Physiological responses of soil crust-forming cyanobacteria to diurnal temperature variation
Abstract
The optimum growth of soil crust-forming cyanobacterial species occurs between 21 and 30 °C. When the temperature decreases below −5 °C, the liquid water in the cyanobacterial cells may freeze. In the natural environment, the temperature gradually decreases from autumn to winter, and the diurnal temperatures fluctuate enormously. It was hypothesized that the physiology of cyanobacterial cells changes in later autumn to acclimatize the cells to the upcoming freezing temperatures. In the present study, an incubation experiment in growth chambers was designed to stimulate the responses of cyanobacterial cells to diurnal temperature variations before freezing in late autumn. The results showed that “light” cyanobacterial soil crusts are more tolerant to diurnal temperature fluctuations than “dark” cyanobacterial soil crusts. After the first diurnal temperature cycle between 24 and −4 °C, the malondialdehyde (MDA) contents increased and the photosynthetic activity decreased. The superoxide dismutase activity increased, more extracellular polysaccharides (EPS) were secreted and the ratios of the light-harvesting and light-screening pigments decreased. With increasing numbers of diurnal temperature cycles, the MDA contents and photosynthetic activity gradually returned to their initial levels. Our results suggest that there are at least three pathways by which crust-forming cyanobacteria acclimate to the diurnal temperature cycles in the late autumn in the Hopq Desert, Northwest China. These three pathways include increased secretion of EPS, regulation of the ratios of light-harvesting and light-screening pigments, and activation of the antioxidant system. The results also indicate that late autumn is a critical period for the protection and restoration of the cyanobacterial soil crusts in the Hopq Desert.
Introduction
Cyanobacterial soil crusts are broadly distributed in various ecosystems throughout the world, especially in semiarid and arid regions. Studies have documented their pioneering and colonizing roles in these ecosystems, which include stabilizing the soil against wind and water erosion, influencing infiltration, enhancing the nutritional status of rangeland soils, and improving the survival of vascular plants stabilizing the soils 1. Cyanobacterial soil crusts are formed mainly by conglutinating and binding soil particles with cyanobacterial sheath materials and cyanobacterial filaments, respectively 2-5.
There are primarily two types of cyanobacterial soil crusts in the desert areas 6. “Light” cyanobacterial soil crusts are dominated by the cyanobacterium Microcoleus, which first colonizes bare soils and lives 1–4 mm below the soil surface 7, 8. Microcoleus lacks UV-protective pigments. During wet periods, it can glide up to the soil surface, while it returns to the depths as soils dry up 8. “Dark” cyanobacterial soil crusts usually occur in a later succession stage, after the “light” cyanobacterial soil crusts 6. They are dominated by the cyanobacteria Scytonema and Nostoc. Scytonema is a small and relatively immobile species that resides on the soil surface. It has a sunscreen pigment in its filament sheaths, which protects it from damage by ultraviolet radiation 6.
The growth of cyanobacteria in the cyanobacterial soil crusts is affected by many environmental factors including temperature 9. The optimum growth of these cyanobacterial species occurs between 21 and 30 °C 9. When the temperature decreases below −5 °C, the liquid water in the cyanobacterial filaments may freeze 10. Many studies revealed a series of thermal adaptations and acclimations of the cyanobacteria. These mechanisms may include maintenance of membrane fluidity, molecular adaptation of enzymes to compensate for the reduction on chemical reaction rates at freezing, and adaptation and acclimation of the photosynthetic electron transport and the energy balance 11-13. In natural states, the temperature fluctuates during the day, especially in the regions where cyanobacteria soil crusts are distributed. Yet, few studies have reported on how crust cyanobacteria respond to diurnal temperature variations, especially to those fluctuations that are in accordance with natural environments, except for thawing and freezing cycles 10, 11, 14.
Crust-forming cyanobacteria in warm deserts, like the Hopq Desert in Northwest China, usually experience dormant stages at higher temperatures (the temperature of the soil surface is up to 65 °C) in the summer and an optimal growth period in the pre-autumn, after which the cyanobacteria in the soil crusts freeze in the pre-winter. We hypothesize that, before the cyanobacterial filaments freeze, significant physiological changes must occur with the gradually decreasing temperature in the face of the upcoming freezing stress. In this study, an incubation experiment in growth chambers was designed to stimulate the responses of cyanobacterial filaments to diurnal temperature variations before freezing in the later autumn.
Materials and methods
Cyanobacterial strains
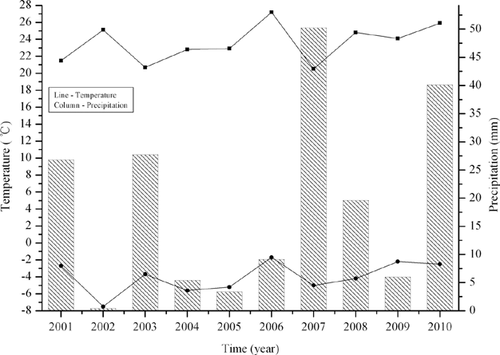
The Hopq Desert (40°21′ N, 109°51′ E) is located in the northwest of China. The mean annual air temperature in the region is 7.5 °C, with a minimum temperature of −34.5 °C and a maximum temperature of 40.2 °C. The mean annual precipitation is 377.6 mm, with 70% occurring in autumn. Fig. 1 shows the maximum and minimum air temperatures and precipitations in later autumn (October), from 2001 to 2010. Two cyanobacterial strains, Microcoleus vaginatus Gom. and Scytonema javanicum Born et Flah, were isolated from their corresponding crusts, using the moisturized ▪pls check!!▪ soil method, in September 2010. The method is as follows: Gently crush the soil clods in the soil sample to a maximum diameter of 5 mm. Place 1 g of soil in a sterile petri dish and saturate the soil with 20 ml sterile deionized water. The cyanobacterial strains can be isolated from the soil under a stereomicroscope. Selected cyanobacterial filaments are plated on sterile agar medium. Inoculate the cyanobacterial fimaments into 50 ml sterile liquid medium (BG11) after 2 months. Then, the cyanobacterial strains are enlarge-cultured successively in 500 ml, 1 l, and 5 l bottles under sterile conditions.
The cultures are grown in BG-11 medium, at 25 ± 1 °C, and illuminated with cool white fluorescent light at 40 μE m−2 s−1.
Experimental treatments
During the mid-exponential growth phase, an aliquot (5 ml; 2.3 μg/ml chlorophyll (Chl) a) of homogenized culture was applied onto a piece of filter membrane (25 mm Whatman GF/F). Eighty samples (filters) were made for each cyanobacterial strain. These samples were kept in the chamber at 24 °C under a light intensity of 40 μE m−2 s−1 for 48 h to allow the cyanobacterial filaments to acclimate to the new environment. The samples were cooled at a rate of 0.5 °C/min to −4 °C during the nighttime (for 8 h; dark), and then warmed up to 24 °C at a rate of 0.5 °C/min during the daytime (for 16 h; light intensity 40 μE m−2 s−1). The cycle of cooling and warming was repeated five times. The chamber was kept at a relative humidity of 80%, and the filaments were kept moisturized by watering with 5 ml of BG-11 medium (10 times diluted) every noon. The characteristics of the pigment content, the extracellular polymeric substance content, lipid peroxidation, antioxidant enzyme and protein contents were measured after 0, 1, 3, and 5 d at noon. Four replicates (filters) were used for each measurement for each of these characteristics. The photosynthetic activity was measured every day at noon and five replicates (filters) were used for each measurement.
Physiological characterization
Photosystem II (PSII) activity was determined from samples, using the Phyto-PAM fluorometer (Walz, Germany) 15. PSII activity was determined using the maximal photochemical efficiency (Fv/Fm) and the effective quantum yield of PSII (yield). Malondialdehyde (MDA), an end-product of lipid peroxidation, was measured using the thiobarbituric acid (TBA) method 16 with slight modifications. A 4 ml reaction containing 1.5 ml extract and 2.5 ml 2% (w/v) TBA in 20% trichloroacetic acid (TCA) was heated in boiling water for 20 min, and then quickly cooled in an ice bath. The absorbance of the supernatant was measured at 532 nm after the mixture was centrifuged at 7000 × g for 10 min. A correction for non-specific turbidity was made by subtracting the absorbance value taken at 600 nm. The MDA levels by the above assay were normalized to the total protein content.
The superoxide dismutase (SOD) activity assay was based on the method described by Giannopotitis and Ries 17. One unit of the enzyme activity was defined as the amount of enzyme required to result in 50% inhibition of the rate of nitro blue tetrazolium reduction measured at 560 nm. These calculated units of SOD activity were normalized for protein content by dividing by the amount of protein (mg) on one filter. The protein content was assayed according to Bradford 18 using bovine serum albumin as the standard. The extracellular polysaccharides (EPS) were quantified according to the phenol-sulfuric acid method of Dubois. The EPS were first extracted before using the phenol-sulfuric procedure: Scrape the cyanobacterial biomass from the filter and put it into 10 ml solution containing 25‰ saline and 100 mMol Na2EDTA, vortex mix to suspend the filaments and incubate for 15 min at 20 °C. Centrifuge at 3620 × g and remove the cyanobacterial filaments. The samples were analyzed using a spectrophotometer (Ultrospec 3000, Pharmacia Biotech, UK) at 480, 486, and 490 nm. A standard curve of the glucose solutions was obtained by plotting the glucose concentration versus the absorbance 19.
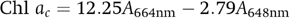

Statistics
All data were evaluated by one-way ANOVA (SPSS 13.0 for Windows). The tests applied were the least significant difference and Tukey's honestly significant difference.
Results
Photosynthetic activity of the two cyanobacterial strains
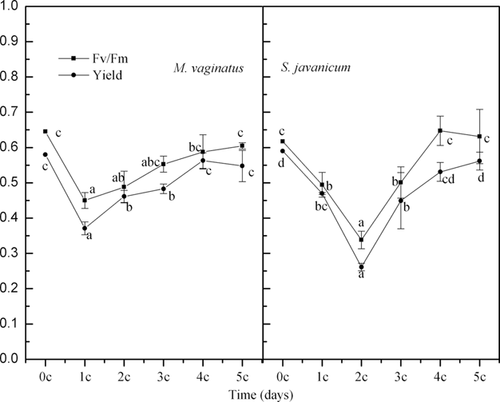
Fig. 2 shows that, with increasing numbers of diurnal temperature cycles, the maximal photochemical efficiencies (Fv/Fm) of both M. vaginatus and S. javanicum first decreased and then returned to their initial levels. Compared with M. vaginatus, the Fv/Fm of S. javanicum decreased more significantly and needed more time to recover during the diurnal temperature cycles. The effective quantum yield of the PSII (yield), which may be considered to be the photosynthetic rate per photon, changed in a manner similar to that of Fv/Fm for these two cyanobacterial strains during the diurnal temperature cycles.
MDA content and SOD activity
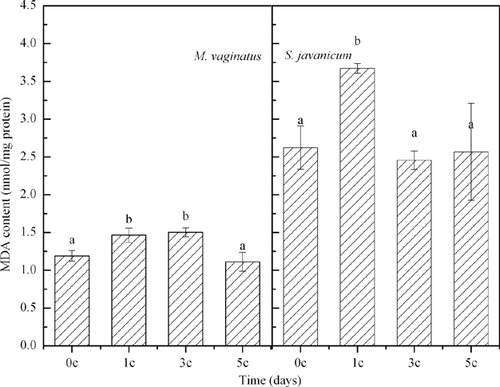
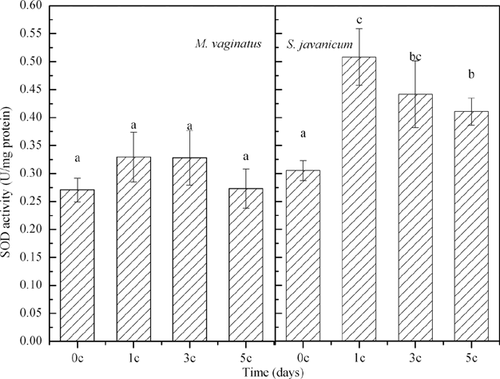
With increasing number of temperature cycles, the MDA contents in the filaments of M. vaginatus and S. javanicum first increased and then returned to their original levels (Fig. 3). The SOD activity of M. vaginatus did not change significantly during the diurnal temperature cycles. However, for S. javanicum, it increased significantly after the first diurnal temperature cycle and then decreased significantly after the third and the fifth diurnal temperature cycles (Fig. 4). At the end of the current experiment, the SOD activity of S. javanicum was still significantly higher than the original level (Fig. 4).
Pigment content
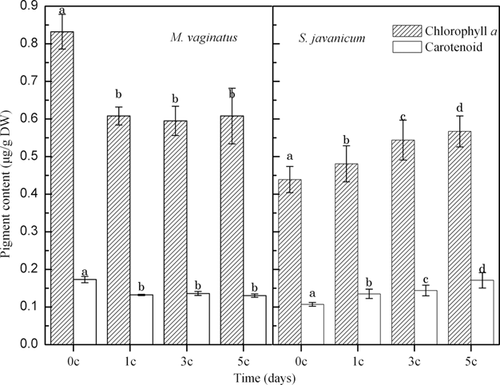
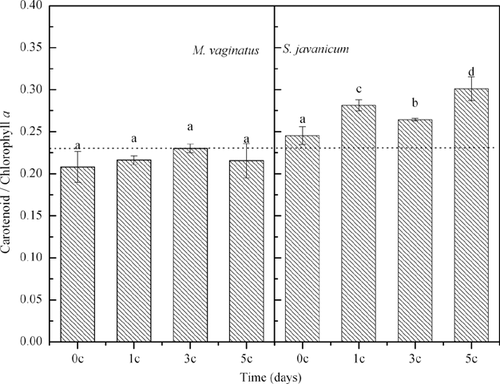
The Chl a and CAR contents of M. vaginatus decreased after one diurnal temperature cycle and then remained steady in the next diurnal temperature cycles (Fig. 5). However, the CAR/Chl a ratio of M. vaginatus did not show any changes during the diurnal temperature cycles (Fig. 5). For S. javanicum, both CAR and Chl a increased with increasing numbers of temperature cycles, as did its CAR/Chl a ratio (Figs. 5 and 6).
EPS content
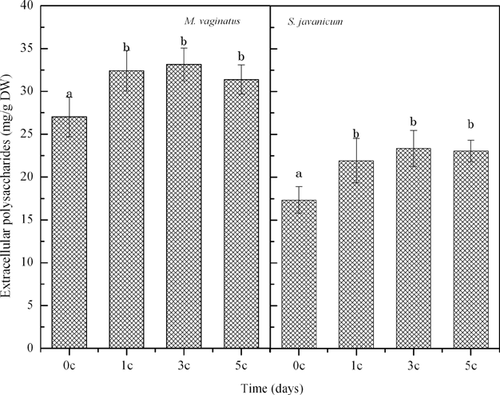
Fig. 7 shows that, after the first diurnal temperatue cycle, the EPS contents of both M. vaginatus and S. javanicum increased significantly and then remained steady after the next diurnal temperature cycles.
Discussion
Photosynthetic activity of the two cyanobacterial strains
The Fv/Fm ratio gives the potential quantum yield or potential quantum efficiency of the cyanobacteria and is thus an indicator of cyanobacterial health 21, 22. A decrease in Fv/Fm indicates the presence of stress, whereas a recovery indicates acclimation. The above-described results imply that M. vaginatus is more tolerant to diurnal temperature fluctuations than S. javanicum. This result was unexpected because S. javanicum, which is distributed in the soil surface in contrast to M. vaginatus which is located in the top soil, tends to experience more diurnal temperature fluctuations than M. vaginatus. However, these results supported the successive theory of cyanobacterial soil crusts, which states that “dark” cyanobacterial soil crusts occurred as a later succession stage after the “light” cyanobacterial soil crusts 6. The changes of yield confirmed the changes in the photosynthetic activity of the two cyanobacterial strains in relation to the diurnal temperature cycles.
MDA content and SOD activity
Lipid peroxidation is a good indicator of oxidative stress in cells 16, so it could be clearly concluded that the diurnal temperature cycles resulted in oxidative stress in the cyanobacterial cells. SOD belongs to the group of enzymes that deal with oxidative stress caused by oxygen itself or by reactive oxygen species (ROS). SOD prevents oxidative damage to the cell, such as lipid peroxidation 23. Our results demonstrated a change in the SOD activity of S. javanicum during the temperature cycles, indicating that the antioxidant system of the cell was initiated at the first diurnal temperature cycle, and then these enzymes helped to scavenge the ROS.
Pigment content
Analysis of the pigment content showed a greater flexibility of S. javanicum relative to M. vaginatus. Differential changes in pigment content can be explained by photoinhibition by photosynthetically available radiation. At low temperatures, cyanobacteria are more susceptible to photoinhibition because of the decreased use of photochemically generated reductants; hence, an increased proportion of excess light is available to PSII 11, 24. Low temperatures are likely to amplify the effects of photoinhibition by depressing all enzymatic processes, including the rate of repair of damage to PSII 25. CAR reduces the damaging effect of high-intensity ▪pls check!!▪ light by screening excess light and by quenching triplet Chl and singlet oxygen excitation 11, 26, 27. Our results agree with those of previous studies which suggest that cyanobacteria acclimate to lower temperatures by regulating the ratios of their light-harvesting and light-screening pigments 13, 28.
EPS content
EPS protect cyanobacterial cells from stress in extreme habitats and from other harmful conditions 3, 12. An increase in EPS indicates that the diurnal temperature cycles could induce the synthesis and secretion of EPS.
Acclimation of crust-forming cyanobacteria to diurnal temperature variations
The results described above show that the cyanobacterial physiology was significantly influenced by the diurnal temperature variations. After the first diurnal temperature cycle, the MDA contents increased and photosynthetic activity decreased, whereas SOD activity increased, more EPS were secreted, and the ratios of light-harvesting and light-screening pigments increased. Subsequently, with increasing numbers of temperature cycles, the MDA contents and photosynthetic activity gradually returned to their initial levels. Our results suggest that there are at least three pathways by which crusts-forming cyanobacteria acclimate to the diurnal temperature variations in the late autumn in the Hopq Desert, Northwest China. These three pathways entail increased EPS secretion, regulation of the ratios of light-harvesting and light-screening pigments, and activation of the antioxidant system. Our results showed that all these pathways were activated quickly within 24 h, which means continuous 24 h soil moisturizing is adequate for cyanobacteria to activate these pathways to acclimatize to the diurnal temperature cycles and the next freezing and thawing cycles. However, more moisturizing time is needed for absolute recovery of the photosynthetic activity. Our results also showed that the function of the EPS pathway is limited, because it did not change after the first diurnal temperature cycle.
Implications
Our results show that “light” cyanobacterial soil crusts were more tolerant to diurnal temperature fluctuations than “dark” cyanobacterial soil crusts. Compared with “light” cyanobacterial soil crusts, “dark” cyanobacterial soil crusts can activate the regulation of the ratios of light-harvesting and light-screening pigment pathways to acclimate to the diurnal temperature variations, in addition to the increased EPS secretion and activation of the antioxidant system. The pigment regulation pathway may be attributed to the fact that “dark” crust-forming cyanobacteria are distributed in the soil surface and not in the topsoil.
In the protection and restoration of cyanobacterial soil crusts, most studies have focused on environmental factors, such as elevation, soil nutrients, topography, climate, vascular plant community structure, and ecological gradients 6. However, few studies reported on the seasonal changes of cyanobacterial soil crusts. A critical period in a typical desert may exist for the protection and restoration of cyanobacterial soil crusts, such as in late autumn in the Hopq Desert, Northwest China. The predicted warming or changes in the precipitation pattern will influence the cyanobacterial soil crusts more markedly in this critical period than in any other period.
Conclusions
The physiologies of two dominant crusts-forming cyanobacteria are significantly influenced by the diurnal temperature variations before their cells get frozen. Ours results show that “light” cyanobacterial soil crusts are more tolerant to diurnal temperature fluctuations than “dark” cyanobacterial soil crusts. The cyanobacterial cells initiated three pathways to acclimate to the diurnal temperature variations. These three pathways included increased EPS secretion, regulation of the ratios of light-harvesting and light-screening pigments, and activation of the antioxidant system. The cyanobacteria could initiate all these pathways within 24 h. However, more moisturizing time is needed for absolute recovery of the photosynthetic activity.
Acknowledgements
The study was supported by the Natural Science Foundation of China (31000061) and the State Key Laboratory of Freshwater Ecology and Biotechnology of China (2010FB16).




