Ion exchange defines the biological activity of titanate nanotubes
Abstract
One-dimensional titanate nanotubes (TiONTs) were subjected to systematic ion exchange to determine the impact of these modifications on biological activities. Ion exchanged TiONTs (with Ag, Mg, Bi, Sb, Ca, K, Sr, Fe, and Cu ions) were successfully synthesized and the presence of the substituted ions was verified by energy dispersive X-ray spectroscopy (EDS). A complex screening was carried out to reveal differences in toxicity to human cells, as well as in antibacterial, antifungal, and antiviral activities between the various modified nanotubes. Our results demonstrated that Ag ion exchanged TiONTs exerted potent antibacterial and antifungal effects against all examined microbial species but were ineffective on viruses. Surprisingly, the antibacterial activity of Cu/TiONTs was restricted to Micrococcus luteus. Most ion exchanged TiONTs did not show antimicrobial activity against the tested bacterial and fungal species. Incorporation of various ions into nanotube architectures lead to mild, moderate, or even to a massive loss of human cell viability; therefore, this type of biological effect exerted by TiONTs can be greatly modulated by ion exchange. These findings further emphasize the contribution of ion exchange in determining not only the physical and chemical characteristics but also the bioactivity of TiONT against different types of living cells.
Introduction
In the past decades, nanosized materials, such as titanate nanostructures with spherical or layered architectures, have already attracted extensive research interest 1. Their unique structural features, like physical stability, high surface area, and environmental benign properties defined substantially their utilization primarily as ion exchangers and as adsorbents. Recent studies verified that the application of these nanostructures can be extended to biomedical, semiconductor, and photocatalytic applications as well 2-11. The high availability of titanate nanotubes (TiONTs) is assured by a fairly economical preparation method from TiO2 using an alkaline hydrothermal process described by Kasuga et al. 12.
Titanates feature a negatively charged framework consisting of TiO6 octahedral units sharing edges at one level combined with similar units above and below, to form zigzag strings of octahedra 13. Modifications of TiONTs could combine the advantageous properties of the original TiONTs (e.g., high specific surface area, chemical inactivity) and those of the modifying moieties to improve their performance and to implement novel desirable properties to the original TiONTs 14. There are several chemical modification options of TiONTs, including ion exchange (e.g., Co2+, Ni2+, Cu2+, and Zn2+) 15, nanoparticle decoration (e.g., Au+) 16, doping TiONTs with a metal (e.g., Co2+) 17 or with a non-metal (e.g., B, C, N, and S) 18, 19 and finally, coupling with narrow band semiconductors, such as CdS or CdSe 20. As cations or positively charged particles are readily exchanged with the intercalated cations within the interlayer of TiONTs such modifications can be easily realized 21. The intercalated materials will not simply alter the composition of TiONTs, but the chemical and physical properties of the resulting nanotubes will be also modified, substantially determined by the chemical nature of the modifying ion 22. Previous studies revealed that bismuth ions influenced crystallization of the tubular titanate structures during calcination 23 and TiONTs decorated with metallic nanoparticles (e.g., Au+, Rh3+) were found to feature extended catalytic application possibilities in photo-oxidation and photocatalytic degradation reactions 24, 25.
Although TiO2 nanoparticles have been studied extensively in recent years 26, the interactions between nanotubular formulation of titanate with different types of living cells have not been reliably characterized. Modified TiONTs are of great interest not only as disinfecting materials, in cosmetic industry and wastewater treatment 27 but also for a broad range of biomedical applications 28. Prior to human implementations it is fundamental to understand how the intercalated ions modify the bioactivity of TiONTs. Therefore in this present study, we carried out a complex screening to determine the human toxicity, the antibacterial, antifungal, and antiviral effects of a series of ion exchanged TiONTs as a function of the exchanged cations.
Materials and methods
Synthesis of TiONTs
TiONTs, pristine were prepared by an alkaline hydrothermal method described previously 12. Briefly, in a typical synthesis, 50 g of titanium(IV) oxide powder (99.8% anatase, Sigma–Aldrich) and 1 L of 10 M NaOH (99.93%, Molar) aqueous solution were mixed until a white suspension was obtained. This suspension was transferred into a closed, PTFE-lined stainless steel autoclave (diameter 4 cm, height 14 cm) and was kept at 155 °C for 24 h under continuous rotation (around the short axis of the autoclave, with a speed of 3 rpm). The as-prepared nanotubes were washed thoroughly first with deionized (DI) water to achieve neutral pH then were rinsed with 0.1 M HCl solution to reach pH 2 and finally with water again to obtain a neutral salt-free product. The resulting H-form TiONTs were dried in air at 60 °C for 24 h.
Preparation of ion exchanged TiONTs
For preparation of different ion exchanged TiONTs 1 g TiONT powder (pristine) was dispersed in 100 ml 1 M of either silver nitrate, or magnesium chloride hexahydrate, bismuth(III) acetate, antimony(III) acetate, calcium chloride dihydrate, potassium chloride, strontium chloride hexahydrate, iron(III) chloride, or copper(II) sulfate (99.0%, Sigma–Aldrich) solution by ultrasonication and stirring for 72 h. After stirring, the suspension was filtered and washed with DI water to obtain neutral pH and finally all the samples were dried at 60 °C for 24 h. The as-synthesized samples were denoted as Ag/TiONT, Mg/TiONT, Bi/TiONT, Sb/TiONT, Ca/TiONT, K/TiONT, Sr/TiONT, Fe/TiONT, and Cu/TiONT.
Characterization of the pristine and the ion exchanged TiONTs
The structure and morphology of both the pristine and the modified TiONTs were characterized first by transmission electron microscopy (TEM). The TEM images were captured by a FEI Tecnai G2 20 X-Twin instrument (operating at an accelerating voltage of 200 kV) using copper mounted holey carbon grids.
Crystal structures were analyzed by X-ray powder diffraction (XRD). The scans were performed with a Rigaku MiniFlex II powder diffractometer using Cu Kα radiation. A scanning rate of 2° min−1 in the 5–60° 2θ range was used.
Energy dispersive X-ray spectroscopy (EDS) was applied to estimate the elemental analysis of the pristine and the ion exchanged nanotube samples. The EDS measurements were carried out on a Hitachi S-4700 scanning electron microscope (30 kV accelerating voltage) equipped with a Röntec energy dispersive X-ray spectrometer.
Cell culture and viability assay
Human cervical cancer HeLa cells were purchased from ATCC and maintained in low glucose DMEM. The medium was supplemented with 10% FBS, 2 mM l-glutamine, 0.01% streptomycin, 0.005% ampicillin and cells were cultured under standard conditions in a 37 °C incubator at 5% CO2 and 95% humidity.
To measure cell viability MTT mitochondrial activity assay was performed. A 104 cells/well were seeded into 96 well plates and treated with the pristine (TiONT) or with ion exchanged TiONTs in 5 mg/ml concentration on the following day. After 24 h treatment cells were washed with PBS and incubated with culture medium containing 0.5 mg/ml MTT reagent (Sigma–Aldrich) for an hour at 37 °C. Formazan crystals were solubilized in DMSO and extinction was measured at 570 nm using a SPECTROStar Nano microplate reader. Absorption corresponding to the untreated control samples was considered as 100%. Viability assays were performed at least three times using four independent biological replicates.
Antibacterial and antifungal activities tests
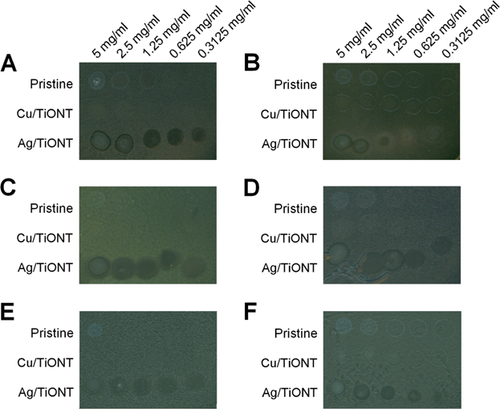
- The antibacterial and antifungal activities of ion exchanged TiONTs were screened by agar diffusion method 29 with a minor modification: titanate nanotube suspensions were prepared in 25 mg/ml final concentration and 5 µl of each suspension was loaded directly onto the agar surface seeded with the test strains. The examined microbes were as follows: Escherichia coli SZMC 0582, Pseudomonas aeruginosa SZMC 0568, Micrococcus luteus SZMC 0264, Bacillus subtilis SZMC 0209, Saccharomyces cerevisiae SZMC 23464, and Candida albicans ATCC 10231. The plates were incubated at 30 °C and to avoid misleading results arising from fluctuating droplet sizes, the width of the inhibition zone was determined in each experiment after 24 h.
- The same agar diffusion method was used to determine the minimum inhibitory concentration of the selected Ag/TiONT and Cu/TiONT against all 6 above mentioned species. The applied concentrations of these ion exchanged nanotubes were as follows: 5, 2.5, 1.25, 0.625, and 0.3125 mg/ml. The pristine TiONTs in the same concentrations were used as control. The plates were incubated at 30 °C for 24 h.
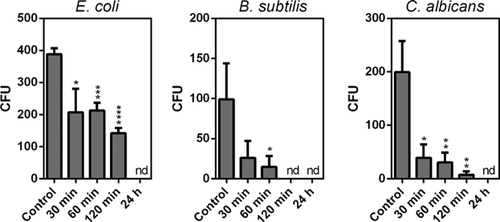
- The number of the colony forming units (CFU) was established to quantify the antibacterial and antifungal effectiveness of Ag/TiONT. Briefly 1 × 106 cells of B. subtilis, E. coli, or C. albicans were exposed to Ag/TiONT at concentration of 0.3125 mg/ml for 30, 60, 120 min, and 24 h. After the incubation time cells were washed out from the Ag/TiONT suspension then were resuspended in sterile water and 20 μl aliquots were spread on the agar surface. Plates were incubated at 30 °C for 48 h and the number of colonies was counted.
The antibacterial and antifungal activity tests were carried out in three independent experiments.
Antiviral tests
Bacteriophage plaque assay
A 104 λ phage particles were incubated in Ag/TiONT solution at 0.3125 and 0.03125 mg/ml concentrations for 60 min. After the incubation time phages were plated onto LB medium according to the method described by Sambrook et al. 30. The experiments were repeated three times using three biological replicates.
Intact capsid test
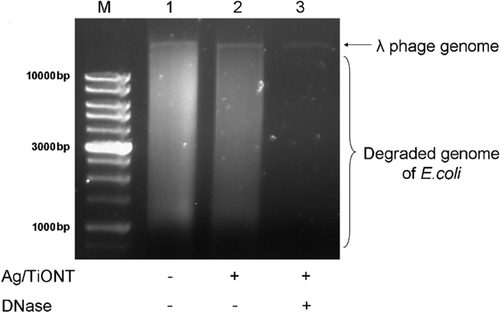
Phage particles were co-incubated with Ag/TiONT solutions at 5, 2.5, 1.25, 0.625, and 0.3125 mg/ml concentrations for 60 min. The samples were divided into two parts. One portion was digested with DNase (50 g/ml, for 30 min at 37 °C) and then extracted with equal volume of phenol–chloroform. The remaining part was extracted only with phenol–chloroform no enzymatic treatment was carried out. Phenol–chloroform extracted phage solution without Ag/TiONT treatment was used as control. Nucleic acids were separated by electrophoresis in a 0.8% agarose gel and were visualized under UV light after ethidium bromide staining.
Results
Synthesis and characterization of ion exchanged TiONTs
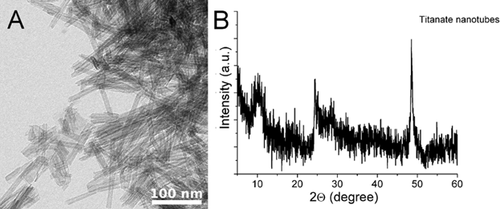
The morphology and structure of the hydrothermally synthesized TiONTs were characterized by transmission electron microscopy (TEM) and X-ray powder diffraction (XRD). The as-synthesized nanotubes are open-ended tubular structures with 90–100 nm length and with 8–10 nm width as shown in Fig. 1A. XRD pattern of the produced nanotubes is represented in Fig. 1B. The XRD pattern did not correspond to anatase, rutile, brookite or their mixtures; however, the crystal structure of the nanotubes is obviously corresponding to trititanate phase. The characteristic reflections of the trititanate nanotubes (H2Ti3O7) are at 2θ = 10°, 24.85°, 28.2°, and 48.8°, among which the 2θ = 10° is the one that belongs to the tubular interlayer distance 31.
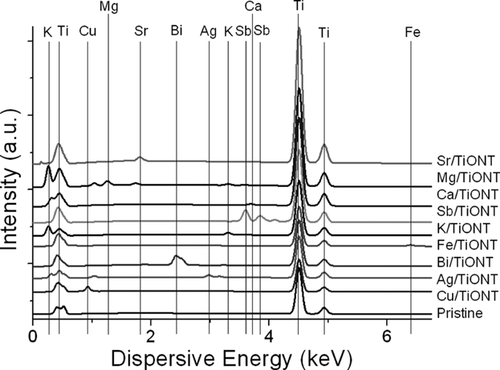
After the respective ion exchange processes, morphological and structural changes did not occur which is in line with the findings on ion exchanged nanotitanate reported previously 23, 32. EDS measurement was carried out to estimate the elemental composition of the different TiONTs (Fig. 2). It can be clearly seen that all samples contained Ti, the ion exchanged variations contained Ca, Mg, Cu, Fe, Sb, Bi, Ag, K, Sr, respectively, and were free from other elements.
Toxicity on human cells
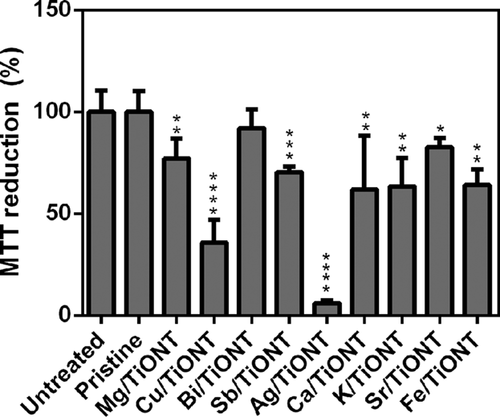
To evaluate how the cytotoxic propensity of TiONTs can be influenced by exchanging different cations within TiONT structures, we performed a cell viability assay on pristine treated and on ion exchanged TiONT treated human HeLa cells. MTT assays revealed that 5 mg/ml concentration of the pristine and of Bi/TiONT did not cause cell death, whereas administration of the same amount of Ag ion exchanged TiONT resulted in complete loss of viability (Fig. 3). Also Cu modified TiONTs had a strong toxic effect on human cells as we observed a severe (∼65%) loss of viability after Cu/TiONT treatments. Interestingly, pristine and Bi/TiONT were non-toxic even in higher doses (data not shown). Mg, Sb, Ca, K, Sr, and Fe modified TiONTs decreased human cell viability moderately, by approximately 20–40% (Fig. 3) when administered in 5 mg/ml concentration. Therefore, we can conclude that modifying TiONTs with metal ions influences greatly the biological activity of the original nanotubes and in some cases the incorporation of metal ions (Ag and Cu ion exchanged TiONT) significantly increases the toxicity of TiONTs.
Antibacterial and antifungal activities
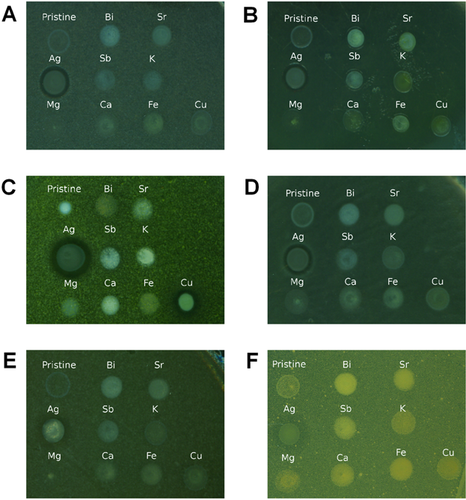
In order to determine whether the as-prepared TiONTs are effective against selected bacteria and fungi and to identify which intercalating ion can enhance the antimicrobial features of TiONTs, we tested the pristine and the ion exchanged nanotubes against Gram negative (E. coli, P. aeruginosa), Gram positive (M. luteus, B. subtilis) bacteria in addition against yeast species (C. albicans, S. cerevisiae). Most of the nanotubes did not exert any antimicrobial activity in the applied concentration (25 mg/ml); however exchange of two ions, namely Ag and Cu, augmented markedly the antimicrobial properties of TiONTs. Ag/TiONT was effective against all the examined microbial species (Fig. 4A–F, Table 1) but Cu/TiONT inhibited only the growth of M. luteus (Fig. 4C). The microbial response to the presence of Ag/TiONT produced clear inhibition zones in the case of bacteria; however, yeasts exhibited cloudy inhibition zones.
| Microorganism | Inhibition zone (mm) |
|---|---|
| B. subtilis | 1.1 ± 0.13 |
| M. luteus | 2.1 ± 0.38 |
| E. coli | 1.6 ± 0.13 |
| P. aeruginosa | 1.0 ± 0.00 |
| C. albicans | 2.3 ± 0.14 |
| S. cerevisiae | 1.8 ± 0.29 |
- Ag/TiONT concentration was 25 mg/ml. Number of samples (n) = 4.
According to the results of the general microbiological screening we found two samples, the Ag/TiONT and Cu/TiONT, which exhibited notable effect on bacteria, therefore we examined more thoroughly their antimicrobial capacities. Agar diffusion method was used to establish the minimum inhibitory concentration of Ag and Cu ion exchanged nanotubes by loading a dilution series of the suspensions onto the surface of inoculated plates (Fig. 5A–F). Contrary to the previously observed inhibitory effect on M. luteus in 25 mg/ml concentration, Cu/TiONT was not effective at these lower concentrations not even against M. luteus. However, Ag/TiONT proved to be both antibacterial and antifungal in lower doses as well (Fig. 5A–F).
The effectiveness of Ag/TiONT was also tested in the function of incubation time. Therefore, we exposed selected microbes (E. coli, B. subtilis, and C. albicans) to Ag modified nanotubes in 0.3125 mg/ml concentration for 30, 60, 120 min, and 24 h. Thirty minutes exposures already decreased the viability of E. coli by 50% and an even more dramatic loss (∼70%) was observed in the viabilities of both C. albicans and B. subtilis (Fig. 6). After treating the cells with the same amount of Ag/TiONT for 24 h no viable cells could be detected in all the three microorganisms.
Antiviral activity
The antiviral activity of Ag/TiONT was investigated in this study by two different methods (Bacteriophage plaque assay and Intact capsid test). Theoretically, structural changes (e.g., damage of the capsid or tail proteins) could be detected by either method. The presence of Ag/TiONT at 0.3125 and 0.03125 mg/ml concentrations did not cause any decrease in bacteriophage plaque counts (Table 2). Phage genome protection was maintained against DNase after treatment of the phage particles with Ag modified nanotubes (Fig. 7). Both assays suggested that the capsid remained intact during treatment of the phage particles with Ag/TiONT therefore no antiviral activity was detected.
| Ag/TiONT (mg/ml) | Number of plaques |
|---|---|
| 0 (control) | 97.67 ± 8.090 |
| 0.03125 | 103.67 ± 9.207 |
| 0.3125 | 106.33 ± 8.686 |
Discussion
The numerous favorable features (e.g., physical stability, high surface area) of TiONTs led to their application in different fields of technology 4, 5. Recent publications proved that chemical modifications (e.g., ion exchange, decoration, and doping) might further improve the physical and chemical properties of TiONTs extending the potential of their practical and industrial utilization 1, 23. In this present study the biological activity of one-dimensional (1D) TiONTs and their ion exchanged variations were investigated. Systematic exchange of cations was carried out to prepare Ag, Mg, Bi, Sb, Ca, K, Sr, Fe, and Cu containing TiONTs in order to measure their activity against a number of bacterial strains, fungi and human cells. The most important question was whether it is feasible to enhance or possibly modulate the biological efficacy of TiONTs by exchanging specific cations into its structure. Prior to microbiological screening the general human toxicity of the as-prepared TiONTs were assayed. Although Magrez et al. have already evaluated the toxicity of the pristine nanotubes, the biological activity of ion exchanged TiONT has not been investigated yet 33. Our results revealed, that in fact incorporation of various ions into nanotubes lead to a mild, to moderate or even to a massive loss of human cell viability, therefore, this type of biological effect of TiONTs can be gradually tailored by ion exchange.
The antimicrobial activity of many different types of metal nanoparticles (e.g., silver, zinc, and copper) is well known and in some cases the mechanisms of bactericidal or bacteriostatic actions have already been elucidated 34, 35. In this study we carried out a complex screening to establish the biological activity, more specifically the antiviral, antibacterial and antifungal features of the ion exchanged TiONTs. As expected, we observed potent antimicrobial activity of Ag/TiONT and Cu/TiONT, however, in the latter case this activity was restricted only to M. luteus. In addition to the powerful antibacterial propensity, Ag/TiONT proved to be strongly antifungal as well.
The observed antimicrobial effect of Ag and Cu exchanged TiONT is owing to the release of ionic silver and copper ions from the nanotube structure into the surrounding media 36, 37 hence these ions are responsible for the witnessed biological activity of these ion exchanged nanotubes.
Although the antiviral effect of silver nanoparticles has been demonstrated in the case of influenza, hepatitis B and HIV-1 viruses 38-40, generally the antiviral activity of nanoparticles is rarely investigated. In our study the antiviral effect of Ag/TiONT was tested by two methods: the bacteriophage plaque assay and the intact capsid test. As we did not see any decrease in bacteriophage plaque counts nor structural damage in the viral capsid during treatment of the phage particles with Ag/TiONT, we concluded that Ag ion exchanged TiONTs do not exert notable antiviral effect.
In summary, our results provide further support that ion exchange can in fact strongly influence not only the physical and chemical characteristics of TiONTs but can also determine their biological activity against different types of living cells.
Conflict of interest
The authors declare that they have no conflicts of interest.




