Antibacterial activities of hexadecanoic acid methyl ester and green-synthesized silver nanoparticles against multidrug-resistant bacteria
Abstract
Antibacterial drug resistance is considered one of the biggest threats to human health worldwide, and the overuse of antibiotics accelerates this problem. Multidrug-resistant (MDR) bacteria are becoming harder to treat as the antibiotics used to treat them become less effective. Therefore, it is necessary to evaluate novel methods to control MDR bacteria. In this study, 40 bacterial isolates were collected from diabetic patients. The sensitivity of 40 bacterial isolates to seven antibiotics was evaluated. Four bacterial isolates were resistant to all antibiotic groups. The MDR pathogenic bacteria were selected and identified morphologically and biochemically and confirmed by VITEK® 2 system as follows: Staphylococcus aureus W35, Pseudomonas aeruginosa D31, Klebsiella pneumoniae DF30, and K. pneumoniae B40. Identification of the most resistant P. aeruginosa D31 was confirmed by the sequencing of a 16S ribosomal RNA gene with an accession number (MW241596). The inhibitory activity of eight types of native grown plant extracts against MDR bacteria was studied. Clove alcoholic extract (CAE) showed the highest inhibitory activity against MDR bacteria. Gas chromatography–mass spectrometry analysis of partially purified CAE at 0.9 Rf detected by thin-layer chromatography showed an active compound named hexadecenoic acid methyl ester with the highest antimicrobial effect against clinical pathogenic bacteria. The formation of silver nanoparticles (AgNPs) by CAE was studied. Evaluation of AgNPs was investigated by X-ray diffraction, UV–Vis, and transmission electron microscopy. The antibacterial effect of AgNPs after 2, 4, and 6 days in light and dark conditions was evaluated. Finally, the AgNPs synthesized using CAE possess good inhibition activity against the tested pathogenic bacteria. As a result, the bactericidal components listed above were promising in reducing MDR bacteria and can be used for treatments of bacterial infection and in the development of safe products with a natural base.
Abbreviations
-
- AgNP
-
- silver nanoparticle
-
- CAE
-
- clove alcoholic extract
-
- MDR
-
- multidrug-resistant
-
- NP
-
- nanoparticle
-
- Rf
-
- retention factor
-
- TLC
-
- thin-layer chromatography
1 INTRODUCTION
Clinical bacterial strain's resistance to different antimicrobial drugs is a severe and prevalent threat to human health globally, and increasing exposure to antibiotics may also lead to death [1]. Staphylococcus aureus is the primary cause of wound infection in hospitals; it is the primary causative pathogen of pharyngitis, meningitis, and pneumonia [2]. Pseudomonas aeruginosa is a Gram-negative bacteria that cause wound infections, ulcers, and diabetic foot in diabetic patients; Klebsiella spp. is also related to burn and wound infections [3]. The development of antibacterial drug resistance led up to the appearance of a postantibiotic period looking for efficient natural antimicrobial compounds free of environmental hazards and toxicity [4].
Some medicinal plants have been known as good sources of natural microbial inhibitory compounds; these compounds can be used as an effective medicine to treat multidrug-resistant (MDR) bacteria. The antimicrobial properties of medicinal plant extracts are due to their pharmacological activities, low toxicity, and economic viability. These plants have diverse bioactive compounds like terpenoids, flavonoids, saponins, and phenolic compounds that can exhibit a specific physiological effect on humans [5].
Clove (Syzygium aromaticum) is a flower bud belonging to the Myrtaceae family. It is characterized by the existence of eugenols and is also rich in nonvolatile oils and antioxidants. Clove has antibacterial, antifungal, and antiviral effects; it has cytotoxic and anti-inflammatory effects; this plant has particular promising results for forming AgNPs due to the presence of natural phytochemical compounds [6].
Metallic nanoparticles are considered the main unit of structures utilized in nanotechnology; these particles have arisen as prominent products due to their useful features such as catalytic properties, good conductivity, electrical and magnetic effect, and chemical stability [7]. AgNPs have proved highly multilateral for various purposes; their activity as antimicrobial means is one of the most critical applications. AgNPs' shape, size, and total surface area play a crucial role in their applications in different fields [8]. Some plants such as A. sativum and Ficus panda can be used for the biosynthesis of AgNPs [9]. Several metallic NPs are accepted as having antimicrobial properties; they can be used in medical devices to prevent contamination of equipment and stop the spread of infectious disease. Metal nanoparticles can be combined with antibiotics to assist in overcoming antibiotic resistance of bacteria and increase antibiotic effects [10]. NPs utilize different mechanisms to inhibit bacterial growth. NPs have been demonstrated to lyse the cell wall of the microorganism and make pores on the membrane surface, which can damage the cell membrane; NPs ions can interfere with enzyme production [8]. The present study aims to (i) estimate the in vitro inhibitory effect of crude ethanolic extract of clove and (ii) stratify the green method for the biosynthesis of AgNPs using clove alcoholic extract (CAE) to evaluate the synergistic effect of silver and clove. Hence, they can be used as alternative medications, safe and inexpensive, to treat diabetic patients infected with MDR bacteria.
2 MATERIALS AND METHODS
Specimens of isolated bacteria were collected from patients who suffered from diabetes mellitus from Zagazig University Hospitals (vascular surgery department).
2.1 Isolation and purification of bacterial isolates
The swabs obtained from diabetic patients were suspended into 100 ml of nutrient broth media and incubated at 37°C for 48 h. This culture was serially diluted and spread on the nutrient agar, blood agar, and MacConkey agar plates (Hi-Media) and incubated at 37°C for 24 h [11].
2.2 Antibiotic susceptibility test
The susceptibility of 40 bacterial isolates to seven antibiotic groups was examined by the standard disc-diffusion method according to Clinical and Laboratory Standards Institute [12]. The following antibiotic discs (Oxoid Ltd.) were used: imipenem (10 µg), nitrofurantoin (300 µg), cefepime (30 µg), cefoxitin (30 µg), norfloxacin (10 µg), amoxicillin/clavulanic acid (30 µg), and neomycin (30 µg). Inhibition zone diameters (IZDs) were assessed by millimeters [13]. MDR occurred when a microorganism was resistant to more than one antimicrobial medication in three or more antimicrobial classes [14].
2.3 Identification of bacterial isolates
The identification of selected clinical MDR bacteria was carried out by using morphological, cultural, and biochemical tests according to Bergey's Manual of Systematics of Bacteria [15] and confirmed by VITEK® 2 system (bioMérieux). Morphological characterizations of the isolates were examined. The ribotyping of most resistant P. aeruginosa was performed on the basis of 16S ribosomal RNA (rRNA) gene sequencing using universal forward primer 27 F (5ʹ-AGAGTTTGATCMTGGCTCAG-3ʹ) and reverse primer 1492 R (5ʹ-TACGGYTACCTTGTTACGACTT-3ʹ). DNA extraction was done using the protocol of GeneJet Genomic DNA Purification Kit (K0721; Thermo Fisher Scientific). The polymerase chain reaction (PCR) product was cleaned up using GeneJet PCR Purification Kit (K0721; Thermo Fisher Scientific). The PCR product was electrophoresed using agarose gel (1%) (Gomhuria). For the purification of the selected DNA bands from the gel, a 0.5 ml punctured Eppendorf tube with a small cotton piece was used. The cotton was embedded with 0.5 × TAE buffer (tris-acetate-EDTA). The piece of DNA-containing gel was placed on the cotton filter and then centrifuged at 5200g for 5 min. The nucleotide sequence of 16S rRNA gene of P. aeruginosa was sequenced by direct PCR sequencing technique using ABI (Applied Biosystems) 3730 X DNA sequencer (Hitachi). The cycling conditions were as follows: 94°C for 10 min and 35 cycles of denaturation at 95°C for 30 s, annealing–extension at 56°C for 1 min, 72°C for 1 min, and an extension at 72°C for 10 min. The 16S rRNA gene sequence was carried out by BLAST analysis with the NCBI GenBank database (http://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastn%26PAGE_TYPE=BlastSearch%26LINK_LOC=blasthome), and a phylogenic tree and cluster analysis was carried out by ClustalX program [15].
2.4 Preparation of plant extracts against bacterial isolates
The plants we used were botanically classified by Dr. Samer Teleb, lecturer of Taxonomy and Flora, Botany and Microbiology Department, Faculty of Science, Zagazig University, Egypt. The plants used in this study were obtained from the local market in Egypt. All chemicals used in this experiment were supplied from Al-Gomhorya Company for chemicals and public procurement Zagazig city, Egypt.
Clove (S. aromaticum), garlic (A. sativum), cinnamon (Cinnamomum zylanicum), ginger (Zingiber officinale), Aloe (Aloe vera) moringa seeds, moringa green leaves, and dry moringa leaves (Moringa oleifera) extracts were studied for their inhibitory activity against MDR bacteria.
The leaves of the collected plants were cleaned, dried in the oven at 45°C, and then pulverized using a sterile mortar (Moulinex). The powders were kept in an airtight plastic container, and 25-g samples of each plant material were extracted with 100 ml of 70% alcohol. The extract obtained was pre-filtered with Whatman No. 4 filter (Grade 4V; Sigma-Aldrich) [16]. Then the filtrate was stored in Eppendorf tubes (Gomhuria Co.) at 4°C until the assessment of its inhibitory effect. The antibacterial activity of plant extracts was evaluated against the selected MDR bacteria by agar well diffusion assay [12].
2.5 Isolation, purification, and identification of the active component of clove
Isolation and purification of the active components of clove were carried out by thin-layer chromatography (TLC) bioautography technique (contact method), whereby TLC was placed in contact with the bacterial culture. For separation of clove constituents, leaving a 1-cm border on the sides of the TLC plate, the origin of TLC (Whatman cellulose chromatography paper) was marked with a pencil and the prepared sample of clove extract was spotted on the marked line. Then, 5 µl of the extract was filled with a band length of 10 mm. The developing running solvent system was ethyl acetate, methanol, and formic acid in a ratio (2:1:0.5 v/v). Paper was placed in an airtight chromatographic chamber saturated with the solvent mixture. Spots were separated by running on the TLC plate in the selected solvent system. The plate was removed before the solvent reached the maximum. The amount that each component of a mixture travels can be quantified using retention factors (Rfs). Rf value is defined as the ratio of the distance the spot shifted above the basic line to the distance the solvent front moved above the origin [17]. After complete drying of the plate, bioautography of different Rf values against pathogenic bacteria was performed by agar well diffusion method [12].
2.5.1 Gas chromatography–mass spectrometry (GC–MS) analysis
The extracted bioactive compounds of purified CAE at 0.9 Rf detected by TLC were analyzed and identified. The GC–MS analysis was performed by using gas chromatography–mass spectrometry instrument: TRACE GC Ultra Gas Chromatographs (Thermo Fisher Scientific Corp.), coupled with a thermo mass spectrometer detector (ISQ Single Quadrupole Mass Spectrometer). The column used was HP-5MS UI (cross-linked 5% methyl phenyl Silox) capillary column [18].
2.6 Biosynthesis and antibacterial activity of silver nanoparticles from clove
The biosynthesis of AgNPs using clove extract was evaluated, different concentrations of CAE (2%, 4%, 8%, 16%) were added to 50 ml of 0.01 M AgNO3 solution (aqueous), and 50 ml of 0.01 M AgNO3 was prepared as a negative control [19]. The reaction mixtures were exposed directly to light and dark conditions for 2, 4, and 6 days for nanoparticle formation. Changes in the color of the reaction mixtures were observed to determine nanoparticles formation, which is characterized by a dark brown color. Further confirmation of formed AgNPs was provided by UV–Vis spectral analysis. The control and nanoparticle were altogether scanned from 200 to 800 nm using a UV–Vis spectrometer, the purification and concentration of AgNPs from the final reaction mixture were assessed, and the size of the nanoparticles was determined using Scherer's equation (D = 0.9λ/β cosθ), where D is the nano-size, λ is the wavelength of X-ray, θ is diffraction angel, and β is full width at half maximum of the peak [20]. AgNPs were tested against selected pathogenic bacteria according to the Kirby–Bauer disc-diffusion method, compared with AgNO3 [12].
2.6.1 Transmission electron microscopy (TEM) for nanoparticles
The sample was examined using a TEM (JEOL JEM-2100) at the National Research Center, Egypt, to evaluate the biosynthesis of AgNPs from CAE.
3 RESULTS
3.1 Antibiotic susceptibility patterns
About 40 clinical sample swabs of the diabetic foot (n = 28), bedsores (n = 7), and wounds (n = 5) were collected from diabetic patients from 2018 to 2020. The antibiotic susceptibility of the bacterial isolates was studied. The selected antibiotics are arranged in the descending pattern according to their inhibitory effect against the pathogenic bacteria: most of the bacterial isolates were susceptible (80%) to imipenem. Thus, it represents the strongest antibiotic. This was followed by norfloxacin (40%), nitrofurantoin (32.5%), amoxicillin–clavulanic acid (27.5%), neomycin, and cefoxitin (12.5%). In contrast, all bacterial isolates (100%) were resistant to cefepime. Out of 40 bacterial isolates, only four were observed to be resistant to all groups of antibiotics (100%), as shown in Table 1. This showed that those isolates were preliminarily approved to be MDR bacteria. So, they were chosen for further studies. The selected MDR bacteria with isolate numbers 30 and 31 were isolated from the diabetic foot, whereas isolate numbers 35 and 45 were isolated from wound and bedsores, respectively. The morphological, physiological, and biochemical identification of the MDR bacteria were according to identification protocols. MDR bacterial strains were identified as P. aeruginosa D31, S. aureus W35, Klebsiella pneumoniae DF30, and K. pneumoniae B40.
| Antibiotic | Conc. (µg/disc) | Resistant bacterial isolate number | Sensitive | Intermediate | Resistant | |||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |||
| Imipenem | 10 | 17, 19, 30, 31, 35, 34, 40 | 32 | 80 | 1 | 2.5 | 7 | 17.5 |
| Norfloxacin | 10 | 2, 5, 8, (13–15), 18, (20–22), 24, 27, 28, 30, 31, 32, 35, 37, 40 | 16 | 40 | 5 | 12.5 | 19 | 47.5 |
| Nitrofurantoin | 300 | (3–7), 9, 12, 16, (19–22), 24, 25, 27, 29, 30, 31, (32–34), 35, (36-38), 40 | 13 | 32.5 | 0 | 0 | 26 | 65 |
| Amoxicillin–clavulanic acid | 30 | (3–7), 9, 11, (13–17), (19–22), (24–27), 29, 30, 31, 32, 33, 35, 36, 37, 39, 40 | 11 | 27.5 | 0 | 0 | 29 | 72.5 |
| Neomycin | 30 | (2–8), 11, 13, (16–24), 28, 29, 30, 31, 32, 34, 35, (37–39), 40 | 5 | 12.5 | 6 | 15 | 29 | 72.5 |
| Cefoxitin | 30 | (1–11), (13–22), 24, 25, (27–29), 30, 31, (32–34), 35, 37, 38, 40 | 5 | 12.5 | 0 | 0 | 35 | 87.5 |
| Cefepime | 30 | 1–40 | 0 | 0 | 0 | 0 | 40 | 100 |
- Note: 30, 31, 35, 40 = isolate numbers of selected MDR bacteria.
- Abbreviation: MDR, multidrug-resistant.
3.2 Antibacterial activity of plant extracts
The inhibitory effect of plant extracts against MDR bacteria is given in Table 2 and Figure 1. CAE demonstrated the highest inhibitory activity against all selected bacteria as IZDs reached 13–17 mm, followed by garlic (IZDs, 10–16 mm), whereas cinnamon and moringa seeds had low antibacterial activity. In contrast, ginger, A. vera, moringa dry leaves, and moringa green leaves extracts did not affect MDR bacteria. As CAE showed the most inhibitory activity against all tested bacteria, it was selected for purification by TLC and then instrumental analysis by GC–MS.
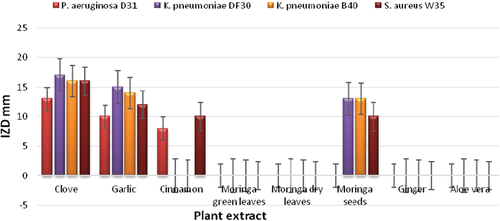
| Plant extracts | IZD (mm) of bacterial isolates | |||
|---|---|---|---|---|
| Pseudomonas aeruginosa D31 | Klebsiella pneumoniae DF30 | K. pneumoniae B40 | Staphylococcus aureus W35 | |
| Clove | 13 ± 0.79 | 17 ± 0.61 | 16 ± 0.24 | 16 ± 0.63 |
| Garlic | 10 ± 0.25 | 15 ± 0.36 | 14 ± 0.72 | 12 ± 0.27 |
| Cinnamon | 8 ± 0.31 | 0 | 0 | 10 ± 0.29 |
| Moringa green leaves | 0 | 0 | 0 | 0 |
| Moringa dry leaves | 0 | 0 | 0 | 0 |
| Moringa seeds | 0 | 13 ± 0.67 | 13 ± 0.35 | 10 ± 0.46 |
| Ginger | 0 | 0 | 0 | 0 |
| Aloe vera | 0 | 0 | 0 | 0 |
- Note: Initial disc = 0.6 mm; resistant (R): no inhibition zone; intermediate (I): 10–15; sensitive (s): >16, according to Kawsar et al. [21]. DF, B, W indicate the source of isolation. 30, 31, 35, 40 are bacterial isolate numbers.
- Abbreviations: B, bedsores; DF, diabetic foot; IZD, inhibition zone diameter; W, wounds.
3.3 Instrumental analysis by GC–MS
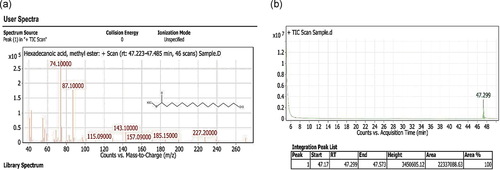
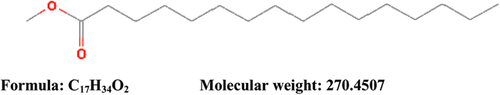
Partially purified CEA at Rf (0.9) detected by TLC showed antibacterial activity against tested bacterial strains (Table 3). Hence, the present work was undertaken to determine the bioactive compound found in 0.9 Rf value by using GC–MS. The active principle with its retention time (RT), molecular formula, and concentration (peak area %) are presented in Figure 2a,b, which shows the existence of only one bioactive compound, hexadecanoic acid methyl ester with chemical formula C17H34O2 and molecular weight 270.4507 (Figure 3). The mass spectra of the extracted compound from the clove are shown in Figure 2a.
| Pathogenic bacteria | Solvent v/v | Rf | IZD (mm) |
|---|---|---|---|
| Pseudomonas aeruginosa D31 | Ethyl acetate:methanol:formic acid (2:1:0.5) | 0.1 | 8 ± 1.5 |
| 0.2 | 10 ± 1.7 | ||
| 0.9 | 13 ± 1.1 | ||
| Klebsiella pneumoniae DF30 | 0.7 | 10 ± 0.57 | |
| 0.8 | 11 ± 1.5 | ||
| 0.9 | 15 ± 0.78 | ||
| K. pneumoniae B40 | 0.9 | 17 ± 1.3 | |
| Staphylococcus aureus W35 | 0.9 | 14 ± 1.7 |
- Abbreviations: IZD, inhibition zone diameter; Rf, retention factor.
3.4 Green biosynthesis of AgNPs from CAE
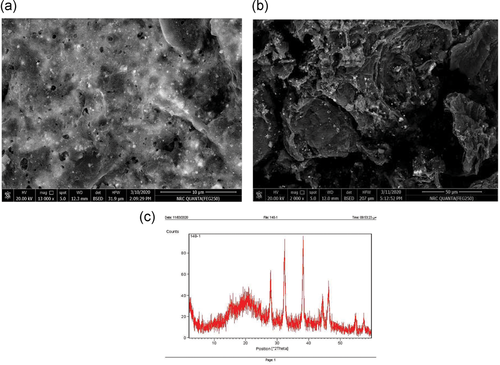
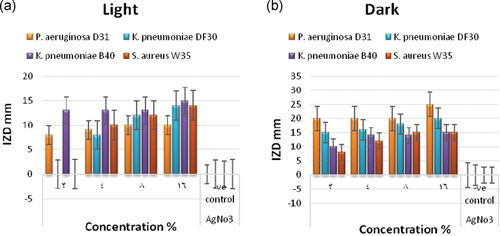
In this study, the color of the prepared mixtures converted from yellow to dark brownish color, indicating the reduction characters of the CEA to the biosynthesis of AgNPs shown in Figure 4a,b. The formation of the prepared nanoparticles was confirmed by X-ray diffraction and examined by TEM (HV = 20 kV; magnification ×13,000, ×2000). The results demonstrated in Table 4 and Figure 5a,b revealed the formation of small particles of AgNPs ranged from 20.17 to 32.13 nm. Figure 5c shows the presence of the X-ray diffraction pattern of the AgNPs. These AgNPs yielded seven dominant diffraction peaks, positioned at 2θ = 27.9°, 32.4°, 38.3°, 44.4°, 46.4°, 54.9°, and 57.5°, which were indexed, respectively, with relative diffraction intensities as follows: 45.93%, 88.94%, 100.00%, 26.68%, 39.32%, 13.54%, 12.30%, respectively. Figure 6 shows the photoluminescence spectrum of the AgNPs. This spectrum showed a peak at a wavelength of 379 nm. The antibacterial effect of green-biosynthesized AgNPs was assessed by disc-diffusion method in the dark and light conditions after 2, 4, and 6 days; the antibacterial effect of the AgNPs was appreciated by measuring IZDs around the discs (Table 5). The results revealed that AgNPs exhibited the highest antimicrobial activity in dark condition after 2 days against P. aeruginosa, with IZDs reaching 20–25 mm (Figure 4c), followed by K. pneumoniae DF (15–20 mm IZDs), K. pneumoniae BS (10–15 mm IZDs), and S. aureus (8–15 mm IZDs) (Figure 7). The obtained results in Table 6 show that there is a highly significant difference (p < 0.009) regarding the antibacterial effect between P. aeruginosa D31 and K. pneumoniae DF30, there is a highly significant difference (p < 0.000) regarding the antibacterial effect between P. aeruginosa D31 and K. pneumoniae B40, there is a highly significant difference (p < 0.000) regarding the antibacterial effect between P. aeruginosa D31 and S. aureus, there is a highly significant difference (p < 0.009) regarding the antibacterial effect between K. pneumoniae DF30 and K. pneumoniae B40, and there is a highly significant difference (p < 0.009) regarding the antibacterial effect between K. pneumoniae DF30 and S. aureus W35 (at concentration 16% after 2 days in dark conditions).


| Peak number | Pos. (2θ) | D-spacing | Height (cm) | FWHM left (2θ) | Rel. int. (%) | β (rad) | Size (nm) |
|---|---|---|---|---|---|---|---|
| 1 | 27.9124 | 3.19652 | 36.02 | 0.3936 | 45.93 | 0.006869 | 22.84 |
| 2 | 32.3604 | 2.76660 | 69.75 | 0.3936 | 88.94 | 0.006869 | 23.89 |
| 3 | 38.2629 | 2.35231 | 78.42 | 0.3149 | 100.00 | 0.005496 | 32.13 |
| 4 | 44.3625 | 2.04201 | 20.92 | 0.4723 | 26.68 | 0.008243 | 23.53 |
| 5 | 46.4051 | 1.95678 | 30.84 | 0.5510 | 39.32 | 0.009616 | 20.17 |
| 6 | 54.8756 | 1.67309 | 10.62 | 0.4723 | 13.54 | 0.008243 | 29.24 |
| 7 | 57.5270 | 1.60213 | 9.65 | 0.4723 | 12.30 | 0.008243 | 31.33 |
- Abbreviation: FWHM, full width at half maximum.
| Bacterial isolates | Condition | Effect of different concentrations % of CAE per 50 ml AgNO3 on bacterial growth IZD (mm) | ||||
|---|---|---|---|---|---|---|
| 2 | 4 | 8 | 16 | AgNO3 −ve control | ||
| Pseudomonas aeruginosa D31 | In light after 2 days | 8 ± 1.5 | 9 ± 1.0 | 10 ± 1.0 | 10 ± 1.0 | 0 |
| Klebsiella pneumoniae DF30 | 0 | 8 ± 1.0 | 12 ± 1.0 | 14 ± 1.0 | 0 | |
| K. pneumoniae B40 | 13 ± 1.0 | 13 ± 1.0 | 13 ± 1.0 | 15 ± 1.0 | 0 | |
| Staphylococcus aureus W35 | 0 | 10 ± 1.8 | 12 ± 0.84 | 14 ± 1.5 | 0 | |
| P. aeruginosa D31 | In dark after 2 days | 20 ± 1.0 | 20 ± 1.0 | 20 ± 1.0 | 25 ± 1.0 | 0 |
| K. pneumoniae DF30 | 15 ± 1.0 | 16 ± 1.0 | 18 ± 1.4 | 20 ± 1.0 | 0 | |
| K. pneumoniae B40 | 10 ± 1.0 | 14 ± 1.0 | 14 ± 1.0 | 15 ± 1.0 | 0 | |
| S. aureus W35 | 8 ± 1.8 | 12 ± 1.5 | 15 ± 0.84 | 15 ± 0.84 | 0 | |
| P. aeruginosa D31 | In light after 4 days | 5 ± 0.87 | 5 ± 1.0 | 8 ± 1.5 | 7 ± 1.0 | 0 |
| K. pneumoniae DF30 | 0 | 7 ± 1.0 | 7 ± 1.0 | 11 ± 1.0 | 0 | |
| K. pneumoniae B40 | 7 ± 1.0 | 5 ± 0.78 | 10 ± 1.0 | 13 ± 1.0 | 0 | |
| S. aureus W35 | 0 | 7 ± 1.3 | 9 ± 0.64 | 11 ± 1.2 | 0 | |
| P. aeruginosa D31 | In dark after 4 days | 18 ± 1.0 | 18 ± 1.0 | 20 ± 1.1 | 22 ± 1.0 | 0 |
| K. pneumoniae DF30 | 13 ± 1.0 | 14 ± 1.0 | 15 ± 1.0 | 18 ± 1.0 | 0 | |
| K. pneumoniae B40 | 7 ± 1.0 | 12 ± 1.0 | 11 ± 1.0 | 13 ± 1.0 | 0 | |
| S. aureus W35 | 6 ± 1.5 | 11 ± 1.0 | 13 ± 0.74 | 11 ± 0.64 | 0 | |
| P. aeruginosa D31 | In light after 6 days | 0 | 0 | 5 ± 1.0 | 5 ± 1.0 | 0 |
| K. pneumoniae DF30 | 0 | 5 ± 0.67 | 5 ± 0.85 | 9 ± 1.0 | 0 | |
| K. pneumonia B40 | 0 | 0 | 8 ± 1.2 | 10 ± 1.0 | 0 | |
| S. aureus W35 | 0 | 5 ± 1.0 | 5 ± 1.0 | 9 ± 1.5 | 0 | |
| P. aeruginosa D31 | In dark after 6 days | 15 ± 1.0 | 15 ± 1.0 | 15 ± 1.0 | 15 ± 1.0 | 0 |
| K. pneumoniae DF30 | 10 ± 1.0 | 10 ± 0.86 | 12 ± 1.0 | 15 ± 1.0 | 0 | |
| K. pneumoniae B40 | 5 ± 1.0 | 10 ± 0.75 | 10 ± 1.0 | 12 ± 1.0 | 0 | |
| S. aureus W35 | 5 ± 1.0 | 10 ± 1.0 | 10 ± 1.0 | 10 ± 1.0 | 0 | |
- Abbreviations: AgNP, silver nanoparticle; CAE, clove alcoholic extract; IZD, inhibition zone diameter.
| 95% Confidence interval | |||||||
|---|---|---|---|---|---|---|---|
| Concentration | (I) strains | (J) strains | Mean difference (I − J) | SE | Sig. | Lower bound | Upper bound |
| 16% in dark condition after 2 days | Pseudomonas aeruginosa D31 | Klebsiella pneumoniae DF30 | 5.00000* | 1.47196 | 0.009 | 1.6057 | 8.3943 |
| K. pneumoniae B40 | 10.00000* | 1.47196 | 0.000 | 6.6057 | 13.3943 | ||
| Staphylococcus aureus W35 | 10.00000* | 1.47196 | 0.000 | 6.6057 | 13.3943 | ||
| K. pneumoniae DF30 | P. aeruginosa D31 | −5.00000* | 1.47196 | 0.009 | −8.3943 | −1.6057 | |
| K. pneumoniae B40 | 5.00000* | 1.47196 | 0.009 | 1.6057 | 8.3943 | ||
| S. aureus W35 | 5.00000* | 1.47196 | 0.009 | 1.6057 | 8.3943 | ||
| K. pneumoniae B40 | P. aeruginosa D31 | −10.00000* | 1.47196 | 0.000 | −13.3943 | −6.6057 | |
| K. pneumoniae DF30 | −5.00000* | 1.47196 | 0.009 | −8.3943 | −1.6057 | ||
| S. aureus W35 | 0.00000 | 1.47196 | 1.000 | −3.3943 | 3.3943 | ||
| S. aureus W35 | P. aeruginosa D31 | −10.00000* | 1.47196 | 0.000 | −13.3943 | −6.6057 | |
| K. pneumoniae DF30 | −5.00000* | 1.47196 | 0.009 | −8.3943 | −1.6057 | ||
| K. pneumoniae B40 | 0.00000 | 1.47196 | 1.000 | −3.3943 | 3.3943 | ||
- * p ≤ 0.05 is statically significant, whereas p ≤ 0.01 is highly statistically significant.
3.5 Confirmation of identification of P. aeruginosa by using 16S rRNA
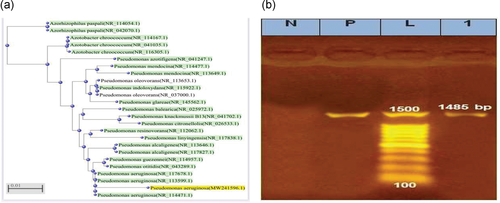
The identification of the most resistant P. aeruginosa was confirmed using 16S rRNA gene sequencing. Sequences of P. aeruginosa strain D-3116S (98%) were submitted to GenBank at the NCBI website as accession number (MW241596). The BLAST program and phylogenetic analysis using the ClustalW muscle algorithm program were used to assess the DNA similarities of the obtained 16S rRNA gene sequence, as shown in Figure 8a,b.
4 DISCUSSION
The increasing prevalence of MDR bacteria, such as methicillin-resistant S. aureus (MRSA) or MDR Gram-negative bacilli, represents global health challenges [22].
There are limited data regarding the distribution of different MDR bacteria, especially Gram-negative bacteria in some developing countries like Egypt [23]. Thus, MDR bacteria must be selected and identified to recognize their types and their antibiotic resistance and search for effective natural antimicrobial compounds as alternative medication that is safe and nontoxic [4]. In this study from bacterial isolates derived from diabetic patients, many microbiological studies were done. Here, isolated antibiotic resistance patterns were assessed and the antimicrobial susceptibility test revealed that the most effective antibiotic was imipenem, followed by norfloxacin and nitrofurantoin, respectively. Imipenem was previously reported to be the most effective and similar effects of these antibiotic categories were also reported [24]. In this study, MDR bacterial strains were P. aeruginosa D31, S. aureus W35, K. pneumoniae DF30, and K. pneumoniae B40 [25].
Some plants like herbs have traditionally been used in medicine. Thus, the essential oils of medicinal plant extracts are used for multiple medical purposes, for example, erosive gastritis and neurological disorders. Kuok et al. [26] showed that the antibacterial activity and clinical safety of bioactive components of some medicinal plants are higher than that of some antibiotics. In our study, eight plant extracts were tested against MDR bacterial isolates. These results showed that the clove and garlic alcoholic extracts had strong efficacy against the resistant bacterial isolates, and the CAE had the highest antibacterial activity against MDR bacteria. Its antimicrobial activity is due to the presence of a phenyl group that is capable of damaging proteins and interacting with the lipids in the cell membrane of the bacteria, leading to bacterial cell death by altering the permeability of the cell membrane [27]. Our results agree with Ismail et al. [28], whose result showed that out of 17 types of plant extracts that were tested for their inhibitory effect against seven microbial strains, only three extracts (clove, thyme, and cinnamon), alcoholic extracts, showed a strong antimicrobial effect against tested bacteria.
GC–MS analysis of purified CAE at 0.9 Rf by running solvent ethyl acetate, methanol, and formic acid in a ratio (2:1:0.5) revealed that hexadecanoic acid methyl ester C17H34O2 was the main active compound [29]. Hexadecanoic acid methyl ester exhibited antibacterial potency against S. aureus W35, P. aeruginosa D31, K. pneumoniae DF30, and K. pneumoniae B45. Our results approximated the results of Lalthanpuii and Lalchhandama [30], who revealed the existence of volatile compounds in Imperata cylindrica; these compounds were analyzed by GC–MS analysis. It was found that some of the analyzed compounds such as hexadecanoic acid methyl ester (35.8%) already have antibacterial effects against tested bacteria, like P. aeruginosa, B. subtilis, and K. pneumoniae. Furthermore, Davoodbasha et al. [31] revealed the presence of hexadecanoic acid methyl ester (23.08%) in Scenedesmus intermedius that had a good inhibitory effect against Gram-positive and Gram-negative bacteria. Hexadecanoic acid methyl ester is a type of fatty acid ester [32]. It can inhibit the growth of pathogenic bacteria. The antimicrobial effect of fatty acids (e.g., hexadecanoic acid methyl ester) is influenced by their structures, shapes, functions of the length of the carbon chains, and the presence, numbers, positions, and orientation of double bonds. Many organisms use the antimicrobial properties of fatty acid methyl ester as a defense means against bacteria. The cell membrane of bacteria is the main target of its action. Also, it interferes with cellular energy production, inhibition of enzyme activity, and finally, direct lysis of bacterial cells. The safety and activity of the fatty acid methyl ester make it a promising antibacterial medication [33].
Eugenol is the main component of the clove responsible for bioreduction in the biosynthesis of (AgNPs) process. In this process, electron pull-out methoxy and allyl groups are present, and the protons from –OH groups are released with the assistance of inductive effect, rendering a negative charge on eugenols. Then, two electrons are released from the resonating structures of the anionic form of eugenols, and this is responsible for the reduction of 2Ag+ ions to 2Ag0. The formation of brownish color after addendum of plant extracts to AgNO3 solution indicates the formation of AgNPs [34]. This mechanism agrees with many researchers, who have synthesized AgNPs from Ocimum and Cinnamomum camphora leaf extract [35].
In our study, AgNPs showed the highest antimicrobial activity against P. aeruginosa D31, followed by K. pneumoniae DF30, K. pneumoniae B45, and S. aureus W35. Our results agree with Ajitha et al. [36]. They showed that biosynthesized AgNPs using clove extract had a higher inhibitory effect against Pseudomonas spp. than that against the other tested microbes [37]. Due to the nano-size and high surface area of nanoparticles, they have high antimicrobial activities. Many mechanisms have been suggested for the inhibitory effect of AgNPs. It was supposed that Ag+ ions from NPs attach to negatively charged bacterial cell wall and rupture it, which causes denaturation of proteins and finally cell death. The accumulation of envelope proteins makes dispersal of proton motive force. AgNPs also displayed destabilization of the outer membrane and rupturing of the plasma membrane [8]. Under the acquired results, it can be concluded that hexadecanoic acid methyl ester from clove and AgNPs synthesized by CAE could be applied as an antibacterial means. They can be used as safe and cheap natural products.
ACKNOWLEDGMENTS
The authors are indebted to Menoufia University, Animal Health Research Institute, and National Research Center, Egypt, for support and facilities for carrying out this study. The authors are indebted to Samer Teleb, Mohsen Askar, and Ahmed El-Azzazy for their help with plant identification, instrumental analysis, and statistical analysis, respectively.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.