Phytophthora cinnamomi causing root rot on Rhododendron lapponicum and control it using potential biocontrol agents
Abstract
Rhododendron lapponicum (R. lapponicum) is a dwarf Rhododendron species, which is severely infected with root rot and wilt in Yunnan province, China. However, the causal agent causing these symptoms was unknown. An isolate, Pci-1 was identified as Phytophthora cinnamomi, based on its morphology and the sequences of β-tubulin, internal transcribed spacer, and Ypt1 genes and verified according to the Koch's postulate. We found that this pathogen could infect 14 species of plants, including Althaea rosea, Viburnum cylindricum, and Brassica napus. Strain Pci-1 could cause R. lapponicum to wither and die; and it grows best in an oat medium with pH 7.0 − 8.0 and an optimum temperature of 27°C. We suggest that the rhizosphere of R. lapponicum treated with biocontrol strains Paenibacillus polymyxoides P2-5 and Trichoderma asperellum Tv-1 showed a significant inhibitory effect on pathogen Pci-1. The inhibitory effect of 70% dimethomorph + cymoxanil was significantly higher with EC50 and EC90 values of 0.1894 and 0.3618 a.i. µg/ml, respectively. Greenhouse experiments revealed that the pathogen load is decreased in the presence of potential antagonists. This study provides fundamentals on risk assessment and theoretical support for the management of P. cinnamomi pathogen and contributes significantly to the planting of forest and horticultural crops in a disease-free environment.
Abbreviations
-
- BLAST
-
- basic local alignment search tool
-
- CTAB
-
- cetyl trimethylammonium bromide
-
- ITS
-
- internal transcribed spacer
-
- LB
-
- Luria–Bertani
-
- MCD
-
- measuring the colony diameter
-
- OA
-
- oatmeal agar
-
- PDA
-
- potato dextrose agar
-
- PRR
-
- Phytophthora root rot
-
- PSA
-
- potato sugar agar
-
- V8
-
- V8 agar medium
1 INTRODUCTION
Rhododendron lapponicum, also recognized as Lapland rosebay, is a dwarf Rhododendron species localized in Asia, Europe, and North America with altitudes from sea level of 1900 m (6200 ft) [1]. The height of this evergreen shrub ranges from 20 to 45 cm (7.9–17.7 in.) with oblong-elliptic or ovate-elliptic to oblong-obviate leaves size of 1.5–6 cm × 2–5 cm. Rhododendron is the largest genus in the family of Ericaceae and is valued in the temperate zone of the world for significant horticultural value [2]. Approximately 571 Rhododendron species have been reported in China accounting for nearly 55% of the world's total [3]. Rhododendron grows in most of China's provinces (except Xinjiang and Ningxia) and is found across 60% of China's land area. Over 74% of Rhododendron members occurring in China are endemic species [3, 4]. Of these provinces, Yunnan, Sichuan, and Tibet have the greatest diversity, with approximately 374, 255, and 227 species, respectively [5].
In addition, Rhododendron is globally cultivated as an ornamental plant [6, 7]. As the largest genus in the family of Ericaceae, they form a major component of the montane ecosystem in the Himalayan subalpine and alpine zones, which has been identified as one of the most fragile ecosystems in the world [8, 9]. These species play a vital role in slope stabilization and watershed protection in the Himalayas. Nevertheless, Rhododendron is one of the most neglected groups of plants in terms of scientific inquiry [9].
Due to its high horticultural value, it has been widely cultivated, but with climate change, habitat differences, and disease and insect infestations, the number of some species has been reduced to the verge of extinction, resulting in the decline of species diversity and serious economic losses [10, 11]. With the extension of planting time and scale, a new disease with symptoms of root rot was observed in Yunnan province, China. However, at an early stage, the causal pathogen was unknown. In this study, we identified the pathogen causing the rot and wilt symptoms in R. lapponicum through different field investigations and pathogen sensitivity to fungicides. This study also addresses an eco-friendly strategy to potentially manage this pathogen in the field and laboratory conditions and provides new insights for disease control in landscape plants.
2 MATERIALS AND METHODS
2.1 Sample collection and pathogen isolation
The plant and rhizosphere soil samples of infected R. lapponicum were collected at a plantation area in Anning City (24°55′08″N, 102°28′41″E), Yunnan province, China in January 2021. Samples were packed in polythene bags and brought to the laboratory for further analyses. Root samples were washed with tap water and surface-sterilized with 75% ethanol for 30 s and further washed with ddH2O three times. Roots were cut into 0.5 cm pieces with sterilized scissors and placed on potato dextrose agar (PDA) and oatmeal agar (OA) medium, containing rifampicin and ampicillin (30 mg/L each) to avoid bacterial contamination, followed by incubation at 28°C for 3–5 days. The mycelium discs at the edge of typical colonies were selected and transferred to the new media plates for purification and cultivation. Pure culture was transferred into the PDA and OA medium glass tubes and added with sterilized paraffin oil after the mycelium grow over the whole slant, then the tubes were stored at 16°C for further use.
2.2 Pathogenicity test
To better understand the pathogenicity of isolated strain, we used healthy R. lapponicum plants as inoculating hosts. The pathogen was cultured for 7 days in a PDA plate (several plates) and the suspension was prepared by adding 50 ml of sterile distilled water. The roots of healthy R. lapponicum were drenched with 20 ml of mycelium suspension through soil. The sterile water was used as a control. Furthermore, all inoculated plants were transferred to the greenhouse (28°C, 75% relative humidity) to observe the disease symptoms. After 45 days of inoculation, the plants inoculated with mycelium suspension were observed for symptoms of leaf yellowing, withered and drooped, and rotten roots. A total of 15 pots were used for these experiments with three biological replicates. The diseased root samples were collected for pathogen confirmation.
2.3 Morphological observation and molecular identification
The candidate pathogen was cultured in an OA medium with 0.1% potassium nitrate at 28°C for 7 days. The plates were kept for 12 h in light and dark each at 28°C for 5 days to induce chlamydospore and sporangium. The morphology of mycelium and spores were observed under a light microscope (Carl Zeiss Microlmaging GmbH 37081). The pathogen was identified and the taxonomic status of the isolate was preliminarily clarified. To get the exact identification of pathogenic isolate, molecular analyses were carried out.
The genomic DNA of the isolate was extracted using cetyl trimethylammonium bromide (CTAB) method [12]. The primer Btub-F1/Btub-R1 was used to amplify the β-tubulin gene [13] and ITS1/ITS4 primers were used to amplify the sequence of internal transcriptional spacer (ITS) of ribosomes [14]. Primer Yph1F/Yph2R amplified the encoding gene of Ypt1 associated with the Ras family of Phytophthora [15]. List of all primers is mentioned in Table 1. The PCR amplification products were sequenced by Shanghai Qingke Biotechnology Co., Ltd. and the sequencing results were compared with the homologous sequence information BLAST (basic local alignment search tool) in GenBank (http://www.ncbi.nlm.nih.gov/balst/) and Phytophthora ID (http://phytophthora-id.org/index.html). Phylogenetic trees were built through MEGA 6 using the neighbor-joining method.
| Locus | Primer | Primer sequence (5′–3′) | Reference |
|---|---|---|---|
| ITS | ITS-1 | TCCGTAGGTGAACCTGCGG | [14,16] |
| ITS-4 | TCCTCCGCTTATTGATATGC | ||
| β-tubulin | Btub-F1 | GCCAAGTTCTGGGAGGTCATC | [13] |
| Btub-R1 | CCTGGTACTGCTGGTACTCAG | ||
| Ypt1 | Yph1F | CGACCATTGGCGTGGACTTT | [15] |
| Yph2R | ACGTTCTCGCAGGCGTATCT | ||
| 16S rDNA | 27 F | AGRGTTYGATYMTGGCTCAG | [17] |
| 1492 R | TACGGYTACCTTGTTACGACTT |
- Abbreviations: ITS, internal transcriptional spacer; rDNA, ribosomal RNA.
2.4 Host range determination
Host range of this pathogen was determined using more than 60 types of common landscape plants and fruit trees and R. lapponicum was used as a positive control. The leaves of tested plants were collected and inoculated with a 7 mm mycelium disc of pathogenic isolate and cultured in a PDA plate with absorbent paper with three replications. All leaves were stored in a 28°C constant temperature light incubator (12 h in light and in dark each, 4000 lux of light), to observe and record disease incidence.
2.5 Biological characteristics of pathogen
2.5.1 Effect of medium on mycelial growth
The mycelium of the 7-day-old culture was punched with a 7 mm perforator and the mycelial discs were picked out with a sterile needle and inverted in fresh 9.0 cm OA, PDA, potato sugar agar (PSA), V8 agar medium (V8), cornmeal agar medium, cucumber tomato agar medium, and incubated at 28°C for 7 days. The effect of different media on mycelial growth was analyzed by measuring the colony diameter (MCD).
2.5.2 Temperature effect on mycelial growth
As described above, the mycelial disc was inoculated onto the center of a fresh OA plate (9.0 cm). The plates were placed at 15°C, 24°C, 26°C, 27°C, 28°C, and 30°C, respectively, for 5 days, and the optimum culture temperature for the colony growth was investigated according to MCD.
2.5.3 Effect of pH value on mycelial growth
In addition, the isolated mycelial disc with a diameter of 7 mm was inoculated in the center of the OA plate with pH values of 4.0, 5.0, 6.0, 7.0, 8.0, 9.0, and 10.0, respectively. After incubation at 28°C for 5 days, the colony diameter was measured to determine the effect of pH value on the mycelial growth of the isolate.
2.6 Screening and identification of potential antagonistic strains
One gram of root tissue and rhizosphere soil of healthy R. lapponicum plant were macerated in sterile mortar and pestle to make different suspension to spread on Luria–Bertani (LB) and PDA plates, respectively. The LB plates were incubated at 37°C for 2 days in the dark and single colonies were picked for marking and preservation. Accordingly, PDA plates were incubated at 28°C for 2–7 days in the dark. The emerging colonies of fungi were picked, purified, and preserved. The candidate bacteria isolated in this experiment were used as indicators. Antagonistic activity of potential candidate strains against the pathogen was evaluated through the dual culture method, followed by the identification of the dominant antagonistic strains by amplifying 16s ribosomal RNA for bacteria and ITS regions for fungi [17]. To prove the potential strains in the greenhouse, the control effect of the pathogen in potted R. lapponicum was also evaluated. The roots of R. lapponicum were inoculated first with mycelium suspension of pathogen Pci-1. After 1 week, the soil substrate of R. lapponicum pot was tested on a selective medium. A total of five spots were taken from each pot and placed on a PDA medium plate. After detection, seedlings carrying pathogen Pci-1 were used to check the biocontrol ability of potential candidate antagonists. Each treatment consisted of 5 pots of seedlings and each pot was irrigated with 100 ml candidate antagonists fermented suspension at a concentration of 1 × 107 cfu/ml. The sterilized water was used as negative control and 40% Dimethomorph(SC) was the chemical (positive) control. Additionally, the soil substrate in the pot was regularly isolated and counted with a selective medium and the pathogen reduction rate was calculated accordingly.
2.7 Toxicity determination of chemical agents
| Fungicide | Regression equation | R² | EC50 | EC90 |
|---|---|---|---|---|
| (a.i. μg/ml) | (a.i. μg/ml) | |||
| 80% Dimethomorph (WG) | y = 2.3259x + 0.0429 | 0.9634 | 0.1965 | 0.3685 |
| 40% Dimethomorph (SC) | y = 2.1641x + 0.1576 | 0.972 | 0.1982 | 0.3431 |
| 30% Flumorph (SC) | y = 0.6165x + 0.0487 | 0.9542 | 0.7320 | 1.3809 |
| 70% Dimethomorph + cymoxanil (WG) | y = 2.3209x + 0.0604 | 0.9516 | 0.1894 | 0.3618 |
| 687.5 g/L Fluopicolide + propamocarb (SC) | y = 0.0641x + 0.1992 | 0.9643 | 4.6927 | 10.9329 |
| 62.5 g/L Mefenoxam + fludioxonil (FS) | y = 0.2879x + 0.1155 | 0.9762 | 1.3355 | 2.7249 |
- Abbreviations: FS, flowable concentrate; R2, determination coefficient; r, Pearson correlation coefficient; SC, suspension concentrate; WG, water dispersible granule.
- p < 0.05.
2.8 Statistical analysis
The software SPSS 24.0 (SPSS Statistics for Windows, version 24.0; IBM Corp) was used for the analysis of variance. Means were subjected to Duncan's multiple range tests at p ≤ 0.05. All figures were processed and analyzed by using Adobe Illustrator CS6 (Adobe Systems Inc.) and GraphPad Prism (8.0.2).
3 RESULTS
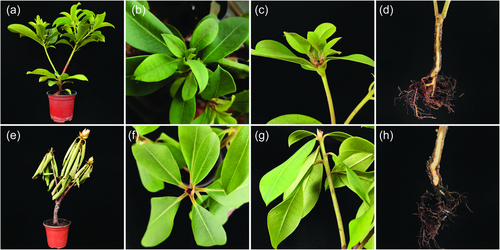
3.1 Symptom description of root rot of Rhododendron lapponicum
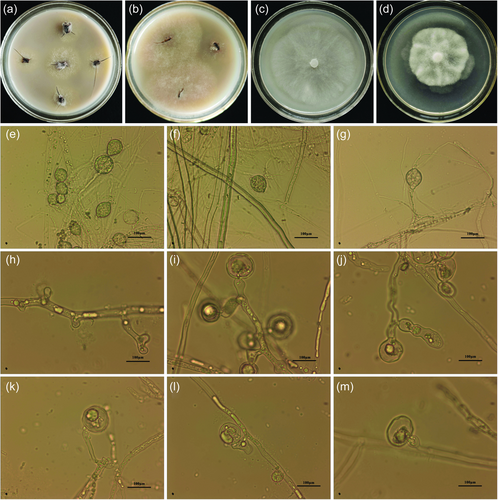
At the early stage of infection, brown spots appeared on the surface of roots and stem and newly emerged leaves turned yellowish. Pathogens spread rapidly in the presence of moist soil. Infected leaves drooped and resulted in enlarged angles between leaves and stem. The root parts turned brown and black at a severe stage of symptoms. Plants started showing reduced growth and all leaves turned yellow, and finally, the whole plant weakened and dieback (Figure 1). Based on the symptoms mentioned above, it was assumed that the causal organism belongs to oomycetes. Furthermore, the pathogen was isolated from the infected root tissue by using the PDA and OA medium of Phytophthora. As a result, white and loose colonies were isolated from the infected roots and designated as a suspected isolate Pci-1 (Figure 2a).
3.2 Pathogenicity test for pathogen confirmation
After the mycelium suspension of isolate Pci-1 had been inoculated to R. lapponicum root for 45 days, the plants' growth stopped, leaves dehydrated, turned yellow, and drooped. In addition, the roots were also dehydrated and drought, turned brown and black, the whole plant withered, and finally died (Figure 1e–h). The symptoms were similar to root rot of R. lapponicum reported previously [7]. The morphological characteristics of the pathogen isolated from the infected plants were observed and the results were exactly the same as that of the first isolated Pci-1 (Figure 2b). Therefore, Koch's postulate confirmed the isolate Pci-1 as the pathogen of root rot on R. lapponicum.
3.3 Morphological characteristics and taxonomic status
3.3.1 Morphological characteristics
The pathogen Pci-1 (Figure 2a,b) isolated from the rotten roots of R. lapponicum grows rapidly in OA medium with well-developed aerial mycelia, radial growth, and loose texture (Figure 2c). On the PDA medium, the growth was slower, the hyphae were dense, and the colonies were petal-like (Figure 2d). The white mycelia had no septum and formed sporangia (35–70 μm). The sporangia were oval or ovoid in shape, basifixed or adnated on the mycelia or sporangiophore and one end of the sporangium formed papillary protrusion or no papillary structure due to slight apical thickening (Figure 2e–g). Based on the above morphological characteristics, it was found that it is similar to oomycetes and belongs to Phytophthora by referring to the Forest Phytophthora of the World (http://forestphytophthoras.org/) and the World Phytophthora Genetic Resource Collection (https://phytophthora.ucr.edu/). The swollen mycelium or thin-walled chlamydospores are usually formed readily in water in greater abundance than sporangia, hyphal swellings are globose and often in botryose clusters (Figure 2h–j). Oogonium is globose and antheridium paragynous or amphigynous (Figure 2k–m).
3.3.2 Molecular identification of candidate Pci-1 strain
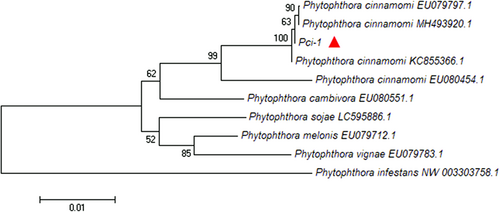
Genomic DNA of strain Pci-1 was used as a template, the amplified sequences of Btub-F1/Btub-R1 (1137 bp), ITS1/ITS4 (727 bp), and Yph1F/Yph2R (476 bp) were submitted to NCBI GenBank database with sequence numbers of MZ905503, MZ901370, and MZ905504, respectively. BLAST results showed that the strain Pci-1 had the closest genetic relationship with P. cinnamomi in GenBank database and the consistency was 100%. The phylogenetic tree based on β-tubulin gene of Pci-1 is displayed in Figure 3 and the phylogenetic trees based on the ITS and Ypt1 gene of Pci-1 are mentioned in Supporting Information: Figures S1 and S2. According to the above-mentioned results, strain Pci-1 was identified as Phytophthora cinnamomi Rands.
3.4 Host range
In this study, more than 60 species were tested (Supporting Information: Table S1), in addition to R. lapponicum, 14 species were infected by P. cinnamomi Pci-1, including Althaea rosea, Viburnum cylindricum, and Brassica napus. The diseased leaves were brown, expanded slowly, and decayed (Figure 4). The leaves resistant to disease had no obvious symptoms and the wounded ones were accrued from sclerenchyma.

3.5 Growth conditions
Isolate Pci-1 could grow significantly on all six media (Figure 5), but it grow most efficiently on the OA plate. The average diameter of the colony reached 69.30 mm after 5 days of culture, followed by V8, and minimal growth was noticed on PSA media. At 15–30°C, the colonies of Pci-1 were expanded on the OA medium, and the optimum temperature was 28°C. The colonies of Pci-1 were not observed on the OA plate when the mycelium was exposed to 40°C for 20 min, showing that the mycelium of Pci-1 could be completely inhibited with this range of temperature (data not shown).
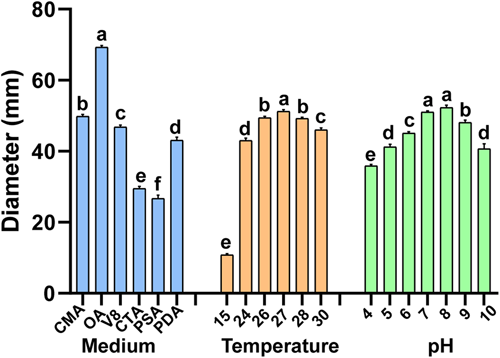
Comparing different pH values, the results showed that Pci-1 could grow in the range of pH 4.0–10.0, and the largest colony diameter was observed on the OA plate with pH value of 7.0–8.0, indicating that the most suitable pH value for the growth of Pci-1 was 7.0–8.0. The growth on the OA plate with pH values of 4.0 and 10.0 resulted in smaller colonies indicating that exceedingly acidic or basic media were not conducive to the growth of Pci-1 on the OA plate.
3.6 Isolation and identification of candidate strains against Pci-1
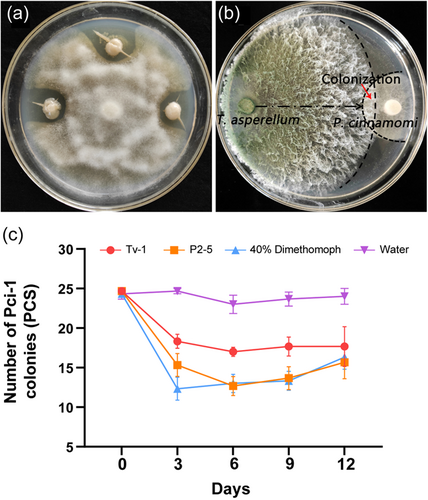
The candidate antagonistic strains of Pci-1 were screened by dual culture method and identified as Paenibacillus polymyxa (MZ771306) (Figure S3) and Trichoderma asperellum (MZ771300) (Figure S4) by 16S rRNA and ITS, respectively. Among them, P. polymyxa P2-5 and Pci-1 were cultured in PDA medium at 28°C for 7 days (Figure 6a). The latter had no complete mycelial structure and was difficult to grow on a special medium. In addition, T. asperellum Tv-1 had obvious inhibitory and parasitic effects on Pci-1, which was limited to the initial colony before contact (Figure 6b). The pot experiment in the greenhouse suggested that the two antagonistic strains had a better control effect on Pci-1 than the fungicide. After 3 days of treatment, the pathogen load reduced significantly, and after 12 days, the pathogen declined to a considerable level of 28.38%–36.49% (Figure 6c).
3.7 Efficacy of fungicides against colony growth of Pci-1
The efficacy of the following six fungicides on Pci-1 was determined by the growth rate method (Table 2): 80% dimethomorph (water dispersible granule [WG], Shanxi Welch crop protection Co., Ltd.), 40% dimethomorph (suspension concentrate [SC], Jiangsu Jianpai Agrochemical Co., Ltd.), 30% flumorph (SC, Shenyang Kechuang Chemicals Co., Ltd.), 70% dimethomorph + cymoxanil (WG, Shandong Rongbang Chemical Co., Ltd.), 687.5 g/L fluopicolide + propamocarb (SC, Bayer Crop Science (China) Co., Ltd.), and 62.5 g/L mefenoxam + fludioxonil (flowable concentrate [FS], Shanghai Hulian Biopharmaceutical Co., Ltd.). The selected fungicides had different degrees of inhibition on the colony growth of Pci-1. The order of EC50 was as follows: fluopicolide + propamocarb > mefenoxam + fludioxonil > flumorph > dimethomorph > dimethomorph > dimethomorph + cymoxanil. Other four fungicides had higher antagonistic activity against isolate Pci-1, except for the two fungicides (Fluopicolide + propamocarb and mefenoxam + fludioxonil) (Table 2). The inhibitory effect of dimethomorph + cymoxanil on the isolate Pci-1 was significantly higher than others and the EC50 value was 0.1894 a.i. µg/ml, EC90 value was 0.3618 a.i. µg/ml.
4 DISCUSSION
Rhododendron plants have been seriously affected by Phytophthora root rot (PRR) as shown by EPPO (https://gd.eppo.int/) data, whereas P. kernoviae and P. ramorum were responsible pathogens. It was reported for the first time that P. hedriandra cause Rhododendron dieback and root rot of common beech in the Czech Republic [19]. Phytophthora cinnamomi is the dominant species to infect Rhododendron and with Phytophthora or Pythium together cause more serious diseases [20]. P. cinnamomi is widely distributed in Oceania, North America, South America, Europe, and Africa (https://gd.eppo.int/). It was also isolated from black locust (Robinia pseudoacacia) in Jiangsu province of China in 1981–1983 marking the first report of the pathogen in the country [21]. Since then, plant root rot caused by P. cinnamomi has been reported in Fujian, Zhejiang, Jiangsu, Shanghai, Guangdong, Hainan, Hebei, Guangxi, and other provinces [22]. However, the study proves that the species and quantity of Phytophthora in the soil where Rhododendron and Quercus grow; and in the rivers of Northwest Yunnan and did not find P. cinnamomi in this area [23]. Before that, the infection of Rhododendron by P. cinnamomi had not been reported in China. This study reported for the first time that the root rot of Rhododendron in Yunnan province was caused by P. cinnamomi. The taxonomic status, pathogenicity, biological characteristics, host range, and control methods of P. cinnamomi were explored to provide a detailed research basis to understand the P. cinnamomi characteristics and mitigate the spread in different areas of Yunnan province, China.
P. cinnamomi is one of the most destructive pathogens for crops and forests in the world. In 1922, stripe canker on the cinnamon tree was described for the first time in Sumatra. Since then, it has spread worldwide with the era of plant exploration and sailing ships. It is reported in more than 70 countries and can infect more than 1000 plants (http://forestphytophthoras.org/species/cinnamomi). Because P. cinnamomi has the widest host range among Phytophthora, it is now widely distributed and continues to cause damage to forests in Australia, Mediterranean countries, Europe, and western North America [24-26]. Since the summer of 2017, in almost all commercial orchards in southern Turkey, it has been found that avocado trees showed severe symptoms after being invaded by P. cinnamomi, and newly planted seedlings had serious dieback [25]. In addition, in the forest where many plants coexist, once the conditions are suitable, it is conducive to develop PRR and aggravates the decline of the forest [26]. In vitro inoculation of the pathogen for pathogenicity identification and resistance, evaluation has the advantage of small workload, simple operation, and so on [26, 27]. In this study, P. cinnamomi was identified as the causal agent of root rot and reduction of R. lapponicum in Yunnan province and its diverse host range was studied. After inoculating the leaves of many plants, it was found that P. cinnamomi Pci-1 could infect many species of plants and cause their leaves to wilt and rot, including Eucalyptus [28] and Rhododendrons [29], flowers, crops, fruit trees, and landscape plants, such as A. rosea, B. napus, Amygdalus persica, Magnolia grandiflora, and so forth. It is of utmost importance to study management strategies to reduce the losses caused by this disease in the future.
Climate change and alien biological invasion are causing global environmental degradation and resource decline, and the spread of PRR is leading to the reduction of forests. The characteristics of P. cinnamomi Pci-1 suggested that it grows efficiently in the OA medium with pH 7.0–8.0 and optimum temperature of 27°C. Before this, few studies suggested that P. cinnamomi has the ability to adapt to coldness and cause diseases at high altitudes and shows plasticity to low temperatures [27]. However, the altitude of Yunnan province is from 76.4 to 6740 m, with a great difference and a high degree of biodiversity. Because of its cold growth environment, R. lapponicum distributes in high altitude areas, with weak disease resistance, and is vulnerable to pathogen invasion. The interaction between P. cinnamomi and Quercus suber in the Mediterranean region was studied and found as soil moisture increase, the incidence of P. cinnamomi also increase resulting in significant damage to plant roots [29]. From the perspective of climate change, the dry climate slows down the invasion speed of P. cinnamomi, and thus reduces the disease spread. In addition, the influence of climate and soil moisture would change with the occurrence of extreme climate, leading to more unpredictable host plant diseases and pathogen transmission.
In the control of plant diseases, biological control is now widely adopted because of its environmental friendliness [30-32]. It is possible to control root rot of Rhododendron by using the antagonistic strains selected in this study. However, efficient chemical control is the main strategy to reduce the losses caused by plant diseases at present. For oomycete diseases, there are many fungicides that can be used, among which metalaxyl is the most common, but it is prone to evolve resistance in pathogens. Among the chemical fungicides used in this study, P. cinnamomi Pci-1 is more sensitive to other oomycete fungicides except for fluopicolide + propamocarb and mefenoxam + fludioxonil. Previous studies have shown that several Phytophthora species such as P. cinnamomi are not sensitive to oomycete fungicides, and their control effect on PRR is reduced [33]. These results provide not only the research basis for the chemical control of P. cinnamomi in the future but also clues for tracing the origin of P. cinnamomi.
Phytophthora has attracted much attention because it causes serious plant diseases and big economic losses. Effective management can reduce the spread of Phytophthora in gardens and natural ecosystems and its ecological impact. At present, China and other countries have actively participated in this study [34-36]. Uncertainty of the original center of many invasive species also hinders the timely management of diseases. Moreover, pathogen management becomes much slower and more difficult due to the extreme susceptibility of natural hosts and the rapid evolution of pathogens. Whether P. cinnamomi found in Rhododendron nursery in Anning City originated from nursery substrate or come from other reported provinces was uncertain and needs further consideration and research. The results of this study may provide a foundation for a detailed and in-depth understanding of PRR disease and guide for risk management assessment of Phytophthora pathogen in the planning and protection of landscape, forest land, and crop planting.
ACKNOWLEDGMENTS
This study was financially supported by Central Government Fund for Local Science and Technology Development (202107AA110007).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are deposited in the GenBank with accession numbers MZ905503, MZ901370, and MZ905504.




