Cloning, characterization, and production of three α-l-fucosidases from Clostridium perfringens ATCC 13124
Abstract
α-l-Fucosidases are key enzymes for the degradation of intestinal glycans by gut microbes. In this work, three putative α-l-fucosidases (Afc1, Afc2, and Afc3) genes from Clostridium perfringens ATCC 13124 were cloned and expressed in Escherichia coli. Afc1 had the α-l-fucosidase domain of glycoside hydrolase (GH) 29 family but showed no enzyme activity toward all the substrates examined. The putative acid/base residue of Afc1, Ser205, was replaced by a glutamic acid which is conserved in GH29-B α-l-fucosidases. However, the mutant Afc1-S205E still failed to show enzyme activity. Afc2 and Afc3 were determined to be 1,3-1,4-α-l-fucosidase of GH29-B subfamily and 1,2-α-l-fucosidase of GH95 family, respectively, and both of them could release fucose from porcine gastric mucin (PGM). When C. perfringens ATCC 13124 grew with the presence of PGM, the transcription of afc1 decreased slightly, while those of afc2 and afc3 increased to 2.2-fold and 1.4-fold, respectively, and the enzyme activities of Afc2 and Afc3 in the culture increased to 2.2-fold and 2.6-fold, respectively. These results suggest that Afc2 and Afc3 are involved in the degradation of intestinal fucosyl glycans by C. perfringens ATCC 13124.
Abbreviations
-
- GH
-
- glycoside hydrolase
-
- CBM
-
- carbohydrate binding module
-
- Lea trisaccharide
-
- Lewis a trisaccharide
-
- Lex trisaccharide
-
- Lewis x trisaccharide
-
- PGM
-
- porcine gastric mucin
Introduction
Human gastrointestinal tract harbors trillions of microbial cells 1. The colonization and inhabitation of these microbes are heavily affected by the available carbohydrates in human gut, such as mucins O-glycans and human milk oligosaccharides (HMOs) 2. Mucin O-glycans are the major host-derived carbon sources, which provide consistent nutrients to gut microbes 3. HMOs are important carbon sources in breast-fed infant gut, which are believed to affect the development of gut microbiota 4. Fucosyl residues are frequently found on the terminal of these carbon sources. It has been reported that 43 of 55 oligosaccharides released from mucins of human ileum are fucosylated 5, and typically over 50% oligosaccharides of HMOs are fucosylated 6-8. Fucosyl residues are generally linked to the terminal galactose (Gal) via α1,2-linkage, and/or linked to N-acetylglucosamine (GlcNAc) or glucose (Glc) via α1,3- or α1,4-linkage. These terminal fucosyl residues often shield fucosyl glycans from sequential digestion, making the removal of fucosyl residue a key step for the utilization of those carbon sources by gut microbes.
α-l-Fucosidases are capable of cleaving α-linked fucosyl residues from the non-reducing end of glycans. Those enzymes have been found in various species 9-18. According to their amino acid sequences, α-l-fucosidases are classified into glycoside hydrolase (GH) families 29 and 95. GH29 enzymes are further divided into two subfamilies, GH29-A and GH29-B 14, 15. GH29-A subfamily contains α-l-fucosidases (EC 3.2.1.51) that can act on synthetic substrates such as p-nitrophenyl-α-l-fucoside (Fucα1-pNP), and these enzymes also have limited hydrolytic ability on fucosyloligosaccharides. GH29-B enzymes, referred to as 1,3-1,4-α-l-fucosidase (EC 3.2.1.111), are specific for α1,3/4-fucosidic linkage of fucosyloligosaccharide with branched Gal residue at the non-reducing end 19. GH95 enzymes, referred to as 1,2-α-l-fucosidase (EC 3.2.1.63), specifically hydrolyze the terminal α1,2-fucosidic linkages 20. α-l-Fucosidases have been cloned and characterized from several intestinal bacteria, including Bifidobacterium 14, 16, 20, Bacteroides 15, 21, and Lactobacillus 22, and the relationship between α-l-fucosidases and the degradation of fucosyl glycans by some of these gut microbes have also been reported 7, 23. It is revealed from these reports that the ability to produce α-l-fucosidases with different substrate specificity plays important roles in the glycan foraging of gut microbes. Nevertheless, these studies mainly regard to probiotics, but rarely involve human-gut pathogens.
Clostridium perfringens is a common opportunistic pathogen in human intestine, which can cause gas gangrene, necrotic enteritis, food poisoning diarrhea, and non-foodborne gastrointestinal infections 24-26. This pathogen is able to produce various glycosidases (http://www.cazy.org/) for glycan foraging and some of them have been clone and characterized, including endo-α-N-acetylgalactosaminidase 27, sialidases 28, α-N-acetylglucosaminidase 29, and endo-β-galactosidases 30-32. There are a few reports up to now about its α-l-fucosidases. A secreted 1,2-α-l-fucosidase was purified from C. perfringens in 1970, but the gene encoding this enzyme has not been reported yet 33. Genes of putative 1,3-1,4-α-l-fucosidase and 1,2-α-l-fucosidase have been found in the genome of C. perfringens and the carbohydrate binding module (CBM) of the putative 1,2-α-l-fucosidase has been characterized by Gregg et al. 34, 35, but the α-l-fucosidase activity of these two enzymes have not been studied. In this work, we performed sequence analysis, molecular cloning, and enzymatic characterization of three α-l-fucosidases of C. perfringens ATCC 13124, from which the classification of GH family and substrate specificity of these enzymes were determined. The production of α-l-fucosidases during cell growth of C. perfringens ATCC 13124 was further studied by transcriptional analysis of enzyme genes in the cell and assay of enzyme activities in the culture. The results obtained in this work expand the knowledge about the α-l-fucosidases of C. perfringens, providing important information to understand the glycan foraging of this pathogen in human intestine.
Materials and methods
Materials and growth condition
Fucα1-2Gal, Lea, and Lex trisaccharides, 3-fucosyl-GlcNAc, 4-fucosyl-GlcNAc, and 6-fucosyl-GlcNAc were purchased from Carbosynth (Carbosynth Limited, Berkshire, UK). Fucα1-pNP and porcine gastric mucin (PGM, Type II) were purchased from Sigma. l-Fucose kit was purchased from Megazyme (Wicklow, Ireland).
C. perfringens ATCC 13124 was grown anerobically at 40 °C in basal medium (BM) contained 5 g yeast extract, 5 g peptone, 5 g sodium acetate, 0.2 g magnesium sulfate, 4 g dipotassium hydrogen phosphate, 0.4 g cysteine hydrochloride, 6.8 g ascorbic acid in 1000 ml of water (pH 7.0). BM-PGM was made by adding 0.5% (w/v) of PGM to BM.
Sequence analysis
Genome of C. perfringens ATCC 13124 was scanned using BLAST program (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Conserved domains were analyzed based on Pfam database (http://pfam.xfam.org/). Multiple alignments were performed using online ClustalW program (http://www.ebi.ac.uk/Tools/msa/clustalw2/ and http://www.genome.jp/tools/clustalw/). Sequence identity was analyzed using ClustalW program of Bioedit software (http://www.mbio.ncsu.edu/bioedit/bioedit.html). Signal peptide and transmembrane region were predicted using online tools (http://www.cbs.dtu.dk/services/SignalP/ and http://www.cbs.dtu.dk/services/TMHMM/).
Gene cloning and heterogeneous expression
To clone Afc1(CPF_0652; GenBank:ABG82807), Afc2 (CPF_2130; GenBank:ABG83106) and Afc3 (CPF_2129; GenBank:ABG82552), DNA fragments encoding each enzyme without putative N-terminal signal peptides were amplified from the genomic DNA of C. perfringens ATCC 13124 by PCR with designed primers (Table 1). PCR was performed using a TP600 Gradient PCR Thermal Cycler Dice (Takara), with initial denaturation at 95 °C for 5 min and 30 cycles of denaturation at 95 °C for 0.5 min, annealing at 55 °C for 0.5 min, extension at 72 °C for 2.5 min, and a final extension at 72 °C for 10 min. The PCR products were gel purified. After digested with NdeI/NheI and XhoI, PCR products were cloned into vectors to get afc1-pET15b, afc2-pET22b, and afc3-pET22b and transformed into E. coli BL21 (DE3), respectively. The proper transformant was cultivated in LB medium containing 50 μg ml−1 ampicillin. Culture was grown at 37 °C to an optical density of 0.6–0.8 at 600 nm. Isopropyl-1-thio-b-d-galactoside was added to give a final concentration of 0.5 mM, and then the culture was incubated at 25 °C for 8 h. Cells were harvested and disrupted by sonication (model VCX500; Sonics & Materials, Inc., Newtown, CT). The enzymes were purified by nickel affinity chromatography (GE Healthcare).
| Name | Description | DNA sequence (5′→3')a | Restriction enzyme site |
|---|---|---|---|
| Afc1F | Cloning primer | CGCCATATGAATACAACTCTAGTAAACCT | NdeI |
| Afc1R | Cloning primer | CCGCTCGAGTCCTAAATATGCACCAATAT | XhoI |
| Afc2F | Cloning primer | CGCGCTAGCCAAGGATCTATATTAACTAA | NheI |
| Afc2R | Cloning primer | CGCGCTCGAGTTTTAATATATCATTCGTT | XhoI |
| Afc3F | Cloning primer | CGCGCTAGCAAGTCTAGTAGATTTAATGA | NheI |
| Afc3R | Cloning primer | CGCGCTCGAGTTTTAGAAGTTCACTAGATAC | XhoI |
| S205E-F | Mutagenesis primer | CGTTGGACATATTATGAAGATATAGAGTCA | |
| S205E-R | Mutagenesis primer | ATAATATGTCCAACGTACATCAGGTCCAAT | |
| afc1-RT-F | RT-qPCR primer | ATTGGACCTGATGTACGTTGG | |
| afc1-RT-R | RT-qPCR primer | ACTTTCTCCAACAATCCAAGTGT | |
| afc2-RT-F | RT-qPCR primer | GGATGGATGGAGCAAAGGGA | |
| afc2-RT-R | RT-qPCR primer | CAGCAAGGCTCTCCTGCATA | |
| afc3-RT-F | RT-qPCR primer | TGAGTCCAAAACTGTGCAGC | |
| afc3-RT-R | RT-qPCR primer | GCTATGGGATGGGAATTTGA | |
| recA-RT-F | RT-qPCR primer | GCTCCTCAAATGGATGCTGT | |
| recA-RT-R | RT-qPCR primer | GCTGCTGCTCCACCTAATTT |
- a Restriction enzyme sites are underlined.
Site-directed mutagenesis
Afc1-S205E was generated according to procedure of GENEART Site-Directed Mutagenesis Kit (Invitrogen). The afc1-pET15b plasmid was used as the template and Phusion polymerase (NEB) was used for PCR. Mutations were confirmed by DNA sequencing and transformed into E. coli BL21 (DE3) cells. Expression and purification of mutants were carried out in the same way as for the wild-type Afc1.
TLC analysis
TLC was performed on silica-gel 60 plates (Merck, Germany) using butanol–ethanol–water [5:3:2 (v/v/v)] as the mobile phase. Sugars were visualized by spraying with a solvent containing 20% sulfuric acid and 0.5% 3,5-dihydroxytoluene, followed by incubation at 120 °C for 5 min.
Enzyme and protein assay
α-l-Fucosidase activity was measured by incubating enzyme sample (0.1 mg ml−1) with 4 mM fucosyloligosaccharides or 1% PGM in 50 mM sodium phosphate buffer (pH 7.0) at 37 °C for 5 min or 2 h, respectively. The reactions were stopped by heating at 100 °C for 3 min. The amount of fucose released was quantified with fucose kit. One unit of enzyme activity was defined as the amount of enzyme required to liberate 1 nmol of l-fucose per minute under the assay conditions. For Fucα1-pNP, the reaction condition was the same as that of fucosyloligosaccharides except for 2.5 mM substrate. The reactions were quenched by adding 1.5-fold of the reaction volume of 1 M sodium carbonate and the amount of p-nitrophenol released was measured by spectrophotometer at 405 nm. One unit of enzyme activity was defined as the amount of enzyme required to liberate 1 µM of p-nitrophenol per minute under the assay condition. The concentration of proteins were quantified by Bradford assay protocol using BSA as the standard.
Biochemical studies
The molecular mass of the enzymes were determined by SDS-PAGE. The hydrolytic substrate specificity of enzymes were detected using various fucosyl-glycans as substrates in the same way as the enzyme assay conditions. Kinetic values of enzymes were determined by incubating each enzyme (0.02 mg ml−1) with varied concentrations of substrates (2–32 mM). The reaction conditions are same as the enzyme assay conditions, but quenched by liquid nitrogen to eliminate the auto-degradation of Lewis trisaccharides at high concentration when heated at 100 °C. The Km and Vmax values were calculated using non-linear regression program of GraphPad Prism software (http://www.graphpad.com/). The optimal pH of enzymes were assayed in the pH range of 3.0–11.0. The effect of pH on stability of enzymes were studied by assessing enzyme activity after incubating enzyme (1 mg ml−1) in the pH range of 2.5–12.0 at 4 °C over 20 h. The optimal temperature of enzymes were measured at 20–70 °C. Thermal stability of enzymes were studied by assessing enzyme activity after incubating enzymes at 20–70 °C for 30 min. All reactions were conducted in triplicate and results were reported as mean values.
Analysis of production of α-l-fucosidases of C. perfringens ATCC 13124
The cell growth curves were tested by sampling aliquots of the culture at regular intervals and measuring the absorbance at 600 nm to determine the cell density. For transcriptional analysis, the cells cultured in BM and BM-PGM were harvested at exponential phase and total RNA was extracted using RNAiso Plus (Takara), respectively. The extracted RNA was converted to cDNA using PrimeScript RT reagent Kit (Takara). Recombinase A (GenBank:Q0TPT0) of C. perfringens ATCC 13124 was used as reference gene 36. Primers used for real-time quantitative PCR (RT-qPCR) were designed using Primer3 (simgene.com/Primer3) (Table 1). RT-qPCR was carried out using PrimeScript® RT-PCR Kit (Takara) in 96-well plates on PTC-200 Peltier Thermal Cycler (Bio-Rad). Threshold cycle values were analyzed using the Opticon Monitor version 3.1 software (www.bio-rad.com/en-us/sku/soft-om-sw-opticon-monitor-software). Relative transcript levels of afc1, afc2, and afc3 were calculated using 2−ΔΔCT method. For the analysis of enzyme activities of α-l-fucosidases, cultures were obtained at the beginning of stationary phase and performed for enzyme activity assay. Fucα1-2Gal was used to determine the 1,2-α-l-fucosidase activity, and Lex and Lea trisaccharides were used to determine the 1,3-1,4-α-l-fucosidase activity.
Results
Sequence analysis of three putative α-l-fucosidases of C. perfringens ATCC 13124
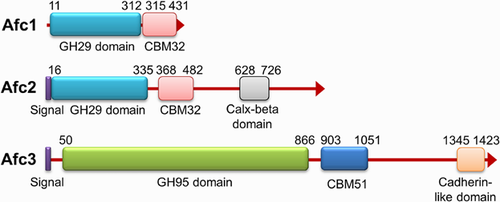
Analysis of the genome of C. perfringens ATCC 13124 by BLAST program educed that three ORFs, CPF_0652 (1.31 kb), CPF_2130 (2.80 kb), and CPF_2129 (4.44 kb), encoded three putative α-l-fucosidases consisting 436 amino acids (named Afc1), 932 amino acids (named Afc2), and 1479 amino acids (named Afc3), respectively. Conserved domain analysis revealed that Afc1 and Afc2 belonged to GH29 family as the presence of GH29 α-l-fucosidase domains (amino acids 11–312 of Afc1 and amino acids 16–335 of Afc2). Both enzymes had CBM32 (amino acids 315–431 of Afc1 and amino acids 365–482 of Afc2) which specifically interacts with terminal galacto-configured sugars 37, 38. Afc2 also had a Calx-β domain (amino acids 628–726) which may have binding capacity of calcium. Afc3 has been predicted to be a GH95 α-l-fucosidase by Gregg et al. 34. We analyzed the amino acid sequence of Afc3 in Pfam database and found similar result. The amino acids 50–866 of Afc3 encoded GH95 α-l-fucosidase domain, and amino acids 903–1051 encoded CBM51 domain which is reported to have similar substrate affinity like CBM32 34. Near the C-terminal, Afc3 had a cadherin-like domain (amino acids 1345–1423) which is deduced to have carbohydrate-binding function.
The predicted signal peptides and transmembrane regions of three enzymes were analyzed. There was no predicted signal sequence at the N-terminal of Afc1, suggesting it might be an intracellular enzyme. The amino acids 1–25 of Afc2 and amino acids 1–26 of Afc3 encoded putative signal sequence at the N-terminal, suggesting Afc2 and Afc3 might be extracellular enzymes. No transmembrane region was found in these three enzymes (Fig. 1).
To further determine the GH29 subfamily that Afc1 and Afc2 belong to, we performed a multiple sequence alignment of the deduced amino acid sequences of the catalytic domains of Afc1, Afc2, and other GH29 enzymes (Supporting Information Table S1). Afc1 showed low identities (9.20–21.8%) with all the GH29 enzymes, but the identities (15.5–21.8%) with GH29-B subfamily were higher than those (9.20–12.7%) with GH29-A subfamily. Afc2 showed 12.1–49.1% identity with all the GH29 enzymes. Likewise, it had higher identities (27.8–49.1%) with GH29-B subfamily than those (12.1–17.4%) with GH29-A subfamily. Based on these results, Afc1 and Afc2 were predicted to be enzymes of GH29-B subfamily.
The putative catalytic residues of Afc1 and Afc2 were also analyzed by aligning their amino acid sequence with that of GH29-B enzymes. GH29 enzymes follow configuration-retaining mechanism which requires a nucleophile residue and an acid/base residue for hydrolysis. The catalytic nucleophile residues are conserved through GH29 family, while the acid/base residues are only conserved in GH29-B enzymes 21. As shown in Fig. 2A, Asp-162 of Afc1 and Asp-203 of Afc2 align with the conserved nucleophile residue. At the conserved acid/base residue, Afc2 had a common conserved glutamic acid residue (Glu-246), while the Afc1 had serine residue (Ser-205).
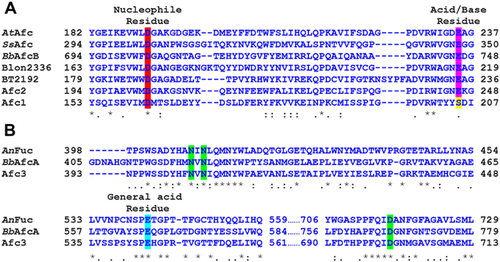
Amino acid sequence of Afc3 was aligned with that of two GH95 enzymes, BbAfcA from Bifidobacterium bifidum JCM1254, and AnFuc from Aspergillus nidulans FGSC A4 20, 39. BbAfcA is the first enzyme classified into GH95 family in 2004 20, which follows a novel configuration-inverting mechanism 40. In this mechanism, BbAfcA uses a glutamic acid (Glu-566) as the general acid residue, but it has no typical general base residue. Instead, Asn-421, Asn-423, and Asp-766 of BbAfcA together perform as the general base catalyst. As shown in Fig. 2B, Glu-544 of Afc3 corresponds to Glu-566 of BbAfcA, and Asn-404, Asn-406, and Asp-700 of Afc3 align with Asn-421, Asn-423, and Asp-766 of BbAfcA, respectively. These results suggest that Afc3 might follow the same hydrolytic mechanism of BbAfcA.
Cloning and characterization of Afc1, Afc2, and Afc3
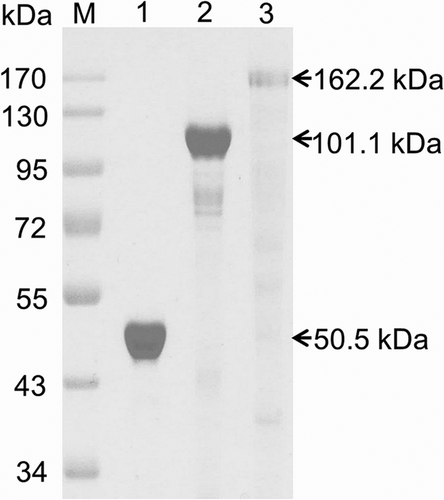
About 1.31, 2.73, and 4.36 kb DNA fragments, encoding Afc1, Afc2, and Afc3 without putative N-terminal signal peptides, were obtained by PCR, respectively. Each of these three DNA fragments was cloned into expression vector and successfully expressed in E. coli BL21 (DE3). After nickel affinity purification, the molecular mass of the recombinant Afc1, Afc2, and Afc3 as determined by SDS-PAGE (Fig. 3) were about 50.5, 101.1, and 162.2 kDa, which were consistent with their calculated molecular weights, respectively.
The substrate specificities of recombinant Afc1, Afc2, and Afc3 were examined (Table 2). Afc1 could not hydrolyze Fucα1-pNP and fucosyloligosaccharides, including Fucα1-2Gal, Lex and Lea trisaccharides, 3-fucosyl-GlcNAc, 4-fucosyl-GlcNAc, and 6-fucosyl-GlcNAc. Afc2 showed enzyme activities exclusively to Lex trisaccharide (12.8 U mg−1) and Lea trisaccharide (12.1 U mg−1). Although 3-fucosyl-GlcNAc and 4-fucosyl-GlcNAc have the same fucosyl linkage with Lex or Lea trisaccharides, they were resistant to the hydrolysis by Afc2 because they do not have the branched Gal residues at the non-reducing end (Table 2). Therefore, Afc2 was concluded to be an 1,3-1,4-α-l-fucosidase. Afc3 showed enzyme activity to Fucα1-2Gal specifically (2.51 U mg−1), but it did not hydrolyze other fucosyloligosaccharides or Fucα1-pNP under the enzyme assay conditions. When the assay time was extended to 40 min, Afc3 exhibited slight hydrolytic activities toward Lea and Lex trisaccharides. Based on the results, Afc3 was concluded to be an 1,2-α-l-fucosidase.
| Activity (U mg−1) | ||||
|---|---|---|---|---|
| Substrate | Structure | Afc1 | Afc2 | Afc3 |
| Synthetic substrate | ||||
| p-Nitrophenyl-α-L-fucoside (Fucα1-pNP) | 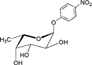 |
–a | – | – |
| Fucosyloligosaccharides | ||||
| Antigen H disaccharide (Fucα1-2Gal) | 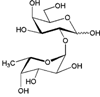 |
– | – | 2.51 ± 0.10 |
| Lea trisaccharide [Galβ1-3(Fucα1-4) GlcNAc] | 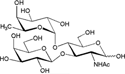 |
– | 12.1 ± 0.7 | Nb |
| Lex trisaccharide [Galβ1-4(Fucα1-3) GlcNAc] | 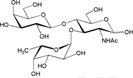 |
– | 12.8 ± 1.6 | Nb |
| 3-Fucosyl-GlcNAc (Fucα1-3GlcNAc) | 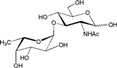 |
– | – | – |
| 4-Fucosyl-GlcNAc (Fucα1-4GlcNAc) | 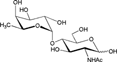 |
– | – | – |
| 6-Fucosyl-GlcNAc (Fucα1-6GlcNAc) |  |
– | – | – |
| Natural substrate | ||||
| Porcine gastric mucin (PGM) | O-glycans with terminal α1,2, α1,3 or α1,4-fucosyl residues | – | 2.91 ± 0.16 | 37.3 ± 1.2 |
- a –, no activity detected.
- b N, marginal activity detected with assay time extended to 40 min.
To study the action of Afc1, Afc2, and Afc3 on natural fucosyl glycans, PGM was used as substrate. PGM has been frequently used as a model for mucin 3, 27, 41 and its O-glycans are generally highly α1,2-fucosylated 42. Each enzyme was incubated with 1% PGM and the fucose released was quantified. Afc1 was unable to release fucose from PGM, while both Afc2 and Afc3 were capable to release fucose from PGM. The enzyme activity of Afc3 (37.3 U mg−1) was 11.8-fold higher than that of Afc2 (2.91 U mg−1).
To determine the influence of pH and temperature on the hydrolytic activity of enzymes, Lea trisaccharide and Fucα1-2Gal were used as substrates of Afc2 and Afc3, respectively. Afc2 showed the highest enzyme activity at pH 7.0 and was stable at pH 5–11. The optimum temperature for enzyme activity was 40 °C, and it maintained over 75% enzyme activity below 55 °C. Afc3 showed the highest enzyme activity at pH 8.0 and was stable at pH 4–11. The optimum temperature for enzyme activity was 60 °C, and it maintained over 95% enzyme activity below 55 °C. The kinetics parameters of Afc2 and Afc3 were shown in Table 3. The Km and kcat values of Afc2 for Lex trisaccharide were determined to be 2.46 ± 0.13 mM and 23.6 ± 5.1 s−1, and those for Lea trisaccharide were 3.10 ± 0.27 mM and 26.9 ± 8.4 s−1, respectively. Thus, the kcat/Km values of Afc2 for Lex and Lea trisaccharide were 9.58 and 8.66 mM−1 s−1, respectively, showing this enzyme had similar catalytic efficiency on α1,3-linked fucose and α1,4-linked fucose. The Km and kcat values of Afc3 for Fucα1-2Gal were determined to be 6.01 ± 1.24 mM and 15.4 ± 1.1 s−1, respectively, and the kcat/Km values of Afc3 for Fucα1-2Gal was 2.56 mM−1 s−1.
| α-l-Fucosidase | Substrate | Km (mM) | kcat (s−1) | kcat/Km (mM−1 s−1) |
|---|---|---|---|---|
| Afc2 | Lea trisaccharide [Galβ1-3(Fucα1-4) GlcNAc] | 3.10 ± 0.27 | 26.9 ± 8.4 | 8.66 |
| Lex trisaccharide [Galβ1-4(Fucα1-3) GlcNAc] | 2.46 ± 0.13 | 23.6 ± 5.1 | 9.58 | |
| Afc3 | Fucα1-2Gal | 6.01 ± 1.24 | 15.4 ± 1.1 | 2.56 |
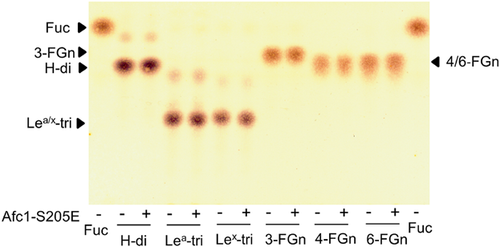
S205E mutagenesis of Afc1
Although Afc1 had the conserved GH29 α-l-fucosidase domain (amino acids 11–312), the recombinant Afc1did not show any enzyme activity on all the substrates tested. To find out whether replacement of amino acid at site 205 of Afc1 is related to the inability to hydrolyze substrates, we constructed a mutant Afc1-S205E by site-directed mutagenesis, and expressed this mutant under the same procedure as the wild-type Afc1. After nickel affinity purification, Afc1-S205E showed single band around 50 kDa on SDS-PAGE analysis, which was consistent with its calculated molecular weights (Supporting Information Fig. S1). To determine its α-l-fucosidase activity, Afc1-S205E mutant was incubated with different fucosyloligosaccharides for 8 h and each reaction was analyzed by thin-layer chromatography (TLC). As shown in Fig. 4, Afc1-S205E mutant does not release fucose from any of these fucosyloligosaccharides. Besides, this mutant could not hydrolyzed Fucα1-pNP.
Production of α-l-fucosidases of C. perfringens ATCC 13124
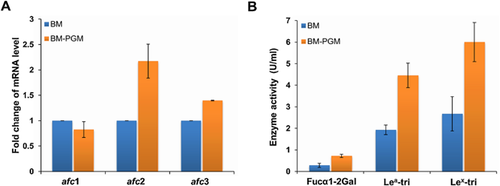
To further understand the production of these three enzymes during cell growth of C. perfringens ATCC 13124, transcription of genes in the cell and enzyme activities of α-l-fucosidases in the culture were determined. At the stationary phase of C. perfringens ATCC 13124 growth, the cell density with the presence of PGM was 18% higher than that without PGM (Supporting Information Fig. S2). With the presence of PGM, transcription of afc1 decreased slightly, while transcriptions of afc2 and afc3 increased to 2.2-fold and 1.4-fold, respectively (Fig. 5A). The activities of 1,3-α-l-fucosidase and 1,4-α-l-fucosidase of Afc2 in the culture with the presence of PGM were 6 and 4.45 U ml−1, respectively, which were about 1.2-fold higher than those without PGM (2.67 and 1.93 U ml−1, respectively). The 1,2-α-l-fucosidase activity of Afc3 in the culture with the presence of PGM was 0.72 U ml−1, which was 1.6-fold higher than that without PGM (0.28 U ml−1).
Discussion
Intestinal mucins are heavily O-glycosylated glycoproteins that cover the epithelium of gastrointestinal tract. They not only function as a barrier that separates epithelial surface from pathogens, but also provide adhesive sites and host-derived nutrients for gut microbes 43, 44. α-l-Fucosidase production is important for the degradation of mucin O-glycans by gut microbes, which facilitate the colonization of commensal or pathogenic bacteria in the intestinal tract. In this work, three α-l-fucosidases from C. perfringens ATCC 13124 were cloned, expressed, and characterized for the first time. The enzyme productions were also investigated.
Gut microbes generally produce α-l-fucosidases with different substrate specificities for the utilization of fucosyl glycans. It has been found that Bi. bifidum JCM1254 14, 20, Bi. longum subsp. infantis ATCC 15697 16, Lactobacillus casei BL23 22, and Bacteroides thetaiotaomicron VPI-5482 15 could produce two or more kinds of α-l-fucosidases. In this work, we found C. perfringens ATCC 13124 possesses three α-l-fucosidases (Afc1, Afc2, and Afc3) in its genome. Afc1 has a conserved GH29 α-l-fucosidase domain, but its enzyme activity could not be detected using all the substrates examined, including Fucα1-pNP, six kinds of fucosyloligosaccharides and a natural fucosyl substrate. It has a serine residue (Ser-205) at the putative acid/base site rather than a glutamic acid conserved in GH29-B α-l-fucosidases. However, the mutant Afc1-S205E constructed subsequently still failed to show α-l-fucosidase activity. Further study about the catalytic ability of Afc1 is under process. Afc2 specifically hydrolyzed Lex and Lea trisaccharides, indicating it is an 1,3-1,4-α-l-fucosidase. This result is consistent with the classification of Afc2 into GH29-B subfamily by sequence analysis. Five 1,3-1,4-α-l-fucosidases have been cloned from plant and bacteria to date, including AtAfc from A. thaliana, SsAfc from Streptomyces sp. 142, Blon_2336 from Bi. longum subsp. infantis ATCC 15697, BT2192 from Ba. thetaiotaomicron VPI-5482, and BbAfcB from Bi. bifidum JCM1254 11, 13-16. Afc2 is the first 1,3-1,4-α-l-fucosidase of GH29-B subfamily from human-gut pathogen. Afc3 specifically hydrolyzed Fucα1-2Gal, indicating it is an 1,2-α-l-fucosidase. Only two bacterial 1,2-α-l-fucosidases, BbAfcA from Bi. bifidum JCM1254 and Blon_2335 from Bi. longum subsp. infantis ATCC 15697, have been cloned and characterized to date 16, 20. BbAfcA specifically acts on the terminal α1,2-fucosyl linkage on linear oligosaccharides, including 2′-fucosyllactose, lacto-N-fucopentaose I, and blood group H trisaccharide. Blon_2335 has strong hydrolytic activity to 2′-fucosyllactose and Fucα1-2 Gal, as well as moderate activity on 3-fucosyllactose. The substrate specificity of Afc3 toward α1,2-fucosyl linkage is similar to those of BbAfcA and Blon_2335. Also this substrate specificity is consistent with that of the 1,2-α-l-fucosidase purified from C. perfringens in 1970 33.
A few studies have reported the production of α-l-fucosidases from gut microbes when cultured with fucosyl glycans. Sela et al. studied the transcription of α-l-fucosidases of Bi. longum subsp. infantis ATCC 15697, a probiotic that grows vigorously in HMOs 7, and found that its 1,2-α-l-fucosidase (Blon_2335) expression is induced to twofold with the presence of HMOs, while its 1,3-1,4-α-l-fucosidase (Blon_2336) expression is only slightly increased 16. Ruas-Madiedo et al. 41 compared the degradation ability of ten strains of Bifidobacterium toward mucin O-glycans and found that Bi. bifidum L22, Bi. bifidum D119, and Bi. breve NCIMB8807 possess outstanding mucolytic activity, whereas other seven strains show no or moderate mucolytic activity. When cultured with PGM as sole carbon source, the transcription of 1,2-α-l-fucosidase gene of Bi. bifidum L22 is induced over than 17-fold. Here we studied the transcription of α-l-fucosidase of C. perfringens ATCC 13124 and further determined the α-l-fucosidase activity in the culture. With the presence of PGM, the transcription of afc1 decreased slightly (about 0.2-fold), while those of afc2 and afc3 increased to 2.2-fold and 1.4-fold, respectively. Simultaneously, the activities of 1,3-1,4-α-l-fucosidase and 1,2-α-l-fucosidase in the culture became higher with the presence of PGM, about 1.2–1.6-fold higher than those without PGM. The results indicated that C. perfringens ATCC 13124 could transcribe these three α-l-fucosidases without substrate induction for the degradation of fucosyl glycans, which might be the result of long-term adaptation to the growth in the intestinal tract. In human intestine, mucin O-glycans have decreased gradient of terminal α-fucosylation from ileum to rectum, but with increased terminal α1,3/4-fucosylation. Especially, α-fucosyl residues on human colonic mucin O-glycans are mostly α1,3/4-linked 5. Therefore, the 1,3-1,4-α-l-fucosidase activity produced would enable C. perfringens ATCC 13124 to remove α1,3/4-fucosyl residues. In addition to α-l-fucosidases, C. perfringens is able to produce a series of other exo-glycosidases, including sialidases 28, α-N-acetylglucosaminidase 29, and β-N-acetylhexosaminidase 37, to release the terminal sialic acid, α-GlcNAc, and α-GalNAc from mucin O-glycans. Endo-β-galactosidases of C. perfringens are able to remove special terminal glyco-epitopes on mucin O-glycans, such as Galα1-3Gal, GlcNAcα1-4Gal, and A/B blood groups 30-32. After removal of the terminal sugar moieties, the endo-α-N-acetylgalactosaminidase from this pathogen is able to hydrolyze the glycosidic linkage between α-GalNAc and Ser/Thr to release core-1 disaccharide, core-2 trisaccharide, and core-3 disaccharide from mucins 27. The cooperation of these glycosidases will equip C. perfringens with broad degradation ability for mucin O-glycans.
In this work, it was proved firstly that C. perfringens ATCC 13124 produces 1,3-1,4-α-l-fucosidase and 1,2-α-l-fucosidase. To find out the prevalence of this character among C. perfringens species, we analyzed the presence of α-l-fucosidases in other C. perfringens strains sequenced, including SM101, str. 13, F262, WAL-14572, B str. ATCC 3626, E str. JGS1987, D str. JGS1721, C str. JGS1495, CPE str. F4969, and NCTC 8239 (Supporting Information Table S2). Ten strains have putative α-l-fucosidases sharing over 96% identity with Afc1, nine strains except for C. perfringens D str. JGS1721 have putative α-l-fucosidases sharing 77.5–99.8% identity with Afc2, and nine strains except for C. perfringens SM101 have putative α-l-fucosidases sharing over 98% identity with Afc3. Therefore, most C. perfringens strains sequenced have ortholog enzymes of Afc1, Afc2, and Afc3. Eight out of ten strains of C. perfringens have both ortholog enzymes of Afc2 and Afc3. The ability to produce these two enzymes is potentially advantageous for C. perfringens to utilize fucosylated carbon sources in human gut, especially for the degradation of mucin O-glycans that could provide continuous nutrient for the strain growth. These results were consistent with the phenomenon that C. perfringens is prevalent and stable colonized in human intestine 45, suggesting that 1,3-1,4-α-l-fucosidase and 1,2-α-l-fucosidase might contribute to the adaption of C. perfringens to the human intestinal environment.
Acknowledgments
This work was supported in parts by the Major State Basic Research Development Program of China (973 Program) (no. 2012CB822102); National High Technology Research and Development Program of China (863 Program) (no. 2012AA021504); the Key grant Project of Chinese Ministry of Education (no. 313033); and Science and Technology Development Project of Shandong Province (no. 2014GSF121006).
Conflict of interest
The authors declare that they have no conflicts of interests.