In vivo monitoring of activated macrophages and neutrophils in response to ischemic osteonecrosis in a mouse model
ABSTRACT
Ischemic osteonecrosis (IO) is caused by disruption of the blood supply to bone. It is a debilitating condition with pathological healing characterized by excessive bone resorption and delayed osteogenesis. Although the majority of research has focused on the role of osteoblasts and osteoclasts in the disease progression, we hypothesize that innate immune cells, macrophages and neutrophils, play a significant role. With the recent development of real-time imaging probes for neutrophils and macrophages, the purpose of this study was to investigate the kinetic immune cell response in a mouse model of IO. Our results show that induction of IO leads to a significant accumulation of activated neutrophils and macrophages at the affected tissue by 48 h after surgery. Additionally, the accumulation of these immune cells remained elevated in comparison to sham controls for up to 6 weeks, indicative of chronic inflammation. Immunohistochemistry confirmed the immune cell infiltration into the necrotic bone marrow and the increased presence of TNFα-positive cells, demonstrating, for the first time, a direct response of these cells to ischemia induced necrotic bone. These new findings support a hypothesis that IO is an osteoimmunologic condition where innate immune cells play a significant role in the chronic inflammation. © 2015 Orthopaedic Research Society. Published by Wiley Periodicals, Inc. J Orthop Res 34:307–313, 2016.
Acute disruption of the blood supply to bone, often a consequence of traumatic injuries such as proximal femoral fractures,1 leads to ischemia induced necrosis of the cells in the bone and bone marrow, a condition known as ischemic osteonecrosis (IO). The treatment of necrotic bone remains a clinical challenge due to the pathological healing process commonly observed in the disease progression. During the early stages of healing, revascularization of the ischemic tissue is accompanied by a large increase in osteoclast activity, resorption of the necrotic bone, and a delay in new bone formation.2 This imbalance in the activity of the bone multicellular unit can significantly compromise the mechanical stability of the bone due to a net bone loss and lead to permanent deformity.
Although the molecular mechanisms involved in the pathological bone healing following IO are currently unclear, we hypothesize that a robust acute and chronic immune response to cell necrosis has a direct role in the pathological bone healing. In recent years, a growing body of research has established that cells undergoing necrosis release a number of inflammatory proteins and signaling molecules, commonly referred to as damage-associated molecular patterns (DAMPs),3, 4 that recruit and activate circulating immune cells, primarily neutrophils and macrophages. In the case of IO, the release of DAMPs may recruit immune cells to infiltrate into the necrotic bone marrow and initiate classical pro-inflammatory signaling cascades. Subsequently, traditional inflammatory signaling molecules, such as tumor necrosis factor-alpha (TNFα), interleukin-1 (IL-1), and interleukin-6 (IL-6) have been shown to stimulate osteoclastic activity both in vitro5-10 and in certain animal models.11, 12 The release of these molecules from immune cells infiltrating into necrotic bone may play a role in the excessive bone resorption associated with IO.
Patients affected with ischemic osteonecrosis often exhibit signs of local inflammation around the affected tissue in the form of synovitis,13 and chronic inflammation has even been associated with poor patient prognosis in a juvenile IO condition called Legg–Calvé–Perthes disease.14 However, the potential role of the innate immune system in the pathogenesis of IO has not been previously investigated. The purpose of this study was, therefore, to determine the temporal response of two major types of innate immune cells, neutrophils and macrophages, following the initiation of IO.
In order to gain a better understanding of the cellular response and the repair process following ischemic osteonecrosis, a mouse model was previously developed.15 In this model, cauterization of the blood vessels supplying the distal femoral epiphysis leads to complete necrosis of the cells in the bone and bone marrow at the local site, as observed by positive TUNEL-staining as early as 24 h after surgery. At 2 weeks following surgery, revascularization into the distal femoral epiphysis can be observed with an accompanying increase in osteoclasts (TRAP-positive cells) and a significant decrease in bone volume and trabecular thickness in comparison to sham surgery control mice (no cauterization of blood vessels). By 6 weeks after surgery, the shape of the distal femur becomes deformed, due to continued weight bearing during the period of excessive osteoclast activity. These features of the mouse model are consistent with that described in both large animal models of IO and juvenile cases of femoral head osteonecrosis.16, 17
In the current study, our mouse model of IO was used in conjunction with a novel in vivo monitoring technique using near-infrared nanoprobes designed to specifically bind to formyl peptide receptors (FPRs) or folate receptors (FRs) on the surface of activated neutrophils or macrophages, respectively. The upregulation of formyl peptide receptors on the surface of neutrophils and folate receptors on the surface of macrophages following cellular activation allows these nanoprobes to preferentially bind to activated cell phenotypes. Previously, these nanoprobes have been shown to have high specificity for activated neutrophils and macrophages in vitro, and safely and effectively facilitate the real-time monitoring of activated neutrophils and macrophages in vivo in response to lipopolysaccharide injections and biomaterial-mediated inflammatory responses.18, 19 We herein report the first use of these nanoprobes as a novel method for studying the recruitment and activation of the innate immune cells in response to ischemia induced necrotic bone tissue.
MATERIALS AND METHODS
This study was approved by the Institutional Animal Care and Use Committees at the University of Texas Southwestern Medical Center and the University of Texas at Arlington.
Surgical Procedure to Induce Ischemic Osteonecrosis in a Mouse Distal Femoral Epiphysis
Four-week-old male BALB/c mice were anesthetized with isoflurane. In the IO group, a dissection microscope and microsurgical instruments were used to visualize and cauterize the blood vessels supplying the distal femoral epiphysis on the right leg of the animal (a branch of the popliteal vessel and branches of medial, central, and lateral genicular vessels). The incision was closed using a combination of 8-0 sutures and surgical glue. In the sham control group, the vessels of the right distal femoral epiphysis were exposed as described above but no cauterization was performed. The mice were euthanized at 48 h, 1 week, and 6 weeks following surgery (n = 4 per time point).
Preparation of Formyl-peptide Receptor Targeting and Folate Receptor Targeting Nanoprobes
Formyl-peptide targeting nanoprobes (FPR-nanoprobes) and folate receptor targeting nanoprobes (FR-nanoprobes) were used to assess the accumulation of neutrophils and macrophages at the necrotic tissue sites in real time, respectively. Both probes were fabricated as described previously.18, 19 To simultaneously monitor the presence of neutrophils and macrophages, FPR-nanoprobes were labeled with Oyster®680 and FR-nanoprobes were labeled with Oyster®800 (Boca Scientific, Boca Raton, FL).
In Vivo Animal Imaging
Twenty-four hours after IO surgery, administration of nanoprobes and in vivo imaging were performed. For imaging analyses, animals were anesthetized by isoflurane inhalation and whole body images were then taken using KODAK in vivo FX Pro system (Kodak Molecular Imaging Systems, New Haven, CT). For imaging neutrophil accumulation, FPR-nanoprobes with excitation filter of 630 nm and emission filter of 700 nm were used as described previously.18 For imaging macrophage recruitment, FR-nanoprobes, excitation wavelength of 760 nm and emission filter of 830 nm, were used as shown in our previous publication.19 After background correction, regions of interest were drawn over the distal femoral epiphysis, and the mean fluorescence intensities for all pixels in the fluorescent images were calculated using Carestream Molecular Imaging Software, Network Edition 4.5 (Carestream Health, Rochester, NY). Our earlier studies have shown that almost all of the imaging probes are removed from circulation by the kidneys and excreted within 8 h after administration. The probes accumulated at the inflammatory tissue typically for the duration of approximately 10–14 days. Only little changes of the probe accumulations were found between 1 and 4 days following probe administration. To avoid the influence of metabolism/pharmacokinetics of the probes on the experimental outcomes, all imaging experiments were carried out 1–4 days following probe administrations.
Immunohistochemical Evaluation of Immune Cell Response
In vivo imaged mice were sacrificed at 48 h, 1 week, and 6 weeks after sham or osteonecrosis surgery for immunohistochemical analysis to validate the in vivo imaging technique. Immediately after sacrifice, the distal femurs were harvested and fixed with 10% formalin for 5 days. The specimens were then decalcified with 10% EDTA for 5 days before being embedded in paraffin and cut at 3 um. Antigen retrieval was performed using 0.25% Trypsin-EDTA enzyme digestion for 3 min at 37°C. Immunohistochemistry was performed to quantify the number of F4/80+ (ab6640 1:500 dilution, Abcam, Cambridge, MA), NIMP-R14+ (ab2557 1:1,000 dilution, Abcam) and TNFα+ (ab6671 1:100 dilution, Abcam) cells. To eliminate endogenous peroxidase activity, samples were incubated in a 3% hydrogen peroxide in methanol solution for 10 min at room temperature. Primary antibodies were incubated overnight at 4°C. For negative controls, non-specific rat IgG was used instead of the specific primary antibodies. After primary incubation, the tissue sections were incubated for 1.5 h at room temperature with a goat anti-rat IgG HRP secondary antibody (AP136P Millipore, 1:250 dilution) or a goat anti-rabbit IgG HRP secondary antibody (12-348 Millipore, 1:250 dilution). DAB peroxidase chromagen was used to give a brown precipitation at the positive antigenic site. Bioquant Osteoimager (Bioquant Image Analysis Corp, Nashville, TN) was used to quantify the number of positive cells within the bone marrow. Three slices were analyzed per animal, four animals per time point.
Statistical Analysis
All results are expressed as a mean ± standard deviation. Student t-tests for comparison of two groups and ANOVA, Tukey's multiple comparison tests for more than two groups. A value of p ≤ 0.05 was considered to be statistically significant.
RESULTS
In Vivo Monitoring of Activated Neutrophils Following Ischemic Osteonecrosis
In order to monitor the neutrophil response to IO, FPR-nanoprobes were intravenously administered to mice 24 h after IO surgery. A sham surgery was used as a control. Starting at 24 h after probe injection (48 h after surgery), whole body images of the mice were recorded at various time points to detect the location and quantity of the FPR-nanoprobes in the mice. As early as 48 h after surgery, a significant increase in fluorescent signal intensity from FPR-nanoprobes was observed around the site of osteonecrosis in the IO group, compared to the sham control group. This signal peaked at 72 h after surgery (Fig. 1A), indicative of a robust accumulation of neutrophils in response to IO, a hallmark of acute inflammatory response.
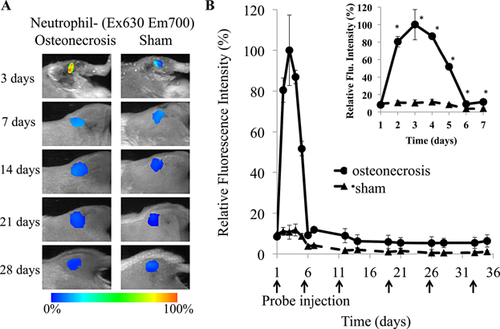
Quantitative analysis determined that ischemic osteonecrosis triggered a significant increase (9.41-fold, p < 0.05) in fluorescent signal from neutrophil specific FPR-nanoprobes compared to sham surgeries (Fig. 1B). By 7 days following surgery, the fluorescent intensity in the IO group had decreased significantly below its peak, yet it still remained significantly increased over the sham control group (2.85-fold), and an increase over sham control group remained for the course of the study.
Immunohistochemical Assessment of Neutrophil Accumulation in Response to Ischemic Osteonecrosis
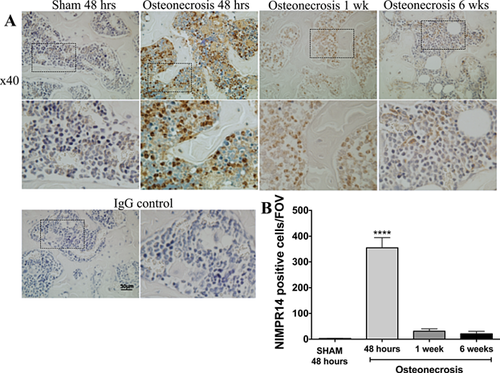
In order to confirm the neutrophil response to IO observed in the in vivo imaging data, immunohistochemistry was performed on tissue sections from mice sacrificed 48 h, 1 week, and 6 weeks following ischemic osteonecrosis surgery. As early as 48 h after IO surgery, the infiltration of neutrophils (NIMP-R14-positive cells) into the periphery of the ischemic bone marrow was observed (Fig. 2A). In corroboration of in vivo imaging data, immunohistochemical analysis using Bioquant showed a significant increase in neutrophils in the distal femoral epiphysis of mice with ischemic osteonecrosis at 48 h compared to sham and the number of neutrophils decreased by 1 week (Fig. 2B). Immunohistochemistry at 6 weeks revealed bone marrow areas restored with hematopoietic marrow, which showed slight positive NIMP-R14 staining.
In Vivo Monitoring of Macrophages in Response to Ischemic Osteonecrosis
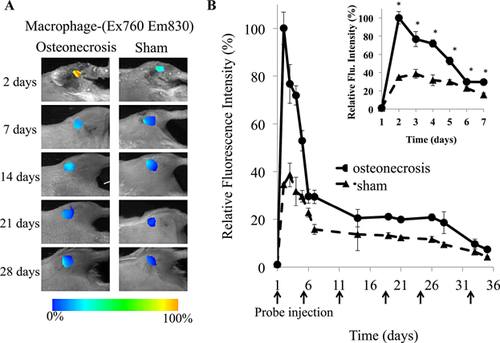
To track the recruitment of activated macrophages in vivo in response to ischemic osteonecrosis, FR-nanoprobes were administered as described for FPR-nanoprobes, and subsequent whole body images of the animals began 48 h after surgery (Fig. 3A). Peak signal intensity from FR-nanoprobes was observed 48 h after surgery, with a significant increase in macrophage specific FR-nanoprobe fluorescent signal (2.85 times sham control, Fig. 3B) around the site of osteonecrosis in the IO group. This strong initial fluorescent signal, correlating to a large accumulation of activated macrophages, further supports the robust innate immune cell response to IO. The acute response dissipated steadily over the first 6 days, yet the fluorescent signal in the IO group remained significantly elevated over the sham control group for an extended period of time. At 4 weeks after surgery, fluorescent intensity in the IO group was still increased 2.11-fold greater than the sham control group indicating increased presence of macrophages, which is a hallmark of chronic inflammatory response.
Immunohistochemical Assessment of Macrophage Accumulation in Response to Ischemic Osteonecrosis
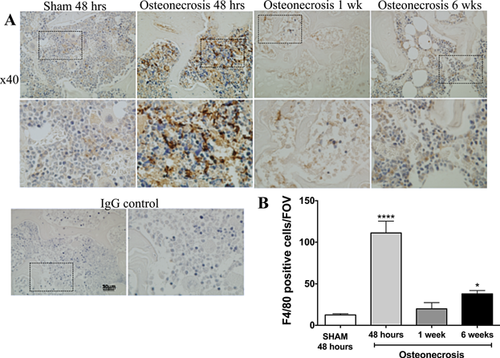
Immunohistochemical analysis of tissue sections from the distal femur of IO mice 48 h, 1 week, and 6 weeks following surgery were used to confirm the in vivo imaging data from FR-nanoprobes. The infiltration of macrophages (F4/80-positive cells) into the periphery of the distal femoral epiphysis was observed in tissue sections from IO mice sacrificed 48 h after surgery (Fig. 4A). Using Bioquant to quantify the macrophage infiltration, a significant increase in the number of F4/80-positive cells was observed at 48 h and 6 weeks after IO surgery compared to sham (Fig. 4B).
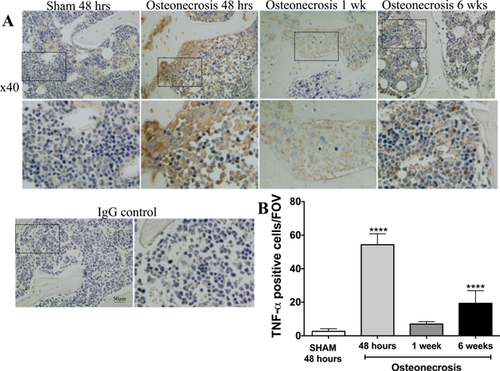
Immunohistochemical Assessment of TNFα-Positive Cells in Response to Ischemic Osteonecrosis
TNFα is secreted by multiple cell types, including neutrophils and macrophages, in response to pro-inflammatory stimuli.20, 21 The secretion of TNFα initiates additional pro-inflammatory cascades and recruits inflammatory cells.22 Immunohistochemistry was performed to determine if cells infiltrating into the osteonecrotic bone marrow exhibited a pro-inflammatory phenotype. As seen in Figure 5, the number of TNFα-positive cells was significantly higher in IO mice as early as 48 h after surgery, and remained higher through the course of the experiment. These results further support the notion of a chronic inflammatory response following IO.
DISCUSSION
The role of the innate immune system and inflammation in the pathogenesis of IO has often been overlooked when examining mechanisms involved in the impaired healing process. In the current study, we monitored the response of neutrophils and macrophages, main cells of the innate immune system, using NIR-nanoprobes previously shown to be highly specific for activated neutrophils or macrophages following the induction of IO in a mouse model. We herein show that surgical induction of IO significantly increases the activation and migration of neutrophils and macrophages to the site of ischemic osteonecrosis as early as 48 h after surgery compared to sham controls and this response remains elevated even 6 weeks after surgery. Immunohistochemical analysis confirmed the time course of cell recruitment observed in the IO group using in vivo imaging and infiltration of neutrophils and macrophages into the necrotic bone marrow space, particularly at the 48-h time point, where cell infiltration into the distal femur from the periphery was observed. Additionally, TNFα staining showed significantly higher levels of pro-inflammatory cells at 48 h after IO surgery than sham control, and this elevation in TNFα remained up to 6 weeks after surgery, a hallmark of chronic inflammation and further corroborating the in vivo imaging. These results provide the first evidence, to our knowledge, of the involvement of innate immune cells, particularly neutrophils and macrophages, in the response to ischemic osteonecrosis.
Although inflammation is often associated with ischemic osteonecrosis, the effect of inflammation on bone remodeling in this condition has not been investigated. However, chronic inflammation has been shown to promote osteoclastic activity in other conditions, such as rheumatoid23 and osteoarthritis,24 leading to deformity of the underlying bone. Additionally, a large number of in vitro studies and animal models have shown the ability of pro-inflammatory signaling molecules, such as TNF-α,20, 25-27 IL-1,10, 28 and IL6,7, 8, 29 to promote osteoclastogenesis and bone resorption. These factors are often secreted by activated neutrophils and macrophages after exposure to DAMPs from necrotic cells.3, 4 Therefore, we postulate that the innate immune response may play an important role in the pathological bone healing following ischemic osteonecrosis, especially in light of our current results. Our findings offer the potential to study new mechanisms controlling disease progression and new therapeutic interventions aimed at modifying the innate immune cell activity.
Due to the dynamic nature of inflammatory responses, monitoring the activity of innate immune cells can be difficult and time consuming using traditional histology techniques. Additionally, many of the antibodies commonly used in immunohistochemistry to detect neutrophils and macrophages will provide strong positive results in the hematopoietic bone marrow, making comparisons between normal and necrotic bone marrow difficult. Therefore, the use of NIR-nanoprobes specific for activated primary innate immune cells offers a powerful method to noninvasively track immune cell activity in real time. These nanoprobes have previously been shown to have good cell compatibility, high affinity for the activated form of their intended cell type, and produce accurate assessment on the degree of inflammatory response at the area of interest in real time.18, 19 It should be noted that near-infrared imaging is limited by the penetration depth of the excitation light as well as the attenuation and scattering of the light by tissue.30 To overcome such drawbacks, our studies have shown that these probes can alternatively be conjugated with radioisotopes and then used in Positron Emission Tomography imaging.30
It is important to note that although this mouse model of IO does show many similarities to observations in humans and other large animal models, including cell necrosis, increased osteoclastic activity, and permanent deformity, the disease progression and repair response are accelerated in mice. In particular, we note the pattern of neutrophil and macrophage recruitment observed in this study differs from the standard cascade of immune cell response to injury, where robust neutrophil recruitment is observed several days before the peak of macrophage infiltration. The significant presence of activated macrophages at such an early time point was higher than anticipated, and may have been influenced by local resident macrophages. However, we believe the intravenous delivery of nanoprobes should primarily target circulating macrophages, and the disruption of the blood supply to the distal femur should limit the amount of local macrophages that would be exposed to the probes. Previously, we observed systemic infusions of radio-labeled anti-resorptive agents had minimal penetration into the femoral head of large animals that had undergone ischemic osteonecrosis surgery, supporting the idea that disruption of the local blood supply prevents the penetration of systemically administered molecules31 and our in vivo imaging results detected immune cells not present within the ischemic bone marrow. However, future studies will be necessary to confirm these results. Lastly, we acknowledge the significant differences in the immune systems of mice and humans,32 such as the ratios of circulating immune cells33 and the gene expression in response to inflammatory stimuli.34 These differences must be kept in mind when interpreting the results from a mouse study to determine clinical relevance, and further necessitate the need to confirm results in larger animal models.
In conclusion, we herein report the successful use of a novel in vivo imaging technique to monitor the temporal changes of neutrophils and macrophages in response to ischemic osteonecrosis in a mouse model. Using this technique, we observed a significant acute and chronic response of these immune cells following the induction of IO. We believe that this technique will be a useful tool for future studies to gain a better understanding of the signaling pathways involved in the pathological healing of necrotic bone and in testing new therapeutic strategies.
AUTHORS’ CONTRIBUTIONS
M.P., L.T., and H.K. designed experiments, reviewed the data, and wrote the manuscript. Y.H. performed the in vivo imaging and analysis, M.P., R.Y., and N.A. performed immunohistochemical analysis, and R.Y. and N.K. performed the animal surgeries. All authors have read and approved the submitted manuscript.
ACKNOWLEDGMENTS
This study was supported by an intramural grant at Texas Scottish Rite Hospital for Children (H.K.) and a grant (EB012575) from the NIH (L.T.). We thank Amanda McLerran for animal care and surgical assistance and Reuel Cornelia and Richard Banlaygas for histological preparation. Authors have no conflicts of interests to disclose.




