Bridging the gap: Bone marrow aspiration concentrate reduces autologous bone grafting in osseous defects
Abstract
Although autologous bone grafting represents an effective tool to induce osteogenic regeneration in local bone defects or pseudarthroses, it is associated with significant donor site morbidity and limited by the amount available for grafting. We investigate the potency of bone marrow aspiration concentrate (BMAC) to augment bone grafting and support bone healing. The functional and radiographic outcome of 39 patients with volumetric bone deficiencies treated with BMAC are presented and evaluated in a prospective clinical trial. A collagen sponge (Col) served as scaffold in 12 patients and a bovine hydroxyapatite (HA) was applied in the other 27 individuals. The minimal follow-up was 6 months. Clinical and radiographic findings were completed by in vitro data. All patients showed new bone formation in radiographs during follow-up. However, two patients underwent revision surgery due to a lack in bone healing. In contrast to the Col group, the postoperative bone formation appeared earlier in the HA group (HA group: 6.8 weeks vs. Col group 13.6 weeks). Complete bone healing was achieved in the HA group after 17.3 weeks compared to 22.4 weeks in the Col group. The average concentration factor of BMAC was 5.2 (SD 1.3). Flow cytometry confirmed the mesenchymal nature of the cells. Cells from BMAC created earlier and larger colonies of forming units fibroblasts (CFU-F) compared to cells from bone marrow aspirate. BMAC combined with HA can reduce the time needed for healing of bone defects when compared to BMAC in combination with collagen sponge. © 2010 Orthopaedic Research Society. Published by Wiley Periodicals, Inc. J Orthop Res 29:173–180, 2011
Autologous bone grafting is the gold standard for the treatment of bone defects. However, due to the limited resources, significant critical size osseous defects, a lack of bone healing ability in elderly patients or diseases which have a high recurrence even after surgical intervention such as aneurymal bone cysts and congenital pseudarthrosis, alternative treatment strategies are required. In addition, donor site morbidity presents a major disadvantage to autologous bone grafting.
Orthobiologics represent a group of clinically available potent agents with the ability to augment bone defect healing. In contrast to the more disappointing efforts to regenerate joint cartilage for clinical application, many experimental investigations and also clinical trials involving orthobiologic agents have shown promising results in augmenting bone regeneration. Clinically, researchers have demonstrated that different orthobiologics such as bone morphogenic proteins (BMPs), cell therapeutics, platelet lysate, or platelet derived growth factor (PDGF) can promote healing in critical sized bone defects and even in the healing of avascular osteonecrosis (AVN).1-8
Due to the different interactions of the hematopoietic and mesenchymal progenitor cells in the human bone marrow, a cell therapy strategy involving using various differentiation stages of the bone marrow cells is a potential candidate to treat tissue defects.9 Especially the stages involving osteoblast differentiation and bone healing induced by human bone marrow cells are currently being investigated.8, 10-14 Encouraged by the positive preliminary results after utilizing BMAC application for clinical bone regeneration,15-17 we initiated a prospective clinical trial to evaluate the osseopromoting potencies of BMAC application in promoting the healing of bone defects utilizing two different scaffolds.
MATERIALS AND METHODS
This prospective study was conducted under the US Food and Drug Administration (FDA) regulations for clinical trails and approved by the local Ethics Committee of the Heinrich Heine University Duesseldorf (study #3096).
Study Eligibility Criteria
From the 75 treated patients, 39 consecutive individuals between the ages of 4–87 years were screened and included in this study. Inclusion criteria for this study were local bone defects with a defect area (length × width) measurement larger than 1 cm × 1 cm such as bone cysts, defects caused by trauma or endoprotheses revision. Before the study enrolment was approved, informed consent stating that the patient was able to comply with all specific care and visit requirements was gathered from the patient, care giver, or legal representative. Exclusion criteria were active malignancy, ongoing treatment with immunosuppressant drugs including glucocorticosteroids, chemotherapy or colchicines, active or chronic infections. Patients were excluded if they were pregnant or during lactation. Also patients with autoimmune deficiency syndrome (AIDS, HIV) or hepatitis, a medical history of alcohol or drug abuse were excluded.
Bone Marrow Preparation
A total of 60 ml bone marrow aspirate (BMA) was obtained by Jamshidi vacuum aspiration from the posterior iliac crest. Bone marrow aspiration concentrate (BMAC) was produced via density gradient centrifugation using the Smartprep2™ centrifuge (Harvest Technologies, Munich, Germany) in accordance with the manufacturer's directions including 2,500 rpm for 3 min and followed by 2,300 rpm for 9 min. Afterwards a total volume of 8 ml BMAC was mixed with porous hydroxyapatite granules (Orthoss®, Fa Geistlich, Wolhusen, Switzerland, inner surface area 97 m2/g, total porosity: 60%, intercrystalline spaces crystal size 10–60 mm, Ca/P 2.03)18-20 or applied onto a porcine collagen sponge (Gelaspon®, Fa. Chauvin Ankerpharm GmbH, Berlin, Germany, 100 mg gelatine, resorption period in vivo 2–3 weeks).21 Both biomaterials have been used for medical application in orthopedic and dental surgery for years. To allow cellular adherence, BMAC was incubated with the HA or Col for 15 min prior to transplantation (Fig. 1). After curettage and soft tissue debridement of the local bone defect 50% of the local defect was treated by autologous cancellous bone grafting. The remaining 50% of the osseous defect was filled with a composite of BMAC-HA or BMAC-Col.
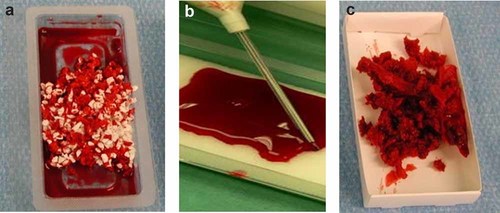
BMAC cultivated for 15 min prior to transplantation with (a) hydroxyapatite (b) on collagen sponge, (c) autologous bone.
Radiographic Controls
For radiological controls pre- and postoperative X-rays were taken in two standard planes (anteroposterior and lateral). Evaluations of the radiographs were performed after 2 and 6 weeks as well as after 6 and 12 months postoperatively by two independent observers (the first and the third author). The initial osseous defect size as well as new bone formation during follow-up was quantified by digital radiographs (Sienet Magic View, Fa. Siemens AG, Munich, Germany) in two planes. Due to the nature of HA and autologous cancellous bone, new bone formation was detected by thickening of cortical bone or by local remodeling within the transplantation site. Cortical thickening ≥2 mm in radiographs (two planes) was valued as a significant new bone formation. Lack of bone formation was documented by progressive resorption of the transplant, osteolysis, and/or insufficient cortical bone thickening.
Assessment of Clinical Parameters
Before surgery and after a minimum of 6 months postoperatively the patients' mobility was assessed. Also, the patients were evaluated concerning their activity level (profession, sports activities, walking distance) during follow-up. Additionally, peri- or postoperative complications and complaints were monitored.
In Vitro Analysis
Control of WBC Concentration
The residual volumes of BMA in the collection bag and of the BMAC in the application syringe were collected and the number of WBC was counted using a cell counter (CellDyn 3500, Abbott, Wiesbaden, Germany). The concentration factor was calculated by the quotient of white blood count (WBC) number in BMAC/BMA.
CFU-F and CFU-ALP Assays
After cell counting, the BMA- and BMAC cells were cultivated in different densities: 5 × 105, 1 × 105, 5 × 104, and 1 × 104 WBC per cm2 in 12-well culture plates (Nunc, Wiesbaden, Germany) with Dulbecco's modified Eagle's medium low glucose (DMEM, PAA Laboratories, Cölbe, Germany) supplemented with 20% fetal bovine serum (PAA), 100 U/ml penicillin, 100 µg/ml streptomycin (PAA) and incubated in 8.5% CO2 at 37°C as previously described.22, 23
Medium was renewed every 3rd day. After 7 and 11 days the non-adherent cells were removed from the wells by PBS washing and the remaining adherent cells were fixed in 4% buffered formalin for 10 min. To detect CFU fibroblasts (CFU-F) the cells were stained with Mayer's hemalaun (Merck, Darmstadt, Germany). Moreover, alkaline phosphatase activity (CFU-ALP) of the cells was measured using a commercial AP kit (Vector, Burlingame, CA). Here, the active enzyme cleaves p-nitrophenol from p-nitrophenyl-phosphate resulting in a detectable color. The cultures were scored for clusters (20–40 cells/aggregate) and colonies (>40 cells) at 6× magnification using a stereomicroscope (SZ61, Olympus, Hamburg, Germany) resulting in number of colonies per 1 × 105 WBC per cm2 after 6 and 10 days.12, 24, 25
Flow Cytometer Analysis
Same amounts of WBC (5 × 106/cm2) of BMA- and BMAC cells were seeded in culture flasks and cultivated as described above. After 3 days the medium was changed, the non-adherent cells were removed, centrifuged, and transferred into new culture flasks (supernatant 1). The same procedure including cell removal and cultivation of the supernatant was repeated 3 days afterwards (supernatant 2). The cells were cultured for 12–20 days until reaching confluency. The adherent cells were removed, counted, and stained for flow cytometric analysis (Coulter XL, Beckmann Coulter, Krefeld, Germany) controlling MSC character according to Dominici.26, 27 Cells were stained against CD34, CD44, CD45, CD73, CD90, and CD105 or the corresponding isotype controls (CD34, CD44: Becton-Dickinson, Heidelberg, Germany; CD45, CD90: Beckmann Coulter; CD 105: Ancell, Bayport, MN) and for alive/dead with DRAQ5 (Biostatus, Leicestershire, UK) and propidium iodide (Becton-Dickinson).
RESULTS
Clinical Trial
Out of 39 patients treated with the BMAC-biomaterial composite (16 males, 23 females) the collagen sponge (Col) was used as a scaffold in 12 patients whereas the remaining 27 patients received BMAC incubated with porous HA granules (Table 1). The osseous defect size of the Col group ranged from 0.54 to 55.6 cm3 whereas the HA group included bone defects varied from 1.3 to 151.2 cm3. Relevant new bone formation, controlled by cortical remodeling and homogenization of the graft was detected in X-rays in all 39 treated patients whereas complete bone healing occurred in 36 cases (Fig. 2). Three patients showed incomplete healing within the 6 months follow-up period or later. In one patient (pseudarthrosis of the medial femoral neck after fracture) the bone defect persisted for a period of 40 weeks. In this case, revision surgery including a second BMAC treatment combined with a BMP-2 application was performed and the defect healed at the latest follow-up (maximal follow-up was 2.5 years) (Fig. 3). The second patient had a recurrence of an enchondroma 26 weeks after the extensive curettage and underwent revision surgery with BMAC. At latest follow-up, the bone defects showed complete healing. A third female patient with a floating pelvis including a critical size defect at ischium and a pseudarthrosis at the pubic bone one year after triple pelvic osteotomy showed insufficient bone healing after BMAC-HA application. However, due to permanent micro- and macromotions of the pelvis the primary precondition for a solid fusion without internal fixation devices was low in this case. The patient is currently planed for ORIF and additionally BMP-2 application as a second line treatment.
| Study Parameters | Collagen (Col) | Hydroxyapatite (HA) |
|---|---|---|
| Number of patients | 12 | 27 |
| Male/female | 6/6 | 10/17 |
| Age range | 4–64 | 9–87 |
| Average age | 30.4 ± 16.9 | 37.7 ± 20.9 |
| Type of bone defects | ||
| Endoprosthetic associated bone defects | 1 | 6 |
| Pseudarthrosis | 4 | 1 |
| Enchondroma | 2 | 5 |
| Unicameral bone cyst | 1 | 3 |
| Aneurismal bone cyst | 2 | 2 |
| AVN | 1 | 4 |
| Others | 1 | 6 |
| Affected area | ||
| Pelvis | 3 | 7 |
| Femur | 7 | 7 |
| Humerus | 1 | 3 |
| Hand | 1 | 1 |
| Tibia/fibula | 0 | 7 |
| Foot | 0 | 2 |
| Osseous defect size (cm3) | 0.54–55.6 | 1.3–151.2 |
| New bone formation (weeks) | 13.6 (SD: 13.8) | 6.8 (SD: 2.7) |
| Bone healing (weeks) | 17.3 (SD: 7.8) | 22.4 (SD: 12.0) |
- Overall number of treatments n = 75 (72 patients). Total of 39 patients were included in our study with BMAC combined with the biomaterials collagen sponge (n = 12) or hydroxyapatite (n = 27).
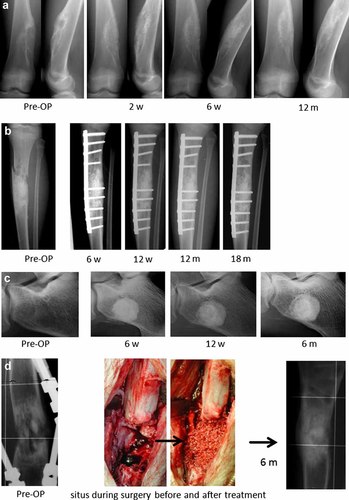
The exemplary radiographic follow-up of four patients with different types of bone defects who were treated with the biomaterial-BMAC composite show a solid healing of the osseous defect. (a) enchondroma of the femur of a 13-year-old male showed progressive healing after BMAC application, (b) pathologic fracture of a 10-year-old female caused by an aneurymal bone cyst showed significant bone remodeling after BAMAC application. (c) Unicameral bone cyst of the calcaneus of a 17-year-old male patient healed during follow up allowing to return to sports (soccer). (d) Persisting tibia pseudarthrosis in a 15-year-old male 1 year after osteotomy for leg lengthening. After BMAC-HA treatment the bone defect healed within 5 months. The patient returned to sports 6 months after treatment.
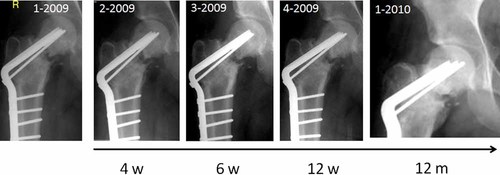
Pseudarthrosis of a femoral neck in a 22-year-old male patient after fracture. Although the application of BMAC did not induce complete bone healing, there was no evidence of avascular osteonecrosis. After second line surgery including autologous bone grafting, BMAC and BMP-2 application the osseous defect showed a progressive healing.
In contrast to the Col group, the postoperative bone formation appeared significantly earlier in the HA group as detected in X-rays (HA group: 6.8 weeks, SD 2.7 vs. Col group 13.6 weeks, SD 13.8). Complete bone healing could be seen in the HA group after 17.3 weeks (SD 7.8) compared to a period of 22.4 weeks (SD 12.0) in the Col group (Table 1). These data imply that the application of BMAC with HA leads to a reduced healing period compared with a gelatine sponge.
Excluding the two revisions mentioned above, there were no other severe postoperative complications. However, one patient showed persisting hematoma, and three other individuals of the collagen group had prolonged wound secretions for more than 7 days. Except for the three mentioned individuals with insufficient bone healing, all treated patients reached their preoperative activity level (add in percentage level).
In Vitro Data
The average concentration of BMAC compared to the initial BMA (amount of WBC in BMAC/BMA) was 5.2 ± 1.3 (n = 17). The amount of WBC in BMA ranged from 6 to 57 × 106 WBC/ml with an average value of 23 ± 13 × 106 WBC/ml and for BMAC from 27 to 220 × 106 WBC/ml with an average of 113 ± 5.2 × 106 WBC/ml (Table 2). The characterization of the BMAC-cells by flow cytometry displayed a positive staining >95% for CD73, CD90, and CD105 whereas CD34 and CD45 positive cells were only detected in <2 %. The cells cultivated from BMA and BMAC could be differentiated into osteoblasts as demonstrated earlier.28
| BMA | 13 | 17 | 19 | 21 | 6 | 15 | 12 | 27 | 26 | 8 | 32 | 46 | 57 | 23 | 32 | 14 | 18 | Ø23 | SD 13 |
| BMAC | 75 | 99 | 72 | 127 | 42 | 98 | 72 | 124 | 134 | 27 | 220 | 294 | 130 | 104 | 119 | 93 | 93 | Ø113 | SD 61 |
| Concentration factor | 5.8 | 5.8 | 3.8 | 6.2 | 6.8 | 6.4 | 5.8 | 4.6 | 5.2 | 3.4 | 6.8 | 6.3 | 2.3 | 4.6 | 3.7 | 6.5 | 5.2 | Ø5.2 | SD 1.3 |
- The high standard deviation indicates the high variability of mesenchymal cells within the human bone marrow.
After 7 days in vitro BMA and BMAC cells presented CFU-F as well as CFU-ALP (Fig. 4). When equal numbers of WBC in BMA and BMAC were cultivated, the BMAC cultures featured a higher number of CFU-F (Fig. 5). After 7 days, an average of 2.7 colonies per cm2 including 20–40 cells were observed in BMAC cultures compared to 2.7 colonies per cm2 in the BMA group. After 11 days 2.8 colonies per cm2 with a number of 20–40 cells and an average of 4.1 colonies with more than 40 cells were counted in the BMAC derived cells whereas in the BMA derived cells presented only 1.9 colonies per cm2 with numbers of 20–40 and an average of 3.0 colonies with more than 40 cells. The flow cytometry analysis, the ability of the cells to adhere to plastic surfaces and their potential to differentiate into osteoblasts (and other lines, data not shown) proved the mesenchymal stem cell character of the bone marrow cells.26
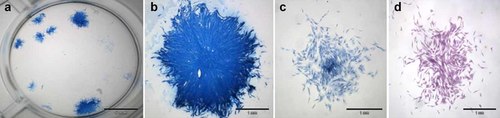
Colony-forming units (CFU) assay: (a) different colonies on a 12-well plate, (b) intense staining of a CFU alkaline phosphatase (ALP) after 11 days, (c) smaller colony after 7 days, (d) HE staining after 7 days.
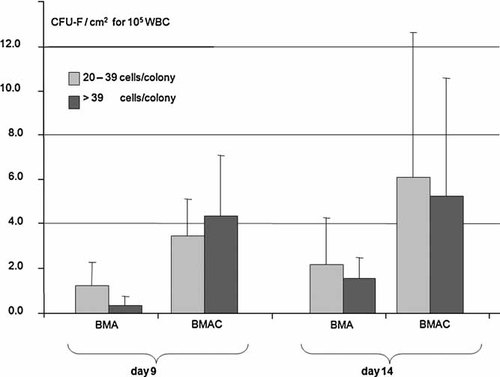
Amount of CFU-F/cm2 for 105 WBC from BMA or from BMAC after 9 and 14 days cultivation. Cells derived from BMAC displayed CFU-F earlier and with larger colony sizes.
DISCUSSION
In the present study, the application of BMAC-biomaterial composite (collagen/hydroxyapatite) expedited bone healing in 36 out of 39 cases within the 6 months postoperative period. However, the use of BMAC-HA lead to faster healing when compared to the BMAC on collagen sponge (17.3 ± 7.8 weeks vs. 22.4 ± 12.0 weeks, add a p-value if possible). The excellent bony ingrowth into porous HA and the osseopromoting potency of BMAC are in conformity with previously published data.19, 29-35 Although HA is only slowly resorbed in vivo, the macroporosity of the HA carrier used in our study allowed bony ingrowth and a solid integration within the transplantation site as evident by radiographs (Fig. 2). Therefore the application of BMAC-HA in weight bearing areas may have advantages over other biomaterials, in which rapid resorption may lead to or increase the risk of fracture. Furthermore, the collagen scaffold promoted cellular adherence, but has no relevant biomechanical function and is resorbed within few days up to 3 weeks in vivo.21 It is hypothesized that collagen-covered HA scaffolds may combine the advantages of a strong cellular adherence with the biomechanical advantages of using solid ceramic bone substitute in areas that require weight bearing.34 Here, in our first in vitro data, cellular adherence and proliferation of bone marrow cells was highest in the HA group with residual collagen when compared to other hydroxyapatite-type bone substitutes without collagen.36
Recent data from the literature have also shown that BMAC can both induce bone formation and also have healing ability in patients with critical limb ischemia.37-40 The finding is that the differentiation pathway of ostoeprogenitor cells including various stages that can be strongly influenced by its local microenvironment41-44 which makes this cell therapeutic strategy a strong candidate for clinical application in different anatomic regions and also provide an alternative treatment option for the regeneration of different types of mesenchymal tissues.
Although it was first suggested by in vitro and animal experiments that bone regeneration in osseous defects may be promoted by BMAC6, 45-48; it is undetermined so far, whether or not bone regeneration in vivo is expedited by supplementing BMAC with other growth factors or agents such as platelet-rich plasma (PRP), PDGF, insulin like growth factor (IGF), or fibroblastic growth factor (FGF) in human patients.
In our flow cytometry data, the ability of cells to adhere to plastic surfaces and the potential to differentiate into osteoblasts demonstrated the characteristics of mesenchymal stem cell in BMAC.26 In regards to the BMAC concentration factor, our presented data are in line with Hermann et al.49 who reported a concentration factor of 4.4 for total nuclear cells using the same system. However, it is evident that in vitro data of the BMAC device used in our study are not applicable to other species. This is evident by Thoesen et al. who showed a sevenfold increase of nucleated cells in 19 adult dogs.50
The average amount of colony-forming units in this study was 2.8 CFU-F/cm2 for BMA and 4.1 CFU-F/cm2 for BMAC, respectively, using a seeding density of 1 × 105 WBC/cm2. These values are comparable to Castro-Malaspina et al.24 with 0.6–1.9 CFU-F/cm2 and Muschler et al.51 with an average of 5.5 CFU/cm2 (seeding density of 1.2 × 105 WBC/cm2). Hernigou et al.12 reported a positive correlation between the volume of mineralized callus at four months postoperatively and the number (p = 0.04) and concentration (p = 0.01) of CFU-F in the graft. He could also show a negative correlation between the time to bone healing and the concentration of CFU-F in the graft (p = 0.04). The fact that the cells from BMAC created earlier (already after 7 days) and larger colonies of forming units fibroblasts (CFU-F) compared to cells from BMA can be explained by the high amount of mesenchymal progenitor cells and also another contributing factor can be due to the reduced number of erythrocytes (Ery) available in the BMAC in comparison to the BMA. Due to the gradient centrifugation, only the supernatant with the WBC is centrifuged again and used for the concentrate in the BMAC production procedure. The values were 3.9 × 106 ± 1.91 Ery/µl for BMAC and 4.0 × 106 ± 0.77 Ery/µl for BMA (n = 18). Considering the average concentration factor of 5.2, the WBC/Ery ratio is smaller for BMAC than for BMA. Therefore the amount of erythrocytes in BMAC is reduced compared to BMA. With the same amount of WBC seeded onto culture dishes, the competition between cells for nutrients will be lower in BMAC compared to the BMA culture. This effect might also play a role in vivo.
Based on recent data and personal experiences in more than 100 cases, we conclude that the application of BMAC is a safe procedure which can harvest a relevant amounts of potent mesenchymal stem cells to differentiate into osteoblasts in vitro. Due to the nature of bone defects, one major limitation of the study is the diversity of indications, transplantation sites, and defect sizes. Therefore it is very hard to generalize these data to all bone defects. However, the excellent bone regeneration by BMAC combined with HA or collagen reduced the amount of autologous bone grafting in our patient population and expedited the healing to within 6 months postoperation. Since the current gold standard of using autograft is accompanied with significant donor site morbidity, the use of BMAC-scaffold composites may contribute to improved patients' outcome while minimizing patient morbidity.
Acknowledgements
Mrs. Lensing-Höhn for technical support and Geistlich Surgery for granting parts of the study.




