Sterigmatocystin production is restricted to hyphae located in the proximity of hülle cells
Abstract
Aspergillus nidulans produces sterigmatocystin, a secondary metabolite mycotoxin, for the protection of its reproductive structures. Previous studies on grazing behavior of fungivore arthropods, regulation of sexual development, and secondary metabolite biosynthesis have revealed the association of sterigmatocystin biosynthesis with sexual reproduction, but the spatial distribution of sterigmatocystin producing hyphae within the colony has never been investigated. In this work, we aimed to locate the site of sterigmatocystin production within the colony by employing a yCFP reporter system. We demonstrated that the stcO promoter is active only in vegetative hyphae that surround groups of hülle cells and the activity decreases and eventually ceases as the distance between the hypha and the hülle cells increases. This phenomenon indicates that the vegetative mycelium might consist of morphologically uniform, but functionally different hyphae.
1 INTRODUCTION
Secondary metabolites of filamentous fungi are widely studied due to either their beneficial (e.g., the β-lactam antibiotic penicillin) or harmful biological effects (such as the carcinogenic mycotoxins aflatoxin and sterigmatocystin). Sterigmatocystin (STC) is a precursor of aflatoxin, however, in Aspergillus nidulans it is the biosynthetic end product as the last two coding genes of the aflatoxin biosynthetic pathway are absent 1, 2. Previous studies revealed that STC production coincides in time with the sexual development due to shared master regulators (reviewed in 3-5). The sexual development of A. nidulans starts with the production of thick-walled hülle cells, which surround, protect and nurse a nest of hyphae that subsequently develops to primordium and cleistothecium. Hülle cell formation requires the expression of the transcription factor NosA 6, which is regulated by several master regulators (such as LaeA 6, 7 and VeA 8) involved in both sexual development and STC biosynthesis. Interconnection of these two biological processes is demonstrated by the lack of STC production in loss-of-function mutants of sexual development regulators VeA, ImeB, RcoA, MpkB, or FlbA 9-13. However, the interconnection of the sexual development and STC production shows environment-specificity, as certain environmental conditions can uncouple these processes. The production of sexual structures in liquid culture does not take place while the STC production is still active. Certain environmental factors, such as light, amino acid availability, carbon- and nitrogen-source availability, salt-content, pH, oxygen-level, and iron-supply could repress or activate sexual- or asexual-development and/or secondary metabolism. According to previous studies, the gene products of rosA, nosA, imeB, cryA, fhbA, fhbB, lsdA, cpcA, cpcB, laeA, fphA, silA, silG, csnD, csnE, indB, and indD repress sexual development (reviewed in 3) under unfavorable environmental conditions. STC production in the deletion mutants of these genes has been reported only in few cases, such as in a strain deleted in imeB encoding a serine threonine kinase involved in the light dependent repression of cleistothecia formation and activation of secondary metabolism 10. In imeBΔ the blockade of sexual development is released under unfavorable conditions. This mutant strain displays artificial hülle cell production even in liquid culture and forms cleistothecia even in light without producing STC. Even though having shared master regulators, sexual development and secondary metabolite production could manifest separately. However, coupling of these two processes might provide an evolutionary advantage. It is conceivable that the STC production during sexual development allows the producer organism to defend its sexual structures from fungivores or to successfully compete against other organisms and thereby protect its environmental niche. According to studies on fungivore insects 14, 15, STC plays a role in the chemical protection of the sexual reproductive structures. Using wild type and STC-deficient strains of A. nidulans, Staaden et al. 14 revealed that the mycetophagous insects Heteromurus nitidus and Supraphorura furcifera avoid the consumption of wild type mycelia. However, these insects sense and feed on the STC deficient mutant fungi, with higher preference to those mutants, in which the STC production is interrupted in the earliest step of biosynthesis. Furthermore, Doll et al. 15 showed that Folsomia candida exclusively grazes on vegetative hyphae and conidia but leaves the fruit bodies untouched. The remarkable avoidance of cleistothecia consumption might be indicative of a heterogeneous, tissue-specific spatial distribution of anti-fungivore compounds within colonies 15.
The genes of STC biosynthesis (stcA-W) are clustered and co-regulated by the cluster-specific transcription factor AflR 16, 17. The AflR-dependent co-regulation was demonstrated for many of the cluster genes (shown for stcA, stcE, stcD, stcU, stcB, stcF, stcJ, stcK, stcL, stcS, stcV, stcW, stcT, stcP, stcN, aflR, and stcO), although experimental evidences of their role in STC biosynthesis (including stcO) were not studied in each case 18-23.
The Aspergillus parasiticus avfA mutant fails to produce aflatoxin and accumulates averufin. This phenotype can be successfully complemented by the expression of its A. flavus homolog avfA (also known as aflI) 24. The counterpart of avfA in A. nidulans is termed stcO, that is considered as the functional equivalent of avfA and thereby might be responsible for the conversion of averufin to versiconal hemiacetal acetate 24, 25. However, the exact role of stcO has not been studied yet. In this recent work we constructed an stcO reporter strain by substituting the stcO gene with a NLS (nuclear localization signal)-tagged cyan fluorescent protein coding gene optimized for yeasts (ycfp). Accordingly, our reporter strain is an stcO gene deletion mutant that is simultaneously suitable to investigate the role of stcO in the STC production of A. nidulans and to monitor the stcO promoter activity by performing microscopic analysis on A. nidulans hyphae. Our results demonstrate that the stcO gene product is essential for STC biosynthesis and the expression of stcO is activated only in hyphae that surround the location of sexual development pinpointed by the presence of hülle cells.
2 MATERIALS AND METHODS
2.1 Strains, media, and growth conditions
The experiments of this study were performed on the following A. nidulans strains: HZS.450, HZS.576 and HZS.606. Strain HZS.450 is a wild type control (riboB2 veA+). HZS.576 (hhoA-mRFP-AfriboB riboB2 pyroA4 pabaA1 veA+ “nkuAΔ::argB+”) is the parental strain of the stcO reporter strain. HZS.576 was obtained by the genetic cross between the strains of LO1516 (hhoA-mRFP-AfriboB riboB2 pyroA4 pyrG89 nkuAΔ::argB+) 26 and HZS.1 (riboB2 pabaA1 veA+). HZS.606 (hhoA-mRFP-AfriboB stcO::NLS-yCFP-AfpyroA+ riboB2 pyroA4 pabaA1 veA+ “nkuAΔ::argB+”) was obtained by the transformation of HZS.576 with a five-partite gene substitution cassette (see below). Loci between double quotes were not checked. Standard genetic markers are described at the following URL: http://www.fgsc.net/Aspergillus/gene_list/. The hhoA-mRFP-AfriboB is a H1 histone-mRFP gene-fusion coupled with the wild type riboB gene of A. flavus. nkuAΔ is the deletion of nkuA that is essential for non-homologous end joining of DNA in double-strand break repair 27. The stcO::NLS-yCFP-AfpyroA+ construct is described below. Minimal Medium with 1% glucose as sole carbon source and 10 mM nicotinic acid (1:100 dilution from 1 M nicotinic acid dissolved in 1 M sodium hydroxide) as sole nitrogen source (nicotinic acid-glucose medium) was used for growth tests and microscopy and complete medium (CM) was used for maintaining the strains 28, 29. Medium with 1.5% lactose as sole carbon-source and 5 mM sodium nitrate as sole nitrogen-source (nitrate-lactose medium) were used both for the extraction of secondary metabolites and growth tests 30. Media were supplemented with vitamins (www.fgsc.net) according to the requirements of each auxotrophic strain. Agar for minimal media was obtained from BD/Difco.
2.2 DNA manipulations
Total DNA was prepared from A. nidulans as described by Specht et al. 31. For Southern blot 32 Hybond-N membrane (Amersham/GE Healthcare) was used and hybridization was done by DIG DNA Labeling and Detection Kit (Roche) according to the manufacturer's instructions. Transformation of A. nidulans protoplasts were done as described by Karácsony et al. 33 using a 4% solution of Glucanex (Novozymes, Switzerland) in 0.7 M KCl. DNA amplifications were carried out in T100 Thermal Cycler (BioRad) with Phusion Hot Start II High-Fidelity DNA Polymerase (Thermo Scientific) according to the manufacturer's instructions.
2.3 Construction of stcO reporter gene-substitution cassette and obtaining of stcO promoter-NLS-ycfp expressing strain
The stcO reporter cassette was constructed by the double-joint PCR method 34. The cassette is composed of five components: i) the stcO (AN7811) promoter and its upstream region (component A, nested size 2436 bp) followed by ii) the nuclear localization signal (NLS) of the transcription factor prnA (component B/1, nested size 550 bp) 35 from wild type A. nidulans (HZS.145) 36; iii) the yCFP (Cyan Fluorescent Protein optimized for yeasts) coding gene (component B/2, 944 bp) amplified from plasmid pKT174 37; iv) the pyroA homologue (component B/3, nested size 2414 bp) of Aspergillus fumigatus (Afu5g08090 from A. fumigatus); and v) the downstream flanking sequence of the stcO gene (component C, nested size 2421 bp). The double-joint PCR 34 was performed by using two consecutive assembly steps (assembly of B/1-B/2-B/3 and A-B1/2/3-C). The five components were amplified with primer pairs stcO up frw (5′-ctgtcgtagtagctgaggtcgc-3′)—stcO up NLS chim rev (5′-gttgatggggcctcgtccatcctgatgtaggattaggatagaatgtatagag-3′) (component A, 3004 nt), NLS frw (5′-catctgttcagcctatctacggac-3′)—NLS CFP chim rev (5′-gaataattcttcacctttagacgaaggtcctccatccgagtctgaac-3′) (component B/1, 911 nt), CFP NLS chim frw (5′-gttcagactcggatggaggaccttcgtctaaaggtgaagaattattcactggtg-3′)—CFP AfpyroA chim rev (5′-cacagtaacatcctcttctgagatcgtgcctgttatccctagcggatctg-3′) (component B/2, 944 nt), AfpyroA CFP chim frw (5′-ccgctagggataacaggcacgatctcagaagaggatgttactgtg-3′)—AfpyroA down rev (5′-gaaattctgtctgccgaaggctc-3′) (component B/3, 2628 nt), and stcO down AfpyroA chim frw (5′-caccctgacagcgccataaggtggacaatattactgctagtcgctc-3′)—stcO down rev (5′-gcgacggatttacgatagagtcagc-3′) (component C, 2893 nt). B/1, B/2, and B/3 components were assembled by using NLS stcO updchim frw (5′-ctctatacattctatcctaatcctacatcaggatggacgaggccccatcaac-3′) and AfpyroA stcO down chim rev (5′-gagcgactagcagtaatattgtccaccttatggcgctgtcagggtg-3′) primers (B1/2/3 component, 3908 bp). The A, B1/2/3, and C components were assembled with stcO upst nest frw (5′-ctcacagaaccaaacaatgccttc-3′) and stcO down nest rev (5′-ggtcaggactacttgttgtttcgaag-3′) primers. Components A and C served for targeting the stcO locus and supporting homologous recombination, while component B1/2/3 provided the nuclearly localised yCFP and the selection marker gene. The five-partite assembled PCR product was transformed into HZS.576 and 9 out of hundreds of pyridoxine prototroph transformants were selected for further analysis. A Southern analysis was performed with a 2421 bp downstream flanking region of stcO (component C, amplified by primer pair stcO down AfpyroA chim frw and stcO down nest rev) as gene probe on BamHI digested total DNA of transformants. Based on the Southern analysis, all selected transformants carried a single copy integration of the five-partite cassette in the targeted stcO locus (data not shown). Single copy integration in transformants was further confirmed by checking the copy number of ycfp using qPCR with CFP frw (5′-ctcacaatgtttacatcatggct-3′) and CFP rev (5′-gtcagctaattgaacagaacca-3′) primers. One of the tested transformants (HZS.606) was selected for microscopic analysis.
2.4 Thin layer chromatography
Agar blocks were excised with a 10 mm diameter cork-borer from 4 day old nitrate-lactose agar cultures and subsequently extracted with 10 ml chloroform. The extracts were concentrated into 1 ml final volume at 65 °C. Samples (15 μL) loaded on Kieselgel 60 (Merck) plates and separated in toluol:ethyl-acetate:formic-acid (60:40:10 [vol/vol/vol]). Secondary metabolites were detected and recorded under UV light, after submerging the plate in 10% AlCl3 dissolved in methanol, and subsequently heating it to 100 °C for 10 min. Identification of sterigmatocystin was accomplished by loading a sterigmatocystin standard (Sigma) on the same plate.
2.5 Microscopy
Solid nicotinic acid-glucose media covered with cellophane sheets were spot inoculated and incubated in dark at 37 °C for 48, 72, and 92 h. Longitudinal sections of colonies on the surface of cellophanes were cut and the cellophane sections were mounted to slides using PBS (150 mM sodium phosphate, 150 mM sodium chloride, pH 7.4) and cover slips. Images were taken by using Olympus BX51 fluorescent microscope U-MNU2 Olympus cube (BP 360–370 excitation filter, DM 400 dichromatic mirror, LP 420 emission filter) and U-M41035 RFP Olympus cube (U-HQ546/12, U-Q560LP, U-HQ6058/75m).
3 RESULTS
3.1 Obtaining the stcO reporter strain by the replacement of the stcO gene with an NLS-tagged ycfp
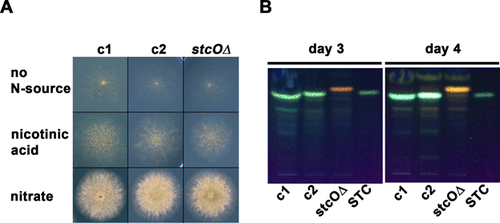
To detect the transcriptional activity of the stc cluster gene stcO, we replaced the stcO coding sequence with the gene of the yeast codon-optimized cyan fluorescent protein (ycfp 37) carrying a built in NLS of prnA at the 5′ end 35 (see Materials and methods for details). Nuclear localization of the yCFP was necessary for the clear detection of the reporter-derived fluorescence. The growth ability of the stcO reporter strain did not differ from that of the control strains on nitrate-lactose and nicotinic acid-glucose media (Fig. 1A). The sexual competence of the stcO reporter strain was monitored on nitrate-lactose and nicotinic acid-glucose media under different environmental conditions (in natural light and dark, with or without oxygen restrictions). We did not observe any deviation from the wild-type in the sexual structures of the reporter strain, which formed cleistothecia with viable ascospores.
3.2 StcO is essential for the STC biosynthesis
Although AvfA, the A. parasiticus and A. flavus counterpart of StcO, is essential for the aflatoxin biosynthesis 25, the exact role of StcO in A. nidulans is still unknown. By investigating the STC production, we found that while the wild-type (HZS.450) and the parental control (HZS.576) strains display normal STC production on nitrate-lactose medium incubated in dark for 3 and 4 days (Fig. 1B), stcO deletion strain (HZS.606) fails to produce STC, indicating an essential role of StcO in the STC biosynthesis in A. nidulans.
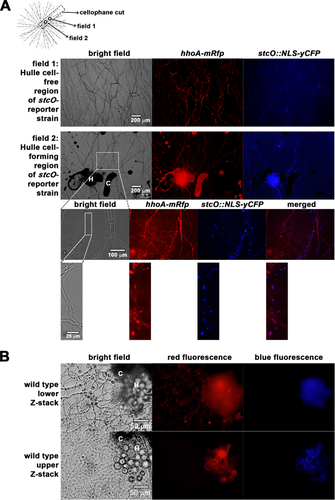
3.3 Localization of stcO promoter activity in the colony is restricted to hyphae encompassing groups of hülle cells
Incubation of A. nidulans with traditionally used nitrogen sources (e.g., acetamide, urea, hypoxanthine, xanthine, allantoin, uric acid, nitrate, ammonium, or arginine) resulted in multiple layers of hyphae at the regions of interest, which made the microscopic analysis less feasible. In order to circumvent this problem, we applied nicotinic acid as sole nitrogen source that is utilized less efficiently than any other nitrogen sources 36. By growing the colonies on nicotinate, we were able to investigate separately running monolayer hyphae even after several days of incubation (Fig. 1A). On the other hand, using nicotinic acid as sole nitrogen source resulted in the production of intracellular compounds with an unusually wide range of autofluorescence after the 3–4 days of incubation (Z. Hamari, unpublished data). This might interfere with microscopic studies performed on fluorescent microscopes equipped with multifunctional filters with a relatively large bandpass. Such filters can detect a much wider emission wavelength range than that is required for the detection of RFP or CFP fluorescent signals (such as the U-MNU2 and U-M41035 Olympus cubes used in this study) (Fig. 2B). In our experimental setup, the unlabeled control strain (HZS.450) grown on nicotinic acid-glucose cellophane plate for 3 days showed red and cyan autofluorescence in the hülle cells (Fig. 2B, bottom) and red autofluorescence in the vacuoles or vesicles of the hyphae (Fig. 2B, top), while the conidia did not show any fluorescence (Figs. 2A and 2B). We monitored three time points (48, 72, and 96 h) and found that the stcO promoter became active only on the third day of incubation (Fig. 2). In the fourth day of incubation the stcO promoter activity ceased and was rarely detected (data not shown). As it is shown in Fig. 2, the stcO promoter is active only in those hyphae, which surround the hülle cells (panel A), and its activity diminishes with the distance of the hyphae to the hülle cells. Monitoring of the stcO promoter activity in the hülle cells was not possible in our experimental setup due to their strong autofluorescence. According to our observations, we propose that STC production is initiated at the core of sexual development.
4 DISCUSSION
The role of STC as protective agent against arthropod fungivores has been well established and the heterogeneous distribution of STC in the colony in association with the sexual structures was proposed previously 14, 15. In addition, studies on sexual development and STC biosynthesis revealed that these processes share common master regulators 9-13 and thereby they are coupled in environmental conditions that allow their manifestation (reviewed in 4). Here we show that StcO plays an essential role in STC biosynthesis (Fig. 1B) and the activation of the stcO gene is an indicator of STC production at the third day of incubation. At the fourth day of incubation, we did not detect any stcO promoter activity (data not shown), however the amount of STC further increased in comparison to the amount of STC detected at the third day (Fig. 1B). The stcO activated hyphae could be seen only in the proximity of groups of hülle cells (forming primordia) and the promoter activity diminished with the distance from the hülle cells. Mutants (e.g., sfaDΔ) with abolished cleistothecia formation and normal hülle cells show no sign of STC production 38, indicating that the presence of hülle cells is not deterministic for STC production. Notably, STC is normally produced in liquid cultures where sexual structures (including hülle cells) are not formed. Uncoupling of these two processes might be governed by a variety of signal transduction pathways, which transduce and transmit environmental signals that determine sexual development and STC production. Sexual development depends on the MAP kinase pathways, while STC production relies on both MAP kinase- and PKA pathways, the latter being repressive on STC production (reviewed in 4). Therefore it is possible that while MAP kinase pathway does not support sexual development, repression of PKA pathway might be relieved and allow AflR activation and subsequent STC production. According to the above mentioned phenomena together with our results, we propose that on solid medium STC production is defined by (an) early sexual developmental factor(s) that locally initiates sexual development. Moreover, according to the observed pattern of the stcO promoter activity, the promoting factors(s)/signal(s) of STC production can be subsequently transmitted to the vegetative hyphae located close to the core of sexual development. The lack of activity in distant hyphae indicates that the vegetative mycelium might consist of morphologically uniform, but functionally different hypha cells. Future works should elucidate the underlying mechanism of signal transmission for STC production from the origin to the nearby vegetative hyphal compartments.
ACKNOWLEDGMENTS
Work was supported by the Hungarian National Research, Development and Innovation Office (NKFI-K16 119516) and by the Széchenyi 2020 Program (GINOP-2.3.2-15-2016-00012).
CONFLICTS OF INTEREST
The authors have declared no conflict of interest.




