Cartilage degeneration and excessive subchondral bone formation in spontaneous osteoarthritis involves altered TGF-β signaling
ABSTRACT
Transforming growth factor-β (TGF-β) has been demonstrated as a potential therapeutic target in osteoarthritis. However, beneficial effects of TGF-β supplement and inhibition have both been reported, suggesting characterization of the spatiotemporal distribution of TGF-β during the whole time course of osteoarthritis is important. To investigate the activity of TGF-β in osteoarthritis progression, we collected knee joints from Dunkin–Hartley (DH) guinea pigs at 3, 6, 9, and 12-month old (n = 8), which develop spontaneous osteoarthritis in a manner extraordinarily similar to humans. Via histology and micro-computed tomography (CT) analysis, we found that the joints exhibited gradual cartilage degeneration, subchondral plate sclerosis, and elevated bone remodeling during aging. The degenerating cartilage showed a progressive switch of the expression of phosphorylated Smad2/3 to Smad1/5/8, suggesting dual roles of TGF-β/Smad signaling during chondrocyte terminal differentiation in osteoarthritis progression. In subchondral bone, we found that the locations and age-related changes of osterix+ osteoprogenitors were in parallel with active TGF-β, which implied the excessive osteogenesis may link to the activity of TGF-β. Our study, therefore, suggests an association of cartilage degeneration and excessive bone remodeling with altered TGF-β signaling in osteoarthritis progression of DH guinea pigs. © 2015 Orthopaedic Research Society. Published by Wiley Periodicals, Inc. J Orthop Res 34:763–770, 2016.
Osteoarthritis, the most common type of arthritis, is characterized by the degeneration of articular cartilage and the excessive remodeling of subchondral bone. In 2010, osteoarthritis of the knee affected 3.64% of the world's population and was the fastest growing cause of disability worldwide.1 Unfortunately, conventional therapies target to relieve symptoms and are ineffective at preventing disease progression, which inevitably leads to surgical intervention.2
Current researches suggest that knee osteoarthritis is a disease of the entire joint, including synovitis,3 meniscal degeneration and malposition,4 periarticular bone overgrowth, and articular cartilage destruction. In response to the altered mechanical environment, the bone–cartilage functional unit adjusts the architecture via cells’ adaptations. However, a discrepancy of repair capacity between the chondrocytes and other skeletal cells is thought to further accelerate the progression of osteoarthritis.5 Furthermore, it is widely appreciated that the subchondral plate6 and trabecular bone7 show different responses, where thickening of the plate can be identified along with osteopenic trabeculae at the advanced stage of osteoarthritis. Longitudinal studies that can monitor the pathological adaptations of the bone–cartilage unit from early to late osteoarthritis may facilitate our understanding of the disease progression.
Human specimens of early osteoarthritis are limited. Therefore, animal models are often used to characterize the pathological alterations over the entire disease time course. Ligament transection and meniscectomy are the most common procedures to induce instability of knee joints and subsequently a rapid destruction of cartilage similar to the post-traumatic osteoarthritis.8 However, animal models with naturally developed osteoarthritis are preferable for investigating the development of primary osteoarthritis. The Dunkin–Hartley (DH) guinea pig has been shown to naturally develop signs of osteoarthritis as early as 3-month old and has been widely used as a spontaneous osteoarthritis model.9 Lesions observed in DH guinea pigs are typical and histo-pathologically similar to human osteoarthritis, including progressive degeneration of articular cartilage and subchondral sclerosis.9, 10
Transforming growth factor-beta (TGF-β) regulates the activities of both chondrocytes and bone cells under physiological and pathological conditions.11-13 TGF-β signal transduction depends on its binding to type I and II receptors (TβR I and TβR II) on cell membrane and subsequently activating the phosphorylation of Smad family members.14 Although TGF-β plays a protective role in maintaining the cartilage integrity, the use of TGF-β supplement for treating osteoarthritis has been a challenge due to its potential in causing synovial fibrosis and osteophyte formation.15 TGF-β has been shown to activate activin receptor-like kinase (ALK)-5 or ALK1/type I receptor, which in turn phosphorylates Smad2/3 or Smad1/5/8. Studies have shown that the ALK5/Smad2/3 route restrains terminal differentiation of chondrocytes, whereas the ALK1/Smad1/5/8 route induces the differentiation. The increase in ALK1/ALK5 ratio in chondrocytes may contribute to the cartilage degeneration.16 On the other hand, TGF-β acts as a coupling factor to induce the migration of mesenchymal stem cells (MSCs) to bone resorption sites, implying its potential function in rebalancing bone resorption and formation.17 Zhen et al. have found that inhibition of TGF-β signaling in subchondral bone resulted in higher bone quality and less cartilage degeneration in an induced-osteoarthritis model.18 However, the activity and impact of TGF-β in spontaneous osteoarthritis awaits to be elucidated.
We hypothesized that TGF-β signaling might play a role in bone–cartilage architectural alterations during spontaneous osteoarthritis. In this study, we monitored the alterations of bone–cartilage unit from disease onset to advanced stages of naturally occurring osteoarthritis in DH guinea pigs and investigated the correlation between the phenotype progression and changes in the activity of TGF-β.
METHODS
Animal Model
Female DH guinea pigs were provided by Laboratory Animal Unit, the University of Hong Kong. They were housed in solid bottom cages (742 × 542 × 250 mm3) with stocking density 2/cage and kept under controlled conditions. Animal health and behaviors were monitored daily. Guinea pigs were euthanized at 3, 6, 9, or 12 months old (n = 8/group). Bilateral knee joints were harvested for micro-CT scan and histological analysis. The study protocols and procedures were conducted under the approval from the local institute.
Micro-CT
Excised hindlimbs were fixed in 10% neutral buffered formalin (NBF) and scanned using a high resolution micro-CT system (model 1076, SkyScan, Kontich, Belgium). Three hundred and twenty-nine projections for each sample were produced with the following settings: Isotropic voxel 18 µm, voltage 100 kV, current 100 µA, and exposure time 320 ms. Using the SkyScan NRecon software (version 1.6.8.0, SkyScan), three-dimensional (3D) reconstructions with a voxel size 18 µm were acquired. The data sets were then reoriented using DataViewer (version 1.4.4.0, SkyScan) to analyze the sample in the coronal plane. After the selection of the regions of interest (ROIs) and binarization of the images with a global thresholding of gray levels (140–255), the calculation of morphological parameters was carried out with the CTAn software (version 1.13.2.1, SkyScan).
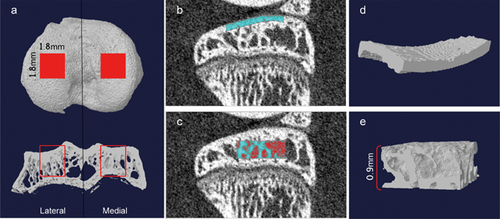
The central regions (1.8 × 1.8 mm2) of lateral and medial tibial plateau were selected for analysis (Fig. 1a). The ROI of subchondral plate was achieved manually slice by slice (Fig. 1b and d), whereas a cuboid underneath with a height of 0.9 mm (Fig. 1c and e) was defined as ROI of subchondral trabecular bone. For the analysis of subchondral plate, the plate thickness (Pl.Th, mm), bone volume fraction (Pl. BV/TV, %), and total porosity (Pl.Po, %) were measured. For the analysis of subchondral trabecular bone, the trabecular thickness (Tb.Th, mm), trabecular number (Tb.N, 1/mm), trabecular separation (Tb.Sp, mm), structure model index (SMI), trabecular bone pattern factor (Tb.Pf, 1/mm), connectivity density (Conn.Dn, 1/mm3), and degree of anisotropy (DA) were calculated. In addition, the bone mineral density (BMD, g/cm3) was calibrated by using the attenuation coefficient of two hydroxyapatite phantoms with defined mineral density of 0.25 and 0.75 g/cm3.
Histological Examination and Scoring
After fixation in 10% NBF for 3 days, the knee joints were rinsed thoroughly with tap water and immersed in 0.5 M ethylenediaminetetraacetic acid disodium salt dihydrate (EDTA-Na2) for decalcification. Fresh solution was changed per 3 days for 4–6 weeks and the end point was determined by X-ray test. Adequately decalcified tissues were embedded in paraffin and sectioned into 5 µm-thick slides. Those slides were stained with Toluidine Blue and Fast Green. Regions not covered by the meniscus were selected to evaluate the disease severity according to the modified Mankin's scoring system.19 Specifically, the osteoarthritis degree was evaluated by grading of the articular cartilage structure, proteoglycan content, cellularity, and tidemark integrity.
Immunohistochemistry and TRAP Staining
For immunohistochemistry, the rehydrated sections underwent heat-induced antigen retrieval in citrate buffer (pH 6.0), followed by incubation with primary antibodies against pSmad2/3 (1:50, Santacruz, Dallas, TX), pSmad1/5/8 (1:100, Santacruz), and osterix (1:500, Abcam, Cambridge, MA) overnight at 4°C. Color was developed using the Vectastain Elite ABC system (Vector, Burlingame, CA) and liquid DAB+ substrate-chromogen system (Dako, Carpinteria, CA). Digital images at 400 magnification were captured using a Nikon Eclipse 80i microscope (Nikon, Japan). The number of positive cells per tissue area (mm2) was counted via Image Pro-Plus software (version 6.0.0.260, MediaCybernetics, Rockville, MD). TRAP staining was performed with a commercial acid phosphatase leukocyte (TRAP) kit (Sigma–Aldrich, St. Louis, MD). After incubation with naphthol AS-BI phosphoric acid and freshly diazotized fast garnet GBC, insoluble maroon dye deposited at sites of activity. The number of positivity per subchondral bone marrow area (mm2) was calculated.
Statistical Analysis
The values are expressed as mean ± SD. For the statistical analysis of the adaptations along with age, one-way analyses of variance (ANOVAs) were performed followed by post hoc Bonferroni's test. The differences in properties between the lateral and medial plateaus were evaluated using paired t-test. p value less than 0.05 was considered significant. SPSS 13.0 software was used in all analyses.
RESULTS
Spontaneous and Age-Related Cartilage Degeneration in DH Guinea Pigs
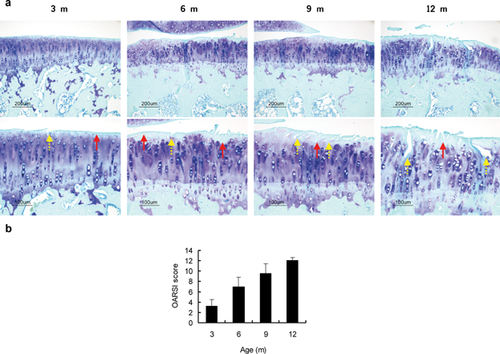
At 3-month old, undulations with matrix loss were found in the superficial zone of cartilage (Fig. 2a). Fissures and decreased proteoglycan content were observed in the superficial zone at 6 months old and extended to the middle zone at 9-months old (Fig. 2a). At age of 12 months, fractures were identified in the deep zone (Fig. 2a) and the distribution of matrix was heterogeneous with a severe loss of proteoglycan content in the interterritorial matrix. Horizontal microcracks were found between uncalcified and calcified cartilage. The total modified Mankin's score was increased with age during the study duration (one way ANOVA test: p < 0.001, Fig. 2b).
 : matrix loss,
: matrix loss,  : fissures. (b) OARSI scores from 3 to 12 months old. One-way ANOVA test: p < 0.001.
: fissures. (b) OARSI scores from 3 to 12 months old. One-way ANOVA test: p < 0.001.Subchondral Plate Sclerosis and Elevated Bone Remodeling in Subchondral Trabeculae
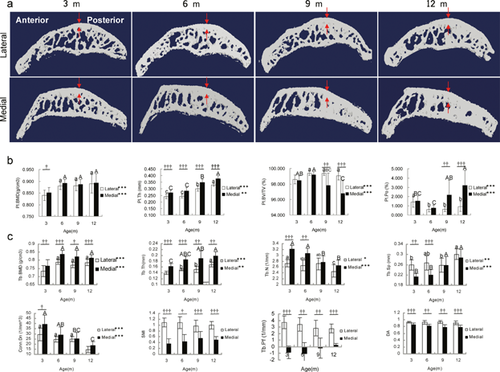
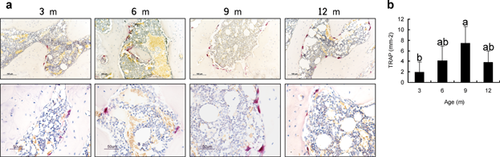
Besides the loss of cartilage, abnormal subchondral bone adaptations were also identified in DH guinea pigs. The thickness of subchondral plate was sharply increased from 6 months old (Fig. 3a and b), which denoted the plate sclerosis in DH guinea pigs osteoarthritis. Meanwhile, followed an initial increase, the bone mineral density (BMD) of subchondral plate maintained relatively stable (Fig. 3a and b). On the other hand, the subchondral trabecular bone showed an age-related increase in trabecular thickness and separation while decrease in trabecular number and connectivity density (Fig. 3a and c). BMD showed a temporal increase from 3 to 6 months old (Fig. 3a and c). Compared with lateral tibial plateau, lower structure model index (SMI), and trabecular bone pattern factor (Tb.Pf) were found in the medial side, which signified superior mechanical properties to the lateral side (Fig. 3a and c). With age, the trabeculae also became less anisotropic. In addition, TRAP staining showed an increase of osteoclasts activity with age (p < 0.001, Fig. 4). This suggested a continuous increase of new bone remodeling sites from 3 to 9 months old and was in accordance with the micro-CT findings.
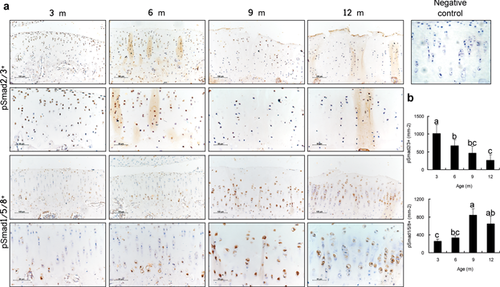
Shifting of Receptor-Smads Signaling in the Degenerated Cartilage
TGF-β plays a pivotal role in maintaining the integrity of cartilage. At early stage of spontaneous osteoarthritis (3-month old), pSmad2/3 expression was found in all zones and pSmad1/5/8 expression was predominantly detected in the hypertrophic chondrocytes at the calcified zone but almost indiscernible in the uncalicified cartilage (Fig. 5a). During the osteoarthritis progression, we found a continual decrease in the number of pSmad2/3+ chondrocytes in the articular cartilage from 3 to 12 months (p < 0.001, Fig. 5b), whereas the number of pSmad1/5/8+ chondrocytes gradually increased (p < 0.01, Fig. 5b). These findings implied a role of TGF-β/Smad signaling in terminal chondrocyte differentiation and degeneration of articular cartilage.
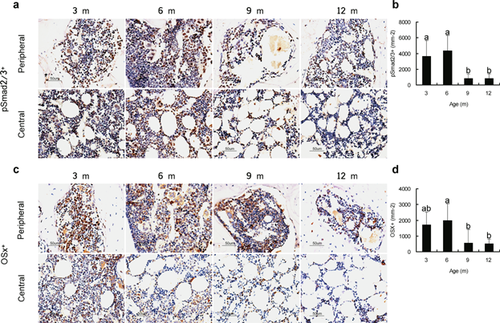
Similar Spatiotemporal Distribution of Active TGF-β and Osteoprogenitors in the Subchondral Bone Marrow
We tested if TGF-β signaling was similarly regulated in the altered bone remodeling. Interestingly, in the subchondral bone marrow, we observed a sharp decrease of pSmad2/3 after the onset of osteoarthritis. The distribution of pSmad2/3+ cells was not equal across different regions of the bone marrow: PSmad2/3 were predominantly found in the marrow adjacent to endocortical surface (Fig. 6a and b). As increased TGF-β activity can induce the migration of MSCs and affect bone formation, osteogenesis in subchondral bone was also investigated. The number of cells positive for osterix, a marker of osteoprogenitors, displayed a decrease by 9 and 12 months (Fig. 6c and d). Similar to that of the pSmad2/3+ cells, the osterix+ cells were also not evenly distributed across the peripheral and central marrow regions.
DISCUSSION
In this study, we confirmed that the female DH guinea pigs, even with lower body weights compared with the male, could develop age-related idiopathic osteoarthritis. By radiological and histological analysis, we demonstrated that DH guinea pigs develop gradual cartilage degeneration, subchondral plate sclerosis, and elevated bone turnover with age. Our findings suggest that a switch of TGF-β signaling mediators progressively from Smad2/3 to Smad1/5/8 during the cartilage destruction. The intensive pSmad1/5/8 expression in the hypertrophic chondrocytes prior to the collapse of articular cartilage suggests the role of TGF-β/Smad signaling during chondrocyte terminal differentiation. With the high level of pSmad2/3 in subchondral bone at early stage of osteoarthritis, and the similar spatiotemporal distribution of pSmad2/3+ cells and osteoprogenitors, represented by osterix positive cells, we propose that the excessive osteogenesis in DH guinea pig subchondral bone might be associated with an alteration of TGF-β/Smad signaling.
Various animal models are established in an attempt to replicate the progression of osteoarthritis in humans so as to study its etiology and pathogenesis. Although the injury-induced models could develop osteoarthritis-related phenotype in a short period of time, the cartilage injury and the alterations of subchondral bone in different models can be disparate depending on the induction procedures.20, 21 We here confirm that female DH guinea pig can be a relevant model to study spontaneous osteoarthritis.
The significant increase of BMD and decrease of porosity in the subchondral plate from 3 to 6 months old is consistent with our previous findings from 1 to 3 months old immature DH guinea pig.22 Different patterns were observed after 6 months, which is temporally coincident with the surcease of bone growth.23 These changes emphasize the importance of mineralization during bone growth. The relatively stable BMD after 6 months might be related to the pronounced elevation of plate thickness and the less calcification of the new bone. Meanwhile, the gain of the total porosity might be partially caused by the increased vascularization at the osteochondral junction. The tidemark advancement is a result of the pathological endochondral ossification at the calcified zone of cartilage.24 In this study, the observed tidemark replication and the increased plate thickness, particularly in the elders, suggests abnormal endochondral ossification in the OA progression.
In the present study, elevated bone turnover was demonstrated in the subchondral trabecular bone as reflected by the altered trabecular thickness, number, and separation. TRAP staining result was also found conformable to the micro-CT findings: New bone remodeling sites were increased. The gain of trabecular thickness but loss of trabecular number imply bone resorption and bone formation are uncoupled in DH guinea pig spontaneous OA. In humans, the same alterations were demonstrated in the femoral trabecular bone of osteoarthritis.25 Here, we also found that the trabecular network became more separated and less interconnected, which was generally caused by the activity of osteoclasts. Interestingly, different appearances of trabeculae between lateral and medial compartments were found (as reflected by SMI and Tb.Pf). The medial compartment presented a higher mechanical strength with plate-like and concave trabeculae, which indicated that the bone of the major load-bearing site developed a better structure. That is in accordance with the Wolff's law of “bone functional adaptation.”26 In addition, the reduced anisotropy implies that the orientation of trabeculae may be adjusted to the longitudinal loading axis. Based on the dynamic changes of architecture of subchondral trabecular bone observed in DH guinea pig model, we propose that sustained abnormal bone turnover occurs during spontaneous OA progression.
TGF-β plays a pivotal role to maintain the homeostasis of articular cartilage via stimulation of the extracellular matrix synthesis and balancing the activities of catabolic factors. Interruption of the TGF-β/Smad3 signaling pathway led to chondrocyte hypertrophic differentiation and cartilage degeneration.27 Furthermore, decreased expression of TGF-β receptor was shown in human28 and rodent animals12 during osteoarthritis. Smad2/3 signaling is essential to repress the hypertrophy of chondrocyte, whereas Smad1/5/8 route, namely bone morphogenetic protein (BMP) pathway, is required for chondrocyte terminal differentiation. Inhibition of the Smad1/5/8 signaling pathway led to reduced or delayed chondrocyte hypertrophy.29, 30 Increase in ALK1/ALK5 ratio was associated with age and osteoarthritis and dominant ALK1/Smad1/5/8 pathway was found in advanced stage of induced osteoarthritis.16 The shift of receptor-Smads signaling during spontaneous cartilage degeneration in guinea pig might be due to the change of ALK1/ALK5 ratio. Furthermore, inhibitory-Smads (Smad6 and 7) and Smad ubiquitin regulatory factors (Smurf1 and 2) function as key negative regulators of receptor-Smads phosphoralytion or stability. Smad7 can block both routes, whereas Smad6 specifically inhibits the BMP pathway.31 Smurf2 mainly targets Smad2, whereas Smurf1 targets Smad1/5.32 As the master regulator of chondrocyte terminal differentiation, the function of transcription factor Runx2 is stimulated by Smad1/533 while blocked by Smad3.34 Based on the altered patterns of Smads activation, we propose a dual role of TGF-β/Smad signaling in chondrocyte differentiation and cartilage destruction in DH guinea pigs.
Bone remodeling is an enduringly dynamic process that is dependent on the spatio-temporal coupling of bone resorption and bone formation. Precise bone remodeling is essential for maintaining bone health and also responsible for bone healing.35 Active TGF-β is released from bone matrix during bone resorption, creating a concentration gradient. High concentration of active TGF-β inhibits the recruitment of osteoclast precursor and prevents further bone resorption.36 The active TGF-β also serves as a critical coupling factor to induce the migration of MSCs to the remodeling sites.17 TGF-β is increased in subchondral bone of post-traumatic osteoarthritis model.18 Elevated TGF-β activity might lead to the loss of the concentration gradient and disrupt the induced migration of MSCs to the adjacent resorptive surface. In the present study, aberrant presence of osteoprogenitor in the marrow cavity, represented by osterix+ cells, have been demonstrated. The parallel temporal and spatial distribution of osterix+ cells and pSmad2/3+ cells in the marrow suggests that osteoprogenitors activity may be linked to TGF-β activity to contribute to the bone phenotype in spontaneous osteoarthritis. Moreover, the intense pSmad2/3+ cells in the subchondral bone at the onset period is constant with the results in the surgery-induced model.18 These findings may provide insights into why only an early blockage of TGF-β signaling could effectively ameliorate the osteoarthritis phenomena.18
A limitation in the current study is the lack of an appropriate control due to the rarity of OA-resistant guinea pig strains. Furthermore, although the Smads are the major mediators in TGF-β signal transduction, the association of the phenotype with Smads-independent pathway remains to be elucidated. Altogether, this longitudinal study has consolidated that the DH guinea pigs presents spontaneous osteoarthritis and suggests the involvement of TGF-β in the cartilage and subchondral bone lesions. These findings warrant further studies to understand the activity of TGF-β signaling pathway in spontaneous osteoarthritis and its potential role in disease-modification.
AUTHORS’ CONTRIBUTIONS
The authors made specific contributions as follows: (i) research design: W. Zhao, T. Wang, C. Wen, K.Y. Chiu, X. Cao, W.W. Lu; (ii) acquisition, analysis, and interpretation of the data: W. Zhao, T. Wang, Q. Luo, Y. Chen; (iii) drafting of the article: W. Zhao; (iv) critical revision of the article: W. Zhao, T. Wang, Q. Luo, V.Y.L. Leung, M.F. Shah, H. Pan, W.W. Lu. All authors have read and approved the final submitted manuscript.
ACKNOWLEDGMENTS
This work was supported by grants from the Research Grant Council (HKU715213), National Natural Science Foundation of China (NSFC81270967), and the ShenZhen Peacock Program (1108110035863317). The funding sources did not influence study design, collection, analysis, and interpretation of data, writing, or the decision to submit the manuscript for publication.




