGF, PGE2, IL-6, and MMP13 and AGEs-activated NF-κB pathway activation. Taken together, these findings indicate that AGEs are capable of inducing VEGF production and inflammatory responses via RAGE-NF-κB pathway activation in human synoviocytes. © 2015 Orthopaedic Research Society. Published by Wiley Periodicals, Inc. J Orthop Res 34:791–800, 2016.
Osteoarthritis (OA) is the most common form of joint disease, which is a top cause of disability in elderly people.1 OA is a slow progressive degenerative joint disease characterized by cartilage breakdown, the formation of body outgrowths at the joint margin (osteophytes), subchondral bone sclerosis, alterations to the joint capsule, as well as inflammation, and neovascularisation of the synovial membrane.2 In OA pathology, erosion of cartilage matrix is thought to be the major results of proteinases synthesis and activation.3 Metalloproteinases (MMPs), synthesized in articular joints by synovial cells and chondrocytes, are considered to play a pivotal role in cartilage destruction.4-7 Several risk factors including obesity, increasing age, trauma, genetic predisposition, and endocrine factors are known to affect the progression of OA.8 Both aging and obesity are recognized as major risk factors for OA.9
Advanced glycation end products (AGEs) are produced irreversibly by the nonenzymatic glycation of reducing sugars with proteins. Once AGEs are formed, they can't be removed from the protein until the protein involved is degraded. AGEs are known to contribute to the pathogenesis of metabolic diseases such as obesity, diabetes, inflammation, ischemic cardiovascular disease, and neurodegenerative disorders.10 AGEs have been observed to accumulate in various organs with aging. Because of the exceptionally long half-life of articular cartilage collagen, joint tissue is especially one of the abundant accumulation sites of AGEs.11-13 Accumulation of AGEs in joint tissue shows the decreased proteoglycan and collagen synthesis, which leads to stiffness and brittleness of cartilage and more prone to mechanical damage.14
The synovial lining is formed by condensed cells with one to three cell layers thick, including fibroblast-like synoviocytes and scattered macrophages-like synoviocytes.15 OA synovial cells are known to be activated by pro-inflammatory cytokines to release cytokines/chemokines, reactive oxygen species (ROS), and other mediators that likely contribute to sustain the inflammatory responses.16 It also releases the proteases such as matrix metalloproteinases (MMPs), which act in a synergistic manner to degrade the components of connective tissue.17 Moreover, it has been demonstrated that human synovial fibroblasts constitutively express receptor for AGEs (RAGE), which is a multi-ligand member of immunoglobulin superfamily of cell surface molecules presented in various cell types.18 It has been found that both RAGE and its ligand, AGEs, are present in the synovial lining, sublinings, and OA synovium specimens.19, 20 RAGE are implicated to play an important role in inducing invasion, cell cycle arrest, and proinflammatory change in synovial fibroblasts.21, 22 However, little was known on the effects of AGEs on synoviocytes. In chondrocytes of OA, AGEs can also up-regulate the production of MMPs that mediate cartilage degradation leading to the joint destruction. Moreover, AGEs has been shown to trigger the expressions of interleukin (IL)-6 and IL-8 through RAGE.23 Our previous study showed that AGEs induced the inflammatory responses, productions of MMP-13 and IL-6, and collagen II reduction in human chondrocytes via a RAGE-regulated p38MAPK/JNK-activated PPARγ down-regulation signaling pathway.24 Therefore, the role of AGEs/RAGE in the activation of synoviocytes is important and needs to be clarified.
Vascular endothelial growth factor (VEGF) is a homodimeric heparin-binding glycoprotein that plays a central role in inflammation and wound healing by controlling both angiogenesis and vascular permeability.25 A previous study showed that primary function of VEGF was considered to be at angiogenesis when binding to its receptors in vascular endothelial cells in the pathological tissues.26 Moreover, binding of VEGF to its receptors on the endothelial cells stimulates the chemotaxis and the production of MMPs.27-29 Enomoto and colleagues have demonstrated that VEGF and its receptors are expressed in OA cartilage and VEGF stimulates OA chondrocytes to produce MMP-1 and MMP-3 by mechanical overload.30 Pufe and colleagues have also suggested that VEGF plays an important autocrine or paracrine role in the progression of OA.25
The normal synovium is highly vascular in order to supply the normally avascular cartilage with nutrients and oxygen. It has been suggested that angiogenesis and inflammation occur at the osteochondral junction as well as within the osteoarthritic synovium, which can contribute to joint damage in OA. Synovial angiogenesis and inflammation are observed across the full range of OA severity. VEGF plays a pivotal role in the process of OA pathogenesis. MMP-mediated cartilage degradation is also an important feature of OA.30 High levels of AGEs are formed in the cartilages not only with diabetic condition but also with aging state.11, 12 However, almost no data are available on the signaling pathway of AGEs-induced VEGF production and inflammatory responses in synoviocytes. In this study, therefore, we investigated the role of RAGE and the signaling pathways involved in AGEs-induced VEGF production and inflammatory responses in human synoviocytes.
MATERIALS AND METHODS
Reagents
Anti-mouse and anti-rabbit IgG-conjugated horseradish peroxidase, rabbit polyclonal antibodies specific for RAGE, inhibitory-κB (IκB)α, and IκB kinase (IKK)α/β (Ser180/181) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse monoclonal antibodies specific for cyclooxygenase (COX)-2, VEGF (VEGF-A), nuclear factor (NF)-κB-p65, α-tubulin, and β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit polyclonal antibodies specific for phospho-IKKα/β (Ser180/181) and phospho-p65 (Ser536) were purchased from Cell Signaling (Danvers, MA). NF-κB (p65) Transcription Factor Assay kit and PGE2 ELISA kit were purchased from Cayman Chemical Company (Ann Arbor, MI). MMP-13 and IL-6 ELISA kits were purchased from eBioscience (San Diego, CA). VEGF ELISA kit was purchased from R&D Systems (Minneaplis, MN). Bovine serum albumin (BSA) and all other chemicals were obtained from Sigma–Aldrich (St. Louis, MO).
Preparation of AGEs
The preparation of AGEs was performed as describe previously.17 Briefly, BSA (1 mg/ml) was incubated under sterile conditions with D-glucose (1 mg/ml) in 0.2 M phosphate buffer (pH 7.4) at 37°C for 8 weeks. After incubation, AGEs were dialyzed against PBS for 24 h to remove unbound sugars and filter-sterilized using a 0.22 µM Millipore filter (Millipore, Billerica, MA). AGEs were identified by Ultraflex III MALDI-TOF/TOF (Bruker) and the AGEs protein concentration was measured by BCA protein assay.
Cell Culture
Primary human synoviocytes were obtained from ScienCell Research Laboratories (Carlsbad, CA) and cultured in Synoviocyte Medium (SM, Cat. No. 4701) supplemented with 10% fetal bovine serum (FBS), synoviocyte growth supplement and antibiotics (100 U/ml penicillin, 100 µg/ml streptomycin) in 5% carbon dioxide (CO2) at 37°C. Cells were amplified during 1 to 3 passages, and the experiments were performed using cells from passages 3 to 6. Synoviocytes (>85% confluent) were serum starved before treatment with different doses AGEs (5, 25, 50, and 100 µg/ml) for 24 h or pretreatment with or without neutralizing antibody of RAGE (10 µg/ml) for 1 h followed by treatment with AGEs (50 µg/ml) for 24 h.
Measurement of PGE2, VEGF, IL-6, and MMP-13 Production
Human synoviocytes (1 × 106/ml) were cultured in 6-well plates in Synoviocyte Medium (Cat. No. 4701) supplemented with 10% fetal bovine serum (FBS), synoviocyte growth supplement and antibiotics (100 U/ml penicillin, 100 µg/ml streptomycin). The levels of PGE2, VEGF, IL-6, and MMP-13 in culture media were quantified by using the commercially available PGE2, VEGF, IL-6, and MMP-13 specific ELISA kits according to the manufacturer's instructions.
Western Blot Analysis
The cellular lysates were prepared. Equal proteins (20–40 µg) were resolved on SDS-PAGE and transferred to immobilon polyvinyl difluoride (PVDF) membranes. The blots were blocked with 4% BSA for 1 h at room temperature and then probed with the primary antibodies against COX-2, VEGF, NF-κB-p65, phospho-IKKα/β, and phospho-p65 (1:1000) overnight at 4°C. The blots were subsequently incubated with the secondary goat anti-mouse antibodies conjugated with horseradish peroxidase (1:1000) for 1 h at room temperature. The blots were visualized by enhanced chemiluminescence using Kodak X-OMAT LS film (Eastman Kodak, Rochester, NY).
Nuclear Extracts Preparation of and NF-κB Activity Measurement
Cells were lysed in a hypotonic buffer on ice for 15 min and centrifuged for 30 s to pellet nuclei. Then the pellet was re-suspended in nuclear extract buffer on ice for 15 min. The lysates were centrifuged at 14,000g for 10 min, and supernatants containing the nuclear proteins were collected. The binding activity of NF-κB to DNA was measured in nuclear extracts using NF-κB (p65) Transcription Factor Assay kit (Cayman, Ann Arbor, MI) according to the manufacturer's instruction at 10 µg of nuclear protein per well. Following color development, absorbance was read at 450 nm within 5 min.
Statistics
The results are presented as mean ± SEM. Each experiment was performed four times to ensure reproducibility. The significant difference from the respective controls for each experimental test condition was assessed by one-way analysis of variance (ANOVA) and two-tailed Student's t-test. The difference is significant when the p-value is less than 0.05. Software used: SigmaPlot 10.0 and GraphPad Prism 5.
RESULTS
Effects of AGEs on the Expressions of COX-2 and VEGF and the Productions of PGE2, VEGF, IL-6, and MMP-13 in Human Synoviocytes
We first investigated the inflammatory effect and related signal molecules by AGEs in human synoviocytes. The concentrations of AGEs at 5–100 µg/ml did not induce the cytotoxicity in human synoviocytes (Fig. 1A). The protein expressions of COX-2 (Fig. 1B) and VEGF (Fig. 1C) were increased in a dose-dependent manner in human synoviocytes cultured with AGEs (25–100 µg/ml) for 24 h (COX-2, from 1.7- to 3.4-fold; VEGF, from 1.8- to 6.0-fold). The secretions of PGE2 (Fig. 2A) and VEGF-A (Fig. 2B) were also dose-dependently increased in human synoviocytes by AGEs for 24 h (PGE2, from 2.6- to 4.3-fold; VEGF, from 2.1- to 3.3-fold).
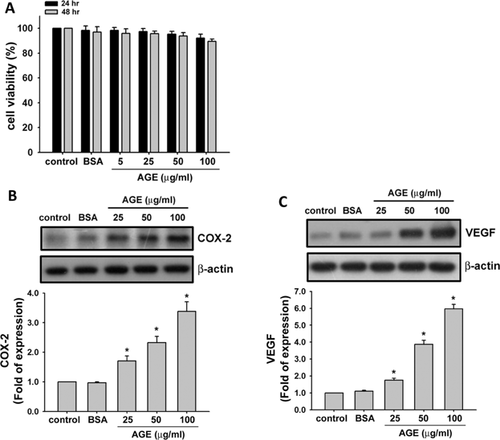
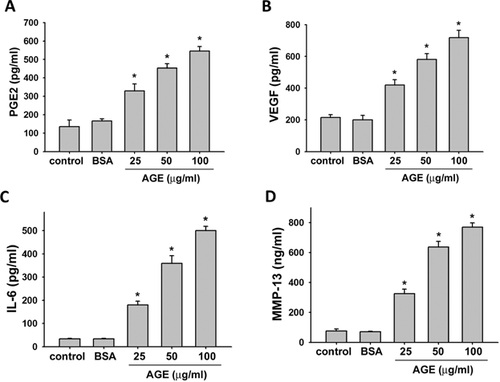
We then evaluated the effects of AGEs on the productions of IL-6 and MMP-13 in human synoviocytes. As shown in Figure 2C and D, AGEs significantly and dose-dependently increased the productions of IL-6 and MMP-13 (IL-6, from 5.1- to 14.2-fold; MMP-13, from 4.8- to 9.7-fold).
Involvement of IKK/IκBα/NF-κB Signaling in AGEs-Induced Inflammatory Responses in Human Synoviocytes
We next investigated the involvement of NF-κB signaling pathway in AGEs-induced inflammatory responses in human synoviocytes. Treatment with AGEs (50 µg/ml) for 0.5–6 h markedly induced the phosphorylations of IKKα/β (Fig. 3A), IκBα (Fig. 3B, upper panel), and NF-κB p65 (Fig. 3C) and the degradation of IκBα protein (Fig. 3B, lower panel) in a time-dependent manner (IKKα/β, from 1.7- to 3.6-fold; IκBα phosphorylation, from 1.2- to 5.1-fold; NF-κB p65, from 1.6- to 3.7-fold; IκBα degradation, from 0.9- to 0.5-fold). Moreover, treatment with AGEs (50 µg/ml) for 1–24 h could also time-dependently induce NF-κB binding activity (Fig. 3D, from 1.2- to 5.0-fold).
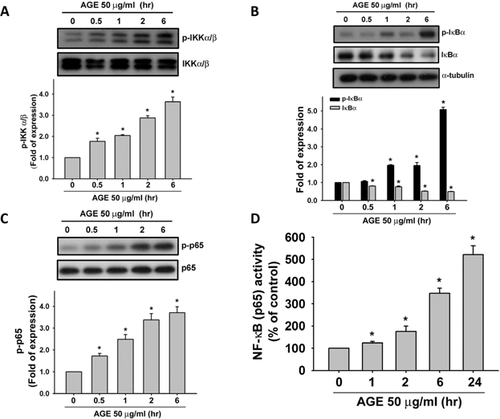
Involvement of RAGE in AGEs-Induced Effects on Human Synoviocytes
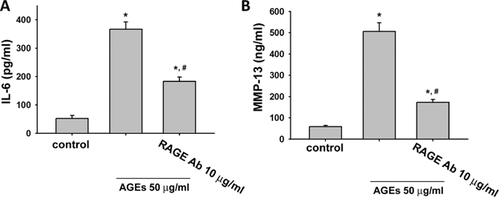
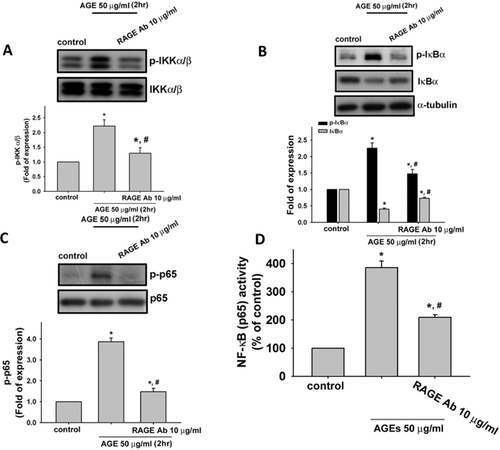
We next tested the role of RAGE in AGEs-triggered inflammatory responses and VEGF production in human synoviocytes. We used neutralizing antibody for RAGE to investigate the role of RAGE. Pretreatment with neutralizing antibodies for RAGE could effectively suppress the AGEs (50 µg/ml)-induced protein expressions of COX-2 (Fig. 4A) and VEGF (Fig. 4B) and secretions of PGE2 (Fig. 4C) and VEGF (Fig. 4D) in human synoviocytes (AGEs group vs. AGEs + RAGE-Ab group: COX-2, from 1.9- to 1.1-fold; VEGF, from 4.2- to 1.7-fold; PGE2, from 5.5- to 3.5-fold; VEGF, from 3.1- to 1.9-fold; p < 0.05). RAGE neutralizing antibody could also effectively reverse the AGEs (50 µg/ml)-induced productions of IL-6 (Fig. 5A) and MMP-13 (Fig. 5B) in human synoviocytes (AGEs group vs. AGEs + RAGE-Ab group: IL-6, from 6.7- to 3.3-fold and MMP-13, from 8.3- to 3.3-fold; p < 0.05). We also tested the effect of RAGE neutralizing antibody on AGEs-induced IKK/IκBα/NF-κB signaling. As shown in Figure 6, pretreatment with neutralizing antibody for RAGE effectively suppressd the phosphorylations of IKKα/β (A), IκBα (B), and NF-κB-p65 (C) and the degradation of IκBα (B) in human synoviocytes (AGEs group vs. AGEs + RAGE-Ab group: IKKα/β, from 2.1- to 1.4-fold; IκBα, from 2.3- to 1.5-fold; NF-κB-p65, from 4.0- to 1.6-fold; IκBα degradation, from 0.4- to 0.7-fold; p < 0.05). Moreover, AGE-triggered NF-κB binding activity could also be inhibited by RAGE neutralized antibody (Fig. 6D; AGEs group vs. AGEs + RAGE-Ab group: from 3.8- to 1.7-fold; p < 0.05).
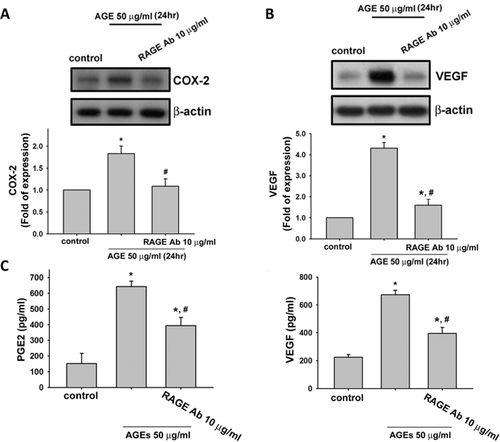
DISCUSSION
OA is the most prevalent type of arthritis and the most common chronic joint disease and the leading causes of pain and disability in the world. Increasing evidence demonstrated an association between OA and metabolic syndrome.31 Several studies have suggested that AGEs accumulation is a possible mechanism for the age-related development of OA.11, 12, 14 The increased levels of AGEs have been found in the cartilages of patients with focal cartilage degeneration.32 AGEs formation has also been shown to be accelerated in diabetic patients.33 AGEs are generated mainly by non-enzymatic glycation of proteins via the Maillard reaction and degrade poorly, leading to their accumulation in the joints of aging populations.34 Several studies have shown that excess AGEs accumulated in joints results in the generation of inflammatory responses and the degradation of cartilage extracellular matrix.17, 32, 34, 35 Nah and colleagues have suggested that AGEs can induce the expression of COX-2 and the production of PGE2 and NO in human OA chondrocytes.35 Huang et al. have reported that AGEs cause collagen II reduction by activating Janus kinase/signal transducer and activator of transcription-3 signaling pathway in porcine chondrocytes.36 AGEs/RAGE have also been shown to trigger the productions of IL-6 and MMP-13 and the expression of COX-2 and the reduction of collagen II expression in human OA chondrocytes.24 However, little is known the effects of AGEs on the synovium, where is the main source of proinflammatory cytokines and degradative enzymes.37 In the present study, we found that AGEs could induce the expression of COX-2 and the productions of PGE2, IL-6, and MMP-13 in human synoviocytes.
The increased pentosidine, an AGEs, has been found in serum and synovial fluid from knee OA patients.38 Increased expression of RAGE has also been shown to be related to various acute and chronic inflammatory diseases including OA.20 Several studies have shown that RAGE plays an important role in the activation of fibroblast-like synoviocytes.39, 40 Hou and colleagues demonstrated that a functional RAGE pathway mediated the binding and degradation of AGEs-modified proteins as well as the induction of MCP-1 in human synovial fibroblasts.39 It has been shown that HMGB1 increases the IL-6 generation in human synoviocytes via the RAGE, c-Src, Akt, p65, and NF-κB signaling pathways.40 In the present study, neutralizing antibody for RAGE effectively reversed the AGEs-induced inflammatory responses and VEGF production in human synoviocytes, indicating an important role of RAGE in the effects of AGEs on synoviocytes. Therefore, the involvement of RAGE in the activation of synoviocytes seems to play an important role in the progression of OA.
IL-6 is a multifunctional cytokine with a wide range of biological activities, including mediation of acute-phase responses and effects on bone metabolism. Patients with OA exhibit elevated IL-6 levels.41 MMPs, a family of zinc-dependent enzymes that can degrade all components of the extracellular matrix, are synthesized in articular joints by synovial cells and chondrocytes.42 MMPs have been demonstrated to be a crucial step, resulting in the destruction of the joints in OA patients. Of all, MMP-13 (collagenase-3) is an important enzyme that preferentially cleaves collagen II in OA cartilage.8 VEGF is implicated to be a destructive factor involved in the progression of OA. The transcriptional regulation of VEGF involves several transcription factors such as NF-κB, LXR, SP-1, AP-1, and so on.43 The NF-κB molecules are a family of ubiquitously expressed transcription factors involved in immunity, stress responses, inflammatory diseases, cell proliferation, and cell death.44 In OA joints, the synovial cells are stimulated by cytokines, matrix fragments originating from cartilage degradation, and cellular stress products and potentiate the canonical NF-κB signaling pathway through their chemokine surface receptors.45 Afterward, the activated NF-κB molecules mediate the synthesis of chemokines (IL-8), cytokines (IL-1β, IL-6, TNF-α), PGE2, MMPs, ADAMTS4, ADAMTS5, and angiogenic factors (VEGF) that cause further cartilage degradation, neoangiogenesis, and inflammation of the synovial membrane (synovitis).46 Synovitis is accompanied with a range of histological abnormalities occurred in synovial membrane, such as infiltration of macrophages and lymphocytes, joint swelling, and stiffness.47 Roman-Blas and Jimenez have reviewed that there are in vitro and in vivo studies to examine the contribution of NF-κB-related pathways to the pathogenesis of OA and rheumatoid arthritis (RA), and large efforts devote to the pharmacological study for the modulation of NF-κB pathways.48 The results of our current study showed that NF-κB activation contributed to the expression of COX-2 and VEGF and the production of IL-6 and MMP-13 in AGEs-treated human synoviocytes. Therefore, NF-κB may be a potential target for OA therapy. Moreover, Tsai et al. have explored that AP-1 activation is involved in high glucose-induced VEGF expression and production in human synovial fibroblasts.49 Berra et al. have also suggested that Sp-1/AP-2 transcriptional factor complexes are involved in MAP kinases-contributed VEGF expression.50 Further studies will be needed to find out if other transcription factors are involved in AGEs-induced effects in human synoviocytes.
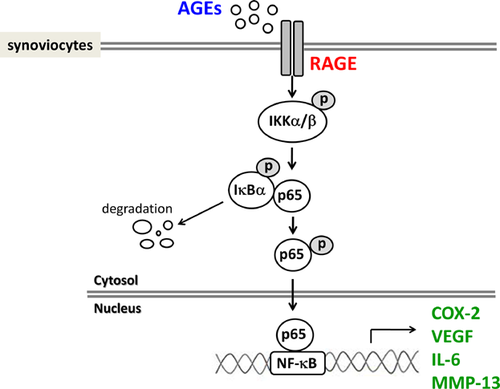
In conclusion, we demonstrated for the first time that AGEs induced the expressions of COX-2 and VEGF and the productions of VEGF, IL-6, and MMP-13 via RAGE-mediated IKKα/β/IκBα/NF-κB signaling activation in human synoviocytes (Fig. 7). These findings may provide a better understanding of AGEs-induced OA pathogenesis. The clinical significance of these findings needs to be clarified in the future.
AUTHORS' CONTRIBUTIONS
YJC and SHL researched data and wrote the manuscript. DCC, CKC, CCW, KCL, SCC, THY, and KST researched data and reviewed the manuscript. DCC, CKC, and RSY reviewed and edited the manuscript. CKC, SHL, and RSY contributed to discussion. SHL and RSY are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors have read and approved the final submitted manuscript.




