Isolation and characterization of a phenol-degrading Rhodococcus sp. strain AQ5NOL 2 KCTC 11961BP
Abstract
In this work, we report on the isolation of a phenol-degrading Rhodococcus sp. with a high tolerance towards phenol. The isolate was identified as Rhodococcus sp. strain AQ5NOL 2, based on 16S rDNA analysis. The strain degraded phenol using the meta pathway, a trait shared by many phenol-degraders. In addition to phenol biodegradation, the strain was also capable of degrading diesel. Strain AQ5NOL 2 exhibited a broad optimum temperature for growth on phenol at between 20 °C and 35 °C. The best nitrogen sources were ammonium sulphate, glycine or phenylalanine, followed by proline, nitrate, leucine, and alanine (in decreasing efficiency). Strain AQ5NOL 2 showed a high tolerance and degradation capacity of phenol, for it was able to register growth in the presence of 2000 mg l−1 phenol. The growth of this strain on phenol as sole carbon and energy source were modeled using Haldane kinetics with a maximal specific growth rate (μmax) of 0.1102 hr−1, a half-saturation constant (Ks) of 99.03 mg l−1 or 1.05 mmol l−1, and a substrate inhibition constant (Ki) of 354 mg l−1 or 3.76 mmol l−1. Aside from phenol, the strain could utilize diesel, 2,4-dinitrophenol and ρ-cresol as carbon sources for growth. Strain AQ5NOL 2 exhibited inhibition of phenol degradation by Zn2+, Cu2+, Cr6+, Ag+ and Hg2+ at 1 mg l−1.
Introduction
Phenol is a very toxic compound, not only to humans but many microorganisms as well 1. However, several microorganisms can tolerate phenol and use it as a carbon and energy source 2. The aerobic biodegradation of phenol by microorganisms has long been an object of intense research globally. Its biodegradation has been studied with mainly Pseudomonas species 3-8. Other microorganisms that have been reported to degrade phenol include fungal species such as Phanerochaete chrysosporium 9, Hormodendrum bergeri, Fusarium oxysporum and Aspergillus flavus var. coulmnaris 10, Penicillium chrysogenum 11, yeast species such as Candida tropicalis 12, Aureobasidium pullulans FE13 13 bacterial species such as Bacillus brevis 14, Alcaligenes sp. 15, Ochrobactrum sp. 16, Rhodococcus species 17-27 and algae species 28, 29. Among these microorganisms, Rhodococcus spp. stand out as promising phenol and phenolics waste degraders this genus has the ability to degrade a multitude of other recalcitrant xenobiotics aside from phenol 17-27. Their versatile metabolic pathway and known resistance to xenobiotics toxicity allowed them to mineralize phenol in the presence of other toxic compounds normally associated with phenolic wastes 18.
In Malaysia, the Department of Environment 30 has reported that 1,709 metric tonnes of phenol and phenolic wastes were generated in 2005. The National Guidelines for Raw Drinking Water Quality had stated that the maximum permissible limit for phenolic compounds is 0.002 mg l−1, and many groundwater wells in Malaysia have phenolic levels exceeding this limit 30, thus indicating a widespread pollution due to phenol and phenolic compounds. In response to this, it is important to isolate efficient phenol-degrading microbes for use in bioremediation. In this work, we report on the isolation of a phenol-degrading Rhodococcus sp. from soil that could register growth on phenol as high as 2000 mg l−1.
Materials and methods
All chemicals were reagent grade; phenol was obtained from Sigma–Aldrich (Steinheim, Germany). Unless otherwise specified, all experiments were conducted in triplicate at room temperature (27 ± 2 °C) under sterile conditions.
Enrichment, isolation and selection of bacterial strains
Soil samples were collected from phenol contaminated sites in Malaysia from October to December 2007. The soils were contaminated with phenolic wastes from industries such as textiles, printing, dyeing as well as pesticides and herbicides. The isolation procedure was as follows: 3 g of soil sample was mixed in 10 ml of sterile nutrient broth (3.0 g l−1 peptone, 5.0 g l−1 beef extract) and incubated at room temperature (RT) on an orbital shaker (Certomat R, USA) at 150 rpm for 24 h. Bacterial strains were purified by subculturing in mineral salt medium (MSM) containing g l−1): K2HPO4, 0.4; KH2PO4, 0.2; NaCl, 0.1; MgSO4, 0.1; MnSO4 · H2O, 0.01; Fe2(SO4) · H2O,0.01; NaMoO4 · 2 H2O, 0.01; (NH4) · 2 SO4, 0.4 in a 250 ml conical flask supplemented with 0.5 g l−1 phenol 31. The phenol was filter-sterilized before added to the medium. The cultures were incubated at RT with shaking at 150 rpm. After five transfers, serial dilutions of the culture were prepared and spread onto mineral medium agar plates supplemented with phenol and incubated at RT for 4 d. In order to obtain pure cultures, a small portion of an individual colony exhibiting distinct colonial morphologies was picked and streaked out on fresh agar plates supplemented with 0.5 g l−1. Routine cultivation was done on phenol agar plates.
As a result of single colony purification, several bacterial strains were obtained. Each isolate was incubated in MS media at 1 × 106 cells ml l−1 initial concentration containing 500 mg l−1 phenol to compare the efficiency of phenol biodegradation by the isolates. Isolate H3 degraded phenol the fastest and was chosen for further studies.
Identification of phenol-degrading Rhodococcus
Several tests were carried out to identify isolate H3. Identification was done mainly on the basis of its cultural and morphological characteristics (micromorphological and macromorphological features) on Nutrient agar, Gram staining, BiologTM Identification System and followed by sequence analysis of the molecules in particular, 16S rRNA. Oxidase activity was monitored through the application of 1% tetramethyl-p-phenylenediamine disks to colonies on TSA plates and observing the formation of a violet color within 10 s. Catalase activity was determined by observing the evolution of O2 upon the addition of 3% H2O2 to single colonies on TSA plates. The 16S rRNA analysis system involves genomic DNA extraction, amplification of the genomic DNA using polymerase chain reaction (PCR) and sequencing of the 16S genes. The genomic DNA was extracted by alkaline lysis using DNeasy® Blood & Tissue Purification KitTM (Qiagen, USA) according to the manufacturer's instructions. The amplification reaction was performed with universal primer 5′-aga gtt tga tca tgg ctg ag-3′ and 5′-acg gtt acc ttg tta cga ctt-3′ synthesized by 1st BASE Sdn. Bhd. (Malaysia). The polymerase chain (PCR) mixture consisted of 2.5 μl PCR buffer (1 ×), 2 μl MgCl2 (25 mM), 0.7 μl dNTP (10 mM), 1.5 μl (100 μM) of each primer solutions, 1 μl Taq Polymerase (Qiagen, USA) and 1 μl of template DNA. The DNA was amplified using a thermal cycler (Biometra, Gottingen, Germany). After an initial denaturation at 94 °C for 4 min, the DNA was amplified during 30 cycles of 94 °C for 1 min, 58 °C for 2 min followed by a final extension at 72 °C for 10 min. Cycle sequencing was subsequently performed with the Big Dye terminator kit (Perkin-Elmer Applied Biosystems) as recommended by the manufacturer. The resultant 1411 bases were compared with the GenBank database using the Blast server at NCBI (http://www.ncbi.nlm.nih.gov/BLAST/). The 16 s rRNA ribosomal gene sequence for this isolate have been deposited in GenBank under the accession number FJ160278. The strain was deposited in the Korean Collection for Type Cultures as KCTC 11961BP.
Phylogenetic analysis
A multiple alignment of 20 16S rRNA gene sequences which closely matched strain AQ5NOL 2 were retrieved from the GenBank and were aligned using clustal_W. A phylogenetic tree was constructed by using PHYLIP, version 3.573 (J. Q. Felsenstein, PHYLIP – phylogeny inference package, version 3.573, Department of Genetics, University of Washington, Seattle, WA. (http://evolution.genetics.washington.edu/phylip.html), with Acinetobacter calcoaceticus as the outgroup in the cladogram. Evolutionary distance matrices for the neighbour-joining/UPGMA method were computed using the DNADIST algorithm program. The model of nucleotide substitution is those of Jukes and Cantor 32. Phylogenetic tree (Fig. 2) was inferred by using the neighbour-joining method of Saitou and Nei 33. With each algorithm, confidence levels for individual branches within the tree were checked by repeating the PHYLIP analysis with 1000 bootstraps 34 by the SEQBOOT program in the PHYLIP package. Majority rule (50%) consensus trees were constructed using the CONSENSE program 35 and the tree was viewed using TreeView 36.
Elucidation of phenol biodegradation pathway
There are two major pathways for phenol biodegradation; ortho- and meta-pathways. In the meta-ring-cleavage pathway, the metabolite 2-hydroxymuconate semialdehyde can be colorimetrically detected in the supernatant due to its intense absorption at 375 nm 37. The strain was grown for 24 hr in the above 100 ml MS media supplemented with 500 mg l−1 phenol on an orbital shaker at 150 rpm at room temperature. The culture was then centrifuged at 15,000 g for 10 minutes and the supernatant was scanned from 200 to 500 nm (Cintra 5) in a quartz cuvette with freshly prepared MSM as the baseline correction.
Statistical analysis
All data were analyzed using Graphpad Prism version 3.0 and Graphpad InStat version 3.05. Values are means ± SE. Comparison between groups was performed using a Student's t-test or a one-way analysis of variance with post hoc analysis by Tukey's test 38. P < 0.05 was considered statistically significant.
Results
Identification of phenol-degrading bacterium
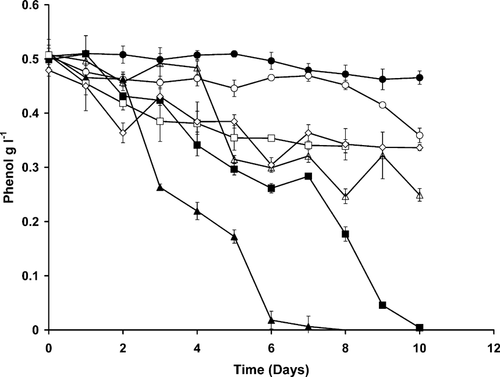
A comparison of the six bacterial isolates showed that isolate H3 was the most potent phenol degrader taking approximately six days to completely degrade 500 mg l−1 phenol. The next best isolate was SA15 which needed 10 days to complete the phenol degradation. Isolate G1 was the least potent with only 30% of the phenol being degraded after the incubation period has ended (Fig. 1). Isolate H3 was chosen for further studies.
Identification of strain H3
Isolate H3 is an aerobic gram positive bacterium. The isolate was catalase positive and oxidase negative. It was a short rod, non-motile, non-spore forming and exhibited variable morphology. Its texture was rugose and radiate, undulate in margin and umbonate in elevation. After a prolonged incubation, all colonies developed orange pigmentation. These characteristics are typical of Rhodococcus colonies 39. The strain was identified as Rhodococcus rhodocrous with a 0.532 similarity using the BiologTM identification system. Aside from phenol, isolate H3 could utilize diesel, 2,4-dinitrophenol and ρ-cresol as carbon sources for growth while chlorinated aromatic compounds such as pentachlorophenol and 2- and 4-chlorophenol were not utilized (Table 1).
| Substrate | Tested concentration mg l−1 | growth |
|---|---|---|
| diesel | 500 | + |
| 2,4-dinitrophenol | 100 | + |
| ρ-cresol | 100 | + |
| pentachlorophenol | 100 | − |
| 2-chlorophenol | 100 | − |
| 4-chlorophenol | 100 | − |
- +, growth; − no growth
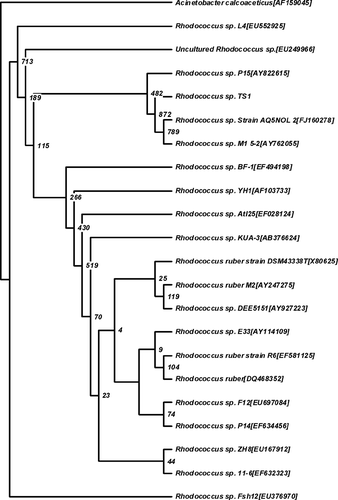
The nucleotide blast search resulted in more than 50 hits with 98% identity. All of these sequences were from the genus Rhodococcus. A phylogenetic tree was constructed based on the alignment of 16s rRNA se-quences to another 20 Rhodococcus strains available in the GenBank. A bootstrap value of 78.9 was obtained linking Isolate H3 to Rhodococcus sp. M1 5-2 (Fig. 2). Thus, based on the morphological, biochemical, and molecular results, isolate H3 was assigned albeit tentatively as Rhodococcus sp. strain AQ5NOL 2.
Optimum temperature and pH for bacterial growth
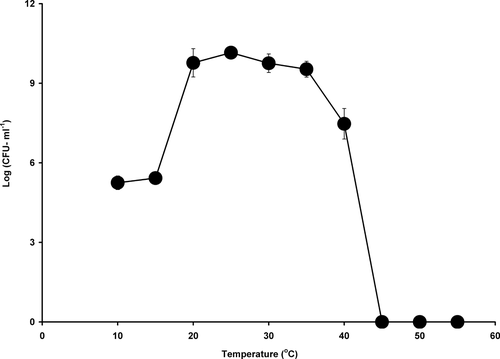
To determine the optimum temperature for bacterial growth, strain AQ5NOL 2 was incubated with 500 mg l−1 phenol at temperatures of 10, 15, 20, 25, 30, 35, 40, 45, 50, and 55 °C. Strain AQ5NOL 2 exhibited a remarkably broad optimum temperature for growth on phenol at between 20 °C and 35 °C (Fig. 3). To determine the optimum pH, an overlapping buffer system consisting of acetate buffer (pH 4.0, 4.5, 5.0, 5.5 and 6.0), phosphate buffer (pH 6.0, 6.5, 7.0 and 7.5), and Tris-HCl buffer (pH 7.0, 7.5, 8.0, 8.5 and 9.0) were used at 50 mM. The optimum pH for growth with strain AQ5NOL 2 was between pH 7 and 8.0 (data not shown).
Effect of carbon and nitrogen sources on growth
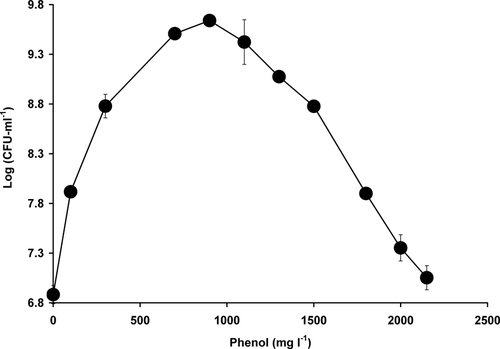
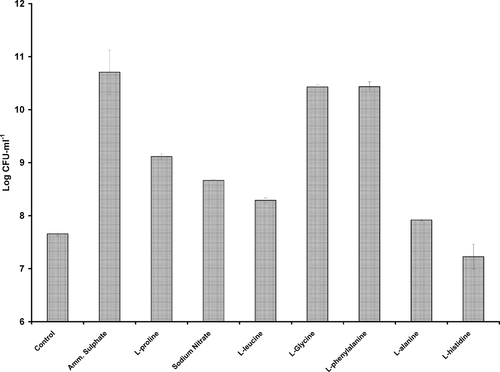
Strain AQ5NOL 2 showed a high tolerance and degradation capacity of phenol for it was able to register growth in the presence of 2000 mg l−1 phenol of about 0.5 log unit (Fig. 4). At this elevated concentration, when supplemented with 0.1% glucose (w/v), growth was doubled after 7 days of incubation at room temperature. Growth at this concentration showed a lag phase of 12 hours while no lag phase was observed when supplemented with glucose. Optimal phenol concentration that supported growth was 900 mg l−1. At 900 mg l−1, the best nitrogen sources were ammonium sulphate, glycine, and phenylalanine with no significant difference (p > 0.05) amongst them in terms of growth on phenol. This is followed by proline, nitrate, leucine, and alanine, with decreasing ability in terms of growth on phenol. L-histidine did not support growth (Fig. 5). The optimum concentration of ammonium sulphate was found to be 0.4% (w/v).
Effect of heavy metals on phenol degradation
Strain AQ5NOL 2 exhibited inhibition of phenol degradation by at least 50% by Zn2+, Hg2+, Cr6+, Ag+ and Cu2+ at 1 mg l−1 (Table 2).
| Heavy metals | % Degradation of phenol |
|---|---|
| Control | 99.93 ± 0.06 |
| As5+ | 99.78 ± 0.17 |
| Zn2+ | 47.59 ± 0.48 |
| Cr2+ | 9.91 ± 7.37 |
| Cd2+ | 99.73 ± 0.06 |
| Cu2+ | 48.72 ± 0.10 |
| Ni2+ | 99.77 ± 0.12 |
| Ag+ | 38.88 ± 1.54 |
| Pb2+ | 99.76 ± 0.10 |
| Hg2+ | 30.88 ± 5.529 |
Growth and degradation kinetics
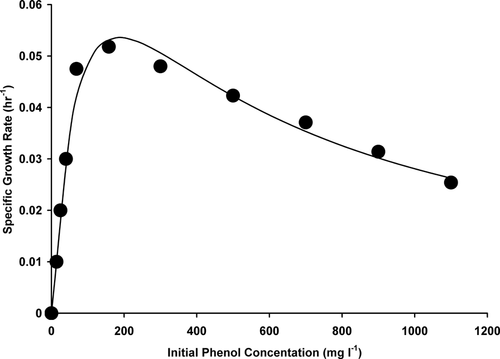
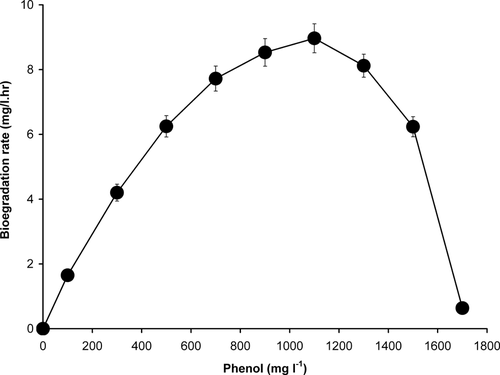
The specific growth rate for each concentration of phenol was calculated from the slope of the linear logarithmic plot of optical density (OD 600 nm) against time. The specific growth rates are then plotted against substrate concentration (Fig. 6). The value for the maximum growth rate (μmax), the inhibition constant Ki and the half saturation constant Ks, defined as the sub-strate concentration that equals to half the maximum growth rate in the Haldane model 25, 26 were obtained using a nonlinear regression analysis available from Graphpad prism. The data fitted the Haldane's model giving a curve with a correlation coefficient of 0.98. The constants derived from the Haldane equation were μmax = 0.1102 hr−1, Ks = 99.03 mg l−1 or 1.05 mmol l−1 and Ki = 354 mg l−1 or 3.76 mmol l−1. The biodegradation rate exhibited by this strain increases with an increase in initial phenol concentration reaching a maximum degradation rate of 8.97 mg l−1 hr−1 at an initial phenol concentration of 1100 mg l−1. At this concentration, the growth rate was severely inhibited (Fig. 7). The strain still exhibited the capability to degrade phenol at an initial phenol concentration of 1500 mg l−1. Almost complete cessation of degradation was observed at an initial phenol concentration of 1700 mg l−1.
Discussion
A phenol-degrading Rhodococcus sp. has been isolated from soil. Upon growth on phenol as the sole carbon source, the UV spectrum of the culture supernatant exhibited an intense absorption at 375 nm, indicating the presence of 2-hydroxymuconate semialdehyde, a product for the meta-degradation of phenol. Studies on phenol degradation by other Rhodococcus species indicates either solely ortho-fission such as in Rhodococcus coprophilus 22, Rhodococcus opacus strain 1G 25 or a mixed of ortho- and meta-fissions as in Rhodococcus sp. DGUM 19 and Rhodococcus sp. strain DK17 20.
Rhodococcus belongs to the group of high-G + C, mycolic acid-containing actinomycetes with the ability to survive in resource-limited situations 18. Gordonia, Nocardia and Mycobacterium are three other GC-rich actinomycetes that are closely related to Rhodococcus 40. Rhodococci possess the ability to degrade a large number of organic compounds including short and long chain alkanes, aromatic, heterocyclic, and polycyclic aromatic compounds. This remarkable property is attributed to their ability to acquire an enormous catabolic genes and their robust cellular physiology 18, 40. Rhodococcus is suitable for bioremediation because it can endure starvation conditions and the absence of substrate repression in the presence of more easily degradable carbon sources 40.
The strain exhibited broad optimal temperatures for growth on phenol and tolerance of a wide range of temperatures is an advantage especially when the microorganism is to be used in a commercial form to cater for a wide range of geographical areas. Many phenol-degraders reported in the literature have a limited range of optimum temperatures supporting growth 2-4, 8, 12, 13, 17-27. On the other extreme, the psychrophilic Pseudomonas putida and a yeast strain could degrade phenol at the optimal temperature of 10 °C 41, while a thermophilic bacterium (Bacillus stearothermophiles) has also been used to effectively degrade phenol at 50 °C 42.
The highest growth at up to 100 hours of incubation was achieved using 900 mg l−1 phenol. Various strains that can tolerate and mineralize phenol at initial concentrations above 1000 mg l−1 have been isolated, most of them during the last 5 years. Very few phenol-degrading bacteria registered growth at phenol concentrations higher than 1500 mg l−1 43. Many phenol-degrading strains exhibited lag phases at phenol concentrations below 500 mg l−1 2-4, 8, 12, 13, 17-27. It has been suggested that the presence of toxic xenobiotics such as phenol had resulted in changes in the fluidity of the Rhodococcus cell envelope and this is probably an important mechanism for the resistance of Rhodococcus against phenol 18, 40. For further optimization studies, we opt for ammonium sulphate as it is found to be the optimum nitrogen source for strain AQ5NOL 2. Ammonium sulphate is also easily available in bulk quantity and is economical for future bioremediation works. Although a majority of phenol-degrader utilize ammonium sulphate as an optimum nitrogen source, an Actinobacillus species was found to optimally use glycine as a nitrogen source 44.
It has been shown that at low concentration of phenol, growth on phenol followed a classical Monod kinetics 5. However, higher concentrations of phenol is inhibitory to growth and the most utilized model for estimating kinetic parameters of growth is the Haldane's model 3-5, 9. The maximum specific growth rate is lower than many of the reported pure culture of phenol-degrading bacterium ranging from 0.15 to 0.542 and the Ks value is also higher 43, 44. These values are consistent with the slow-growing properties of the genus Rhodococcus 17-27. On the other hand the Ki value is moderately high for a bacterial pure culture 43. The highest Ki reported for a bacterial pure culture is Bacillius brevis at 2437 mg l−1 14 while the highest Ki reported so far is at 3600 mg l−1 in the yeast Candida albicans 45. The high inhibition constant indicates a better adaptation and higher tolerance towards phenol.
Although the specific growth rate was severely inhibited in this strain at high phenol concentrations, degradation continues as evident from the degradation rate profile, indicating that the strain is directing its cellular machinery for phenol detoxification instead of growth. The biodegradation rate of bacterial pure cultures are generally lower than mixed culture with maximal values ranging from 0.5 to 10 mg l−1 hr−1 at initial phenol concentrations ranging from 50 to 400 mg l−1 46. Bacterial mixed cultures and phenol-degrading yeasts have higher maximum degradation rates of between 5 to 24.5 mg l−1 hr−1 at initial phenol concentrations of between 300 to 400 mg l−1 13, 43, 47. Although the maximal growth rate for this strain is low, the strain is able to exhibit maximal degradation at high initial concentrations of phenol reflecting an efficient cellular detoxification machinery of the Rhodococcus genus.
In conclusion, we have succeeded in isolating a phenol-degrading Rhodococcus sp. with a high tolerance towards phenol. The isolate was identified as Rhodococcus sp. strain AQ5NOL 2 based on 16S rDNA analysis. The strain degraded phenol using the meta pathway, a trait shared with other phenol-degraders. In addition to phenol biodegradation, the strain was also able to exhibit growth on diesel and several other aromatic compounds. The strain exhibited a broad optimum temperature and high phenol tolerance but is susceptible to several heavy metals.
Very few studies have addressed the effect of heavy metals on phenol degradation. Studies have shown that phenol biodegradation is suppressed by Cu2+, Cd2+, Hg2+, Cr6+, and Ag+ 48, 49. The sensitivity of this strain to these heavy metals indicates that future isolation of phenol-degrading microbes should take into account heavy metals' tolerance as phenolic wastes often contain metallic pollutants 50. Otherwise, a bioremediated site must be supplemented with chemicals that could reduce metal bioavailability and mobility in metal-contaminated sites such as thiosulphate, sulphur, calcium carbonate, phosphate, manganese oxide, and magnesium hydroxide 51, 52.
The Haldane's model was used in describing the growth kinetics of this strain under various initial phenol concentrations and the results indicated good tolerance and degradation towards high concentration of phenol. Due to the strain's high tolerance towards phenol, its ability to grow on several xenobiotic compounds, known metabolic diversity and robustness of the Rhodococcus genus, this strain is a good candidate for the remediation of phenol wastewaters that usually harbor other toxic xenobiotics as well. Work is underway to study the biodegradation kinetics of this strain in its immobilized form, and preliminary results have shown an increased performance in terms of degradation at higher phenol concentrations and tolerance towards heavy metals.
Acknowledgement
This project was funded by the Ministry of Science, Technology and Innovation, Malaysia (MOSTI) under NBD top down Project No: 07-05-16-MGI-GMB05 and the National Science Fellowship (NSF).




