High-density mapping in catheter ablation for atrial fibrillation in Asia Pacific region: An observational study
Abstract
Background
Few clinical studies of atrial fibrillation (AF) have focused on Asian patients; data are lacking on current mapping and ablation strategies in the Asia Pacific region (APAC).
Objective
The HD Mapping Observational Study (NCT04022954) was designed to characterize electroanatomic mapping (EAM) with market-released high-density mapping (HDM) catheters in subjects with AF in APAC.
Methods
Subjects undergoing HDM and indicated for radiofrequency ablation (RFA) to treat AF were prospectively enrolled in APAC. Data included mapping strategy and ablation targets. EAM was performed using one of two commercially available HDM catheters (Advisor™ HD Grid, Sensor Enabled™, Abbott [GRID] or Inquiry™ AFocus II™ Double Loop, Abbott [DL]). Procedure-related adverse events were collected.
Results
Two hundred subjects were enrolled at 15 centers: 164 with symptomatic paroxysmal (PAF) and 36 with symptomatic persistent (PersAF) AF for de novo ablation. GRID and DL were used in 186 and 14 cases, respectively. All subjects underwent voltage mapping, with conservative thresholds (low voltage ≤0.5 mV and very low voltage/electrical scar ≤0.1 mV) used in 60.2% and 35.4% of maps, respectively. Focal impulses, rotors, complex fractionated electrograms, and other substrate targets were each searched for in <3% of subjects. Median time to generate a map was 9.0 (Q1: 5.0, Q3: 13.0) minutes. Ablation strategy included pulmonary vein (PV) isolation in all, and non-PV triggers in 75/200 (37.5%) subjects. Five serious adverse events were reported.
Conclusions
The study demonstrated an efficient strategy with the feasibility and safety of using HDM during AF ablation procedures in APAC.
1 INTRODUCTION
Atrial fibrillation (AF) is the most common sustained arrhythmia and poses a significant burden to patients. It represents a significant risk factor for cardiogenic thromboembolism leading to ischemic stroke and substantial disability.1
In patients with AF, early intervention with rhythm control therapies, as opposed to standard care, demonstrated a decreased risk of combined outcomes.2 Compared to usage of antiarrhythmic drugs (AAD) alone, catheter ablation lowers the morbidity and mortality.3
Pulmonary vein isolation (PVI) remains the cornerstone of treatment. However, apart from this initial set of lesions, the clinical outcomes from various strategies have shown variations in both paroxysmal (PAF) and persistent (PersAF) AF.4 After PVI, areas in left atrium (LA) with low voltage areas (LVAs), corresponding to fibrotic regions, have been reported as predictors of recurrent AF.5, 6 Substrate based ablation strategies following PVI may provide a chance to improve long-term outcomes,7, 8 but rely heavily on correct identification and characteristics of scars. Therefore, left atrial voltage mapping is performed to identify atrial arrhythmogenic substrates and is important to assess the extent of LVAs.
High-density mapping (HDM) has been employed to differentiate potential triggers and instigators of AF, which encompass regions with low voltage, fibrosis, local reentry, dyssynchrony, and rotational drivers.9
Although prevalence of AF has been known to be lower in Asia than in Western countries; the reported prevalence of AF in Asia Pacific countries varies from 0.49% to 5.4%. The incidence of AF has risen due to a growing elderly population and improved detection methods.10 There is a scarcity of clinical studies that have specifically centered on Asian patients with AF, and there is an absence of data regarding the mapping and ablation techniques presently employed in the Asia Pacific region (APAC). Therefore, this study was created to assess the effectiveness of using electroanatomic mapping (EAM) with commercially available HDM catheters (HDMC) among individuals with AF in the real-world environment of the Asian population.
2 METHODS
2.1 Study design
The High Density Mapping Observational Study (NCT04022954) was designed to quantify and characterize outcomes of radiofrequency ablation (RFA) after, and the utility of, EAM with the market-released HDMC Inquiry™ AFocus II™ Double Loop (DL, Abbott Laboratories) and Advisor™ HD Grid, Sensor Enabled™ (GRID, Abbott Laboratories) with the EnSite Precision™ Cardiac Mapping System and the EnSite™ AutoMap module in subjects with AF in the real-world environment of the Asian population. Figure 1 displays HDMC.
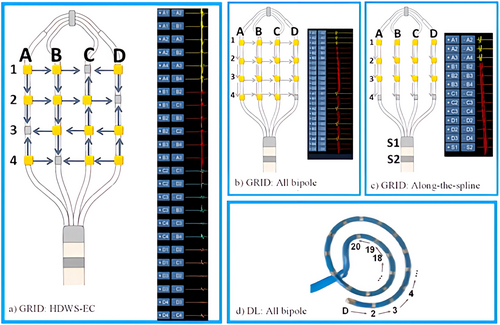
Abbott Laboratories designed and sponsored the study. Local Ethics Committee approved the study at each investigational site. Sponsor executed data collection, monitoring, and principal data analysis in partnership with publication committee. This clinical investigation was conducted in accordance with the study protocol, Declaration of Helsinki, ISO 14155:2011 standards and appropriate local legislations.
2.2 Population
Patients with documented symptomatic PAF or PersAF undergoing HDM with the specified catheters and indicated for RFA were prospectively enrolled in APAC from 2019 to 2020. PAF was defined as AF that terminates spontaneously or with intervention within 7 days of onset; PersAF as continuous AF sustained beyond 7 days.3 Key exclusions were previous ablation/surgery in LA, and presence of left atrial appendage occluder. Complete eligibility criteria are provided in Table S1. Patients provided written informed consent and were considered enrolled once HDMC was inserted.
2.3 Procedures
AAD and anticoagulation medications were documented at baseline and procedure. Pre-procedure thrombus assessment was required and performed per investigator's standard-of-care (SOC). HDM and RFA procedure was performed per SOC. Instructions for use were followed for all devices, including intraprocedural anticoagulation requirements. HDMC was used with EnSite and AutoMap to create baseline model and voltage map of LA. Additional maps were created per SOC. For GRID, use of HD Wave Solution™ electrode configuration (HDWS-EC) was recommended as described previously11, 12 (Figure 1(A)). Care was taken to assess HDMC contact with cardiac wall (e.g., catheter flex on fluoroscopy, adjusting interior projection setting on EnSite) while mapping. Percentage of time with sufficient contact was defined as all (100%), most (91%–99%) some (51%–90%), occasional (11%–50%) or rare (0%–10%). Frequency of ectopic beats or other arrhythmias noted as a result of mapping with HDMC was rated on a scale from 1 to 5 (1—None, 2—Minimal, 3—Half the time, 4—Most of the time, 5—Contact tachycardia). Specific ablation techniques/targets such as PVI, non-PV ablation, complex fractionated atrial electrograms (CFAE), and superior vena cava (SVC) isolation were used per SOC and documented, however no specific technique was mandated in this observational study. Acute procedural success (APS) was defined as termination of AF to sinus rhythm, or non-inducibility (using SOC procedures) of the clinical arrhythmia after ablation. Cardioversion was allowed prior to inducibility check. At the end of the procedure, the inducibility test was performed under isoproterenol infusion at a rate of 2–4 mcg/min, determined based on the operator's preference and the patient's condition, with stimulation applied via the coronary sinus (CS). All stimulation protocols included burst stimulation at decremental cycle lengths until atrial refractoriness was achieved.
Mapping efficiency (expressed as used mapping points per minute) and procedure, radiofrequency, and fluoroscopy times were collected.
2.4 Follow-up and arrhythmia monitoring
Subjects were seen at baseline, procedure, pre-discharge, 6 and 12 months, and during unscheduled visits when arrhythmia recurrence was suspected. Arrhythmia monitoring consisted of protocol-specified 24-h Holter monitor at 12-month visit (Holter). Any atrial arrhythmia recurrence >30 s documented by SOC recordings was also captured. AAD usage post-ablation was per SOC. A pre-specified 90-day post-procedure blanking period (BP) was employed. Quality of Life (QOL) survey (EQ-5D-5L) was administered at baseline, 6 and 12 months. Device-, procedure-, and death-related adverse events were collected. The EQ-5D-5L system consists of five dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Each dimension has five levels, which are determined based on the patient's own judgment and represent the patient's health state.
2.5 Outcomes
The primary endpoint of this observational study will be freedom from documented AF episodes >30 s, including pre-specified descriptive acute (APS) and long-term outcomes endpoints. Acute success is defined as termination to sinus rhythm or non-inducibility after ablation at the procedure's end. For the definition of long-term success, data will be reported for both “AAD-free” freedom from AF and regardless “with AAD” freedom from AF. Long-term clinical success was defined as freedom from AF, atrial flutter (AFL), and atrial tachycardia (AT) episodes >30 s on Holter, regardless of Class I/III AAD usage. Long-term AAD-free success followed same definition except subjects also needed freedom from Class I/III AAD usage between end of 90-day BP and 12-month visit. For post-hoc analysis, subjects with AF/AFL/AT episodes >30 s documented by SOC recordings after BP were also counted as failures. Subjects with repeat ablation targeting clinical study arrythmia after BP were included as failures. Secondary endpoints will include the incidence of periprocedural complications, procedure duration, mapping time, total collected mapping points, fluoroscopy time, and the measurement of QOL.
2.6 Statistical analysis
Continuous variables are reported as number of observations, mean, standard deviation, or median and first (Q1) and third (Q3) quartiles. Categorical variables are reported as patient counts and percentages. All data available among analysis population was used. Missing data was not imputed. No formal sample size calculation was performed. All analyses were performed using SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA). The p-values presented are two-sided, and p < .05 (not adjusted for multiplicity) was considered statistically significant.
3 RESULTS
3.1 Enrollment and analysis population
Two hundred subjects were enrolled at 15 sites in APAC, including 165 (82.5%) with PAF and 35 (17.5%) with PersAF. All underwent study-specified mapping and ablation procedure. One PersAF subject was later identified as having AF duration >12 months but was retained in the study. All subjects were negative for intracardiac thrombus before ablation.
Baseline demographics (Table 1) included mean age 63.0 ± 11.0 years, 138 (69.0%) male, and all subjects reported a race of Asian. Most PAF subjects (119/161, 73.9%) were in sinus rhythm on baseline ECG, while over half of PersAF subjects (18/35, 51.4%) were in AF. Over half (105/200, 52.5%) had history of hypertension. History of Class I/III AAD use was present in 135 PAF (81.1%) and 20 PersAF (57.1%) subjects.
| Characteristic | PAF (N = 165) | PersAF (N = 35) | All subjects (N = 200) |
|---|---|---|---|
| Age (Years) | 63.9 ± 11.0 (165) | 58.5 ± 9.8 (35) | 63.0 ± 11.0 (200) |
| Sex (Male) | 67.9% (112/165) | 74.3% (26/35) | 69.0% (138/200) |
| Height (cm) | 165.4 ± 9.9 (164) | 168.5 ± 9.2 (35) | 166.0 ± 9.8 (199) |
| Weight (kg) | 67.5 ± 13.8 (164) | 75.8 ± 16.6 (35) | 69.0 ± 14.6 (199) |
| Race | |||
| Asian, Chinese | 26.7% (44/165) | 48.6% (17/35) | 30.5% (61/200) |
| Asian, Indian | 0.6% (1/165) | 0.0% (0/35) | 0.5% (1/200) |
| Asian, Japanese | 61.2% (101/165) | 0.0% (0/35) | 50.5% (101/200) |
| Asian, Korean | 11.5% (19/165) | 51.4% (18/35) | 18.5% (37/200) |
| Heart rate on ECG (bpm) | 71.1 ± 19.7 (160) | 81.1 ± 21.2 (35) | 72.9 ± 20.3 (195) |
| Rhythm on ECG | |||
| Sinus rhythm | 73.9% (119/161) | 22.9% (8/35) | 64.8% (127/196) |
| Atrial fibrillation | 11.8% (19/161) | 51.4% (18/35) | 18.9% (37/196) |
| Sinus bradycardia | 6.8% (11/161) | 8.6% (3/35) | 7.1% (14/196) |
| Atypical AFL | 1.9% (3/161) | 11.4% (4/35) | 3.6% (7/196) |
| Typical AFL | 1.9% (3/161) | 2.9% (1/35) | 2.0% (4/196) |
| Other | 3.7% (6/161) | 2.9% (1/35) | 3.6% (7/196) |
| History of Heart Failure | 6.1% (10/165) | 14.3% (5/35) | 7.5% (15/200) |
| Number of Class I/II/III/IV/Not Evaluated | 4/3/1/0/2 | 2/0/0/0/3 | 6/3/1/0/5 |
| Cardiomyopathy | 3.0% (5/165) | 2.9% (1/35) | 3.0% (6/200) |
| Valvular Heart Disease | 7.3% (12/165) | 14.3% (5/35) | 8.5% (17/200) |
| Hypertension | 52.1% (86/165) | 54.3% (19/35) | 52.5% (105/200) |
| Stroke/TIA/Thromboembolism | 9.3% (15/161) | 0.0% (0/35) | 7.7% (15/196) |
| Conduction Disease | 4.8% (8/165) | 0.0% (0/35) | 4.0% (8/200) |
| Additional Arrhythmia History | |||
| Typical AFL | 10.9% (18/165) | 8.6% (3/35) | 10.5% (21/200) |
| Atypical AFL | 0.6% (1/165) | 5.7% (2/35) | 1.5% (3/200) |
| Type Unknown Flutter | 3.0% (5/165) | 0.0% (0/35) | 2.5% (5/200) |
| AT | 1.2% (2/165) | 0.0% (0/35) | 1.0% (2/200) |
| AVNRT | 0.6% (1/165) | 0.0% (0/35) | 0.5% (1/200) |
| Idiopathic Ventricular Tachycardia | 0.6% (1/165) | 0.0% (0/35) | 0.5% (1/200) |
| PVCs | 4.8% (8/165) | 0.0% (0/35) | 4.0% (8/200) |
| History of Class I/III AAD use | 81.8% (135/165) | 57.1% (20/35) | 77.5% (155/200) |
3.2 HDMC
During enrollment, GRID was not available commercially at two participating centers. GRID and DL were used in 186 (93.0%) and 14 (7.0%) subjects to create 394 and 30 maps, respectively. Among GRID subjects, primary electrode configuration used was HDWS-EC (152, 81.7%), all bipole (31, 16.7%), or along-the-spline (3, 1.6%).
For GRID and DL respectively, sufficient contact was achieved either all (36/186, 19.4%; 0/14, 0%), most (127/186, 68.3%; 11/14, 78.6%), or some of the time (23/186, 12.4%; 3/14, 21.4%), with no investigators reporting occasional or rare contact. Investigators reported difficulty reaching specific areas in 28/186 (15.1%) GRID procedures and 6/14 (42.9%) DL procedures, with most frequent location of difficulty reported as RIPV for GRID (17/186, 9.1%) and LIPV for DL (3/14, 21.4%). When attempted, HDMCs were successfully maneuvered into PVs during all procedures (GRID: 185/185, 100%; DL: 14/14, 100%). For GRID and DL respectively, frequency of ectopic beats or other arrhythmias was reported as either none (73/186, 39.2%; 4/14, 28.6%), minimal (108/186, 58.1%; 10/14, 71.4%), or more than minimal (4/186, 2.2%, 0/14, 0%). Ablation targets were identified with the HDMC in 151/186 (81.2%) GRID and 12/14 (81.5%) DL procedures.
3.3 Mapping characteristics
Mapping characteristics are summarized in Table 2. There were 424 maps created with HDMCs, including 343 in PAF and 81 in PersAF subjects. Maps were created pre-, post-, and/or during ablation, with HDM at all three timepoints in 18 subjects (9.0%). Among the 424 maps created, 57.3% were created in sinus rhythm, 15.6% in paced rhythms, 9.4% in AF, 1.7% in AT, 1.7% in typical atrial flutter, and 14.4% in other unspecified rhythms. Median time to generate map was 9.0 (Q1:5.0, Q3:13.0) minutes. Median total mapping points used was 1929 (Q1:1148, Q3:2730), for a median 209 (Q1:147.4; Q3:292.4; n = 423 maps) used mapping points per minute. Excluding maps with only manual mapping, median time to generate map and mapping efficiency were 10 (Q1:6.0; Q3:12.0) minutes and 226.7 (Q1:166.8; Q3:306.4) used mapping points per minute, respectively. Conservative thresholds for border zone (<= 0.5 mV) and electrically inactive tissue (<= 0.1 mV) were used in 60.2% and 35.4% of maps, respectively (range across all subjects: 0.1–5.0 mV and 0.05–1.0 mV, respectively), with low voltage detected in 100% of patients. Substrate characteristics such as CFAE and focal impulses were identified in <5% of subjects (Table S2).
| Variable | Maps in PAF subjects (N = 165) | Maps in PersAF subjects (N = 35) | All maps in all subjects (N = 200) |
|---|---|---|---|
| Proportion of maps by timepoint | |||
| Pre-ablation | 51.0% (175/343) | 38.3% (31/81) | 48.6% (206/424) |
| During ablation | 12.2% (42/343) | 9.9% (8/81) | 11.8% (50/424) |
| Post-ablation | 36.7% (126/343) | 51.9% (42/81) | 39.6% (168/424) |
| Time to generate map by timepoint (minutes) | |||
| All timepoints | 9.0 (5.0, 12.0) | 9.0 (6.0, 13.0) | 9.0 (5.0, 12.0) |
| Pre-ablation | 10.0 (9.0, 14.0) | 14.0 (10.0, 15.0) | 11.0 (9.0, 15.0) |
| During ablation | 3.8 (2.0, 8.0) | 9.0 (7.5, 12.5) | 4.3 (2.0, 9.0) |
| Post-ablation | 6.0 (4.0, 10.0) | 6.5 (4.0, 8.0) | 6.0 (4.0, 10.0) |
| Total mapping points collected | 9176.0 (4704.0, 13224.0) | 8579.0 (3845.0, 11808.0) | 8913.5 (4665.5, 12938.5) |
| Total mapping points used | 1965.0 (1148.0, 2671.0) | 1931.0 (1393.0, 2788.0) | 1957.5 (1160.0, 2730.0) |
| Used mapping points per minute | 210.0 (152.1, 288.8) | 233.2 (151.5, 318.1) | 213.5 (151.8, 296.5) |
| Mapping points edited | |||
| No editing | 66.8% (229/343) | 50.6% (41/81) | 63.7% (270/424) |
| <10 | 16.3% (56/343) | 13.6% (11/81) | 15.8% (67/424) |
| 10–50 | 7.0% (24/343) | 23.5% (19/81) | 10.1% (43/424) |
| 51–100 | 1.7% (6/343) | 6.2% (5/81) | 2.6% (11/424) |
| >100 | 8.2% (28/343) | 6.2% (5/81) | 7.8% (33/424) |
3.4 Ablation and procedural characteristics
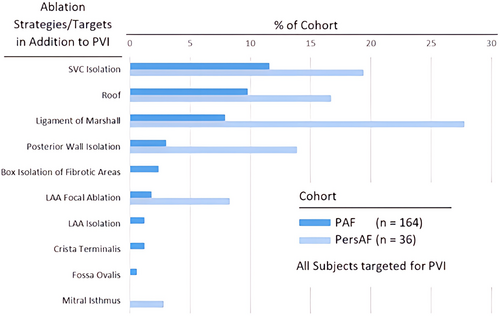
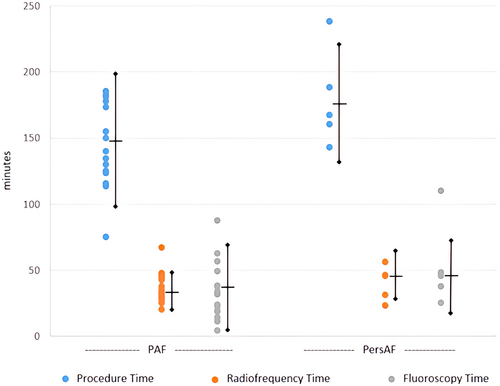
Contact force sensing RFA catheters were used in 146 subjects (73.0%, including TactiCath™ Contact Force Ablation Catheter, Sensor Enabled™ [112/200, 56.0%] and TactiCath™ Quartz Contact Force Ablation Catheter [34/200, 17.0%]) with the remaining using a flexible tip radiofrequency catheter [FlexAbility™, Sensor Enabled™ (54/200, 27.0%)]. PVI was attempted in all subjects, with 120/165 PAF (72.7%) and 28/35 PersAF (68.6%) receiving ablation in addition to PVI. Figure 2 summarizes the additional ablation strategies utilized. Average procedure time was 147.8 ± 49.8 and 176.7 ± 46.0 min for the PAF and PersAF cohorts, respectively. Procedure, RFA, and fluoroscopy times were significantly different across investigational centers (Figure 3).
3.5 Acute procedural success and safety
Acute success, defined as termination to sinus rhythm or noninducibility after ablation, was achieved in 145/165 (87.9%) PAF and 28/35 (80.0%) PersAF subjects (Table 3). Of 168 subjects checked for inducibility at procedure end, 164 (97.6%) were non-inducible for AF. Ten adverse events occurred, including five serious in 5/200 (2.5%) subjects (Table S3). Two cases of pericardial effusion and one case of retroperitoneal hematoma were considered procedure-related adverse events. Two cases of cerebrovascular accidents occurred, but the events were not considered procedure-related by the investigator. No deaths occurred.
| Variable | Paroxysmal AF subjects (N = 165) | Persistent AF subjects (N = 35) | All subjects (N = 200) |
|---|---|---|---|
| Acute Successa | 87.9% (145/165) | 80.0% (28/35) | 86.5% (173/200) |
| Components of success (either or both)b | |||
| Termination to sinus rhythm | 62.8% (91/145) | 50.0% (14/28) | 60.7% (105/173) |
| Non-inducible | 80.0% (116/145) | 75.0% (21/28) | 79.2% (137/173) |
| Inducibility checked | 84.8% (139/164) | 82.9% (29/35) | 84.4% (168/199) |
| Documented non-inducibility | 97.1% (135/139) | 100.0% (29/29) | 97.6% (164/168) |
- a Acute success defined as proportion of subjects who receive HDM and radiofrequency energy delivery resulting in acute termination of clinical arrhythmia, defined by termination to SR (or AT if being treated for PersAF) or non-inducibility of clinical arrhythmia after ablation (cardioversion allowed prior to inducibility attempt). Denominator is number of subjects undergoing procedure with investigational catheter used for mapping and radiofrequency energy delivered.
- b Subjects may fall into more than one category. Denominators in percentages is the number of subjects that experienced acute success.
3.6 Holter compliance and long-term success
Holter was received from 182 subjects. Average total analyzable time was 1392.1 ± 165.3 min. Long-term clinical success based on Holter alone (per-protocol analysis) was achieved in 141/151 (93.4%) PAF and 27/31 (87.1%) PersAF subjects. Long-term AAD-free success was achieved in 91/156 (58.3%) PAF and 13/33 (39.4%) PersAF subjects. Counting repeat ablations and recurrences documented on SOC recordings outside BP as failures, long-term clinical success (post-hoc analysis) was achieved in 130/151 (86.1%) PAF and 25/31 (80.6%) PersAF subjects (Table 4).
| Endpoint | Paroxysmal AF subjects (N = 165) | Persistent AF subjects (N = 35) | All subjects (N = 200) |
|---|---|---|---|
| Long-term clinical successa | |||
| Per-protocol | 93.4% (141/151) | 87.1% (27/31) | 92.3% (168/182) |
| Post-hoc | 86.1% (130/151) | 80.6% (25/31) | 85.2% (155/182) |
| Long-term AAD-free successb | |||
| Per-protocol | 58.3% (91/156) | 39.4% (13/33) | 55.0% (104/189) |
| Post-hoc | 54.5% (85/156) | 36.4% (12/33) | 51.3% (97/189) |
| Failure Modes | |||
| Repeat ablation after BPc | 3.8% (6/157) | 2.9% (1/35) | 3.6% (7/192) |
| Recurrence documented by SOC recording after BP | 8.6% (13/151) | 6.5% (2/31) | 8.2% (15/182) |
| Recurrence documented by study-specified 12-month Holter | 6.6% (10/151) | 12.9% (4/31) | 7.7% (14/182) |
| Use of AAD after BP | 38.5% (60/156) | 57.6% (19/33) | 41.8% (79/189) |
- Note: AF denotes atrial fibrillation; AAD Class I/III antiarrhythmic drug; BP 90-day blanking period; SOC standard-of-care.
- a Per-protocol: freedom from AF/AFL/AT >30 seconds (recurrence), as documented by Holter. Post-hoc: freedom from per-protocol failures, recurrence documented on standard-of-care (SOC) recordings after BP and repeat ablation after BP. Long-term clinical success was calculated without regard to Class I/III antiarrhythmic drug (AAD) use. Denominator is the number of subjects that have completed Holter.
- b Per-protocol: freedom from recurrence as documented by Holter and off Class I/III AAD after blanking period. Post-hoc: freedom from per-protocol failures, recurrence documented on SOC recordings after blanking period, and repeat ablation after blanking period. Denominator is the number of subjects that have completed Holter or have documented Class I/III AAD use between the end of the blanking period and 12-months.
- c Proportion of subjects with an additional ablation procedure to treat indicated cardiac arrhythmia outside of the blanking period. Denominator is the number of subjects that have completed 12 months of follow up or experienced an additional ablation procedure.
3.7 QOL
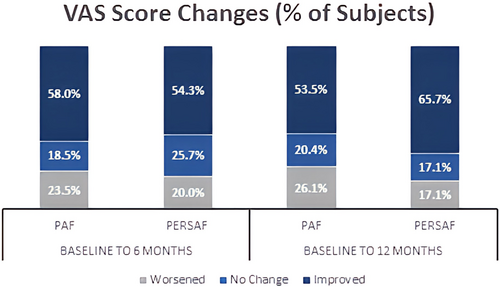
Mean values ± SD of the VAS scores at Baseline, 6 months and 12 months were as follows, respectively: PAF subjects: 78.0 ± 14.5, 84.4 ± 12.6, 81.9 ± 15.1; PersAF subjects: 78.6 ± 15.7, 86.4 ± 8.4, 89.6 ± 6.6. From Baseline to 6 months, VAS Scores improved 6.4 ± 14.2 (PAF, p < .0001) and 7.8 ± 15.0 (PersAF, p = .0040) points; 12-month improvements were 4.1 ± 17.0 (PAF, p = .0027) and 11.1 ± 16.3 (PersAF, p = .0003). Figure 4 displays percentage of subjects with improved, unchanged, or worsened VAS Scores at each timepoint.
4 DISCUSSION
4.1 Main findings of the study
In recent years, there has been an introduction of multiple-electrode contact-based catheters in clinical use.13 Apart from other studies that predominantly focused on Western countries, this is the first real-word prospective, non-randomized multicenter observational study focused on APAC examining the utilization of HDMCs for EAM in both PAF and PersAF patients receiving RFA. The main findings of this study highlighted effectiveness and safety with the HDMCs. We included patients mostly from East Asia; more than half of patients were Japanese, followed by Chinese. Nevertheless, age and gender distribution in this study population were representative of the AF population and similar to other epidemiologic studies in Asia.14, 15 All patients enrolled in this study were de-novo AF without previous ablation or surgery in LA. Compared to other studies, there were fewer concomitant diseases in this study population, primarily in patients with persistent AF, among whom only 14% had a history of heart failure (HF), predominantly with NYHA class I.
4.2 Role of HDM in AF ablation procedures
Compared with point-by-point voltage maps, multipolar maps have been shown to record higher bipolar voltage amplitude with shorter electrogram duration.13, 16 Through the automatic annotation of recorded electrograms, HDM systems can swiftly create electroanatomic maps with high point density.17 A total of 424 maps were created with the HDMCs, using a median mapping time of 9 min, collecting a median of 8913.5 points. The conventional voltage cut-off is set at <0.5 mV for defining scar areas that have been targeted during ablation.18, 19 Scar maps obtained by EAM have emerged as a useful tool to guide personalized AF substrate modification in patients undergoing AF ablation.19 Among maps created in our study, conservative threshold with low voltage ≤0.5 mV was used in only 60.2% of maps, with low voltage detected in 100% of patients. Electrical scar threshold of ≤0.1 mV was used in 35.4% of maps, with detection in only 28.5% of subjects. Available literature suggests that use of multipolar mapping catheters offers an important addition to the voltage map, helping clinicians better understand the arrhythmias. However, substrate characteristics such as focal impulses, rotors, CFAE and other substrate targets were each searched for in <3% of subjects in our study. This is relevant especially in the context that our study enrolled patients receiving index procedure for AF and most patients did not have concomitant heart disease.
PVI was attempted in all subjects and some non-PV related ablation concepts are emerging.20 It is still challenging to elucidate the relative contribution of an arrhythmogenic substrate and PV triggers to AF in the individual patient. Following primary ablation strategy with PVI, there were 33.9% of PAF and 54.3% of PersAF patients who received PVI and additional lines, excluding carvotricuspid isthmus (CTI) ablation. Additional ablations were targeted at SVC, roof area, and ligament of Marshall. Additionally, CTI ablation occurred in 61.2% of PAF and 74.3% PersAF subjects.
4.3 Efficacy and safety with HDM
Acute success defined in this study, suggesting acute termination or non-inducibility of clinical arrhythmia after ablation was 86.5%, considering cardioversion allowed prior to inducibility attempt. 92.3% of patients, with 93% of PAF and 87% of PersAF, respectively, were free from atrial recurrence as documented by 12-month Holter monitoring at the follow up visits. Furthermore, 55.9% of patients were free from the long-term use of AAD, and there was a high percentage of patients (96.4%) who did not receive a repeat ablation procedure after the BP. In comparison to other reported studies focused on Western countries, such as a real-world prospective, non-randomized multicenter observational study that enrolled patients from 25 international sites within Europe, Canada, Australia, and South Africa, investigating the use of the Advisor™ HD Grid mapping catheter in patients undergoing RF ablation for PersAF. They focused on a single HD mapping catheter, studying a total of 334 subjects with PerAF. Acute success, defined similarly to our study, was achieved in 85.9% of subjects. At the 12-month follow-up, 87.4% of patients were free from all atrial arrhythmias lasting more than 30 s, as documented by a 48-h Holter monitor, whether on or off AAD, although details regarding long-term AAD usage were not provided. Additionally, 97% of patients avoided needing an additional procedure specifically targeting AF after the BP. These results indicate non-inferiority to our study in terms of both acute success and long-term outcomes, except for long-term freedom from AAD use.21
Efficiency of HDM-based ablation procedures was also reflected in improvement of quality-of-life measure by EQ-5D-5L VAS Score as in other studies,22 which was more significant over an extended follow-up period for PersAF patients. QoL progressively improved with a significant change evident after 12 months in the PersAF group, which initially had significantly worse baseline QoL. In contrast, symptoms in the PAF group were milder at baseline. However, some patients developed symptoms associated with the inflammatory reaction to ablation, leading to worsening compared to baseline. These findings underscore the practicality of using HDM to identify ablation strategies that lead to successful ablation procedures and an enhanced QOL.
Only a total of 2.5% subjects experienced serious adverse events. Both pericardial effusion and retroperitoneal hematoma were considered procedure-related adverse events occurring in only 2 and 1 patients, respectively. However, two cerebrovascular accident events were not considered associated with the HMDC. These data emphasize the safety of the commercially available HDMCs.
4.4 Limitations
Although this study was designed to exclusively collect data from patients in APAC, it still primarily focused on the East Asia region, particularly in countries with relatively better economic and healthcare conditions. This also resulted in a varying number of patients enrolled from individual hospitals, with a total of only 200 patients across 15 centers. Second, it's important to note that the choice of AF ablation technique and strategy should be individually based on heterogeneous conditions in different countries, and the expertise of physicians. AF procedure technique strongly depends on the skills and experience of physicians, which was reflected by the significant difference in procedure times across investigational centers. Third, the patients enrolled in this study had fewer pre-existing medical conditions, and all were diagnosed with de-novo AF, which may have contributed to the higher success rate of PVI as originally anticipated. Additionally, the HDMCs we selected for this study were GRID and DL, using multiple configurations, and have different shapes, electrode size and spacing, and corresponding angle of the incoming wavefronts to the catheter, which potentially introduced variability to electrogram voltages and the visualization of areas of scar regions. Further research is needed with larger samples focused on more diverse racial groups and conducted in more recent years to evaluate long term clinical efficacy.
4.5 Conclusions
The utilization of GRID was shown to generate comprehensive maps and guide ablation strategies for the treatment of AF with favorable acute and long-term outcomes. This study illustrated an effective approach that shows the practicality and safety of employing HDM during AF ablation procedures in APAC.
ACKNOWLEDGMENTS
The authors gratefully acknowledge all HD Mapping Observational Study Investigators and thank Christopher G. Williams and Feiyi Jia for performing statistical analyses.
FUNDING INFORMATION
This study was sponsored by Abbott.
CONFLICT OF INTEREST STATEMENT
Yamaguchi: Received honoraria from Abbott Medical Japan; Medtronic Japan. Affiliated with Department of Advanced Management of Cardiac Arrhythmia, Saga University, sponsored by Abbott Medical Japan, Nihon Kohden Corporation, Medtronic Japan, Japan Lifeline, Boston Scientific Japan, and Fides-ONE Corporation. Fukaya: Received speaker honoraria from Daiichi-Sankyo, Bayer Yakuhin, Nippon Boehringer Ingelheim, Johnson & Johnson K.K., Abbott Medical Japan, Medtronic Japan, and Japan Lifeline. Soejima: Lecture honorarium Abbott Japan. Morishima: Honoraria from Abbott Medical Japan. Choi: Consulting/Speaker/Teaching fee or honoraria from Boehringer-Ingelheim; Abbott; Daiichi Sankyo; Sanofi; Menarini; Novartis; Samjin pharma; Yuhan; Chong Kun Dang; Hanmi Pharmaceutical; Daewoong; Ildong; Roche. Research grant from Sanofi Genzyme; Medtronic; Chong Kun Dang; Samjin pharma. Horvath: Abbott Employee. All authors except Huang, Horvath: Abbott provided Holters solely loaned for this study. Huang, Pak, Hiroshima, Chen, Yan, Shizuta, Okubo, Zheng, Jiang, Ieda, Lo: no other disclosures.
ETHICS STATEMENT
N/A.
CONSENT
N/A.
Open Research
DATA AVAILABILITY STATEMENT
Data supporting the findings of this study are available from the corresponding author on reasonable request.




