3830 lead extraction from two patients with left bundle branch area pacing
Left bundle branch area pacing (LBBAP) is a physiological pacing method that is currently being used worldwide. The lead is screwed deep inside the interventricular septum to capture the left bundle branch. However, due to the deep septal location of the ventricular lead, major concerns regarding the impact of lead extraction remain. We describe two cases of device infection in patients with LBBAP, in whom successful 3830 lead extraction was performed.
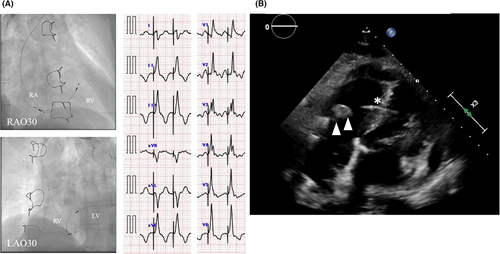
A 72-year-old man presented to our institution with syncope due to sick sinus syndrome. The patient had a history of atrial septal defect closure, tricuspid annuloplasty, atrial tachycardia ablation, and diabetes mellitus. Apixaban 5 mg twice daily, teneligliptin 20 mg once daily, glimepiride 1 mg once daily, and voglibose 0.2 mg thrice daily were prescribed. He underwent LBBAP with SelectSecure lead (Medtronic, Minneapolis, Minnesota, USA). The details of the implantation procedure are shown in Figure 1A. Eleven months after the implantation, he presented to our institution with a high-grade fever of 38.5°C. Transthoracic echocardiography (TTE) demonstrated a 12 mm large, mobile tricuspid valve vegetation associated with the pacemaker lead (Figure 1B). A computed tomography (CT) scan on day 3 revealed a pulmonary embolism due to vegetation. Despite antibiotic treatment, the vegetation became larger (20 × 21 mm) on day 7. The patient was refractory to antibiotic treatment and had a vegetation of >20 mm, which was considered a risk of occlusion of the main trunk of the pulmonary artery in the event of a further pulmonary embolism. We decided to perform a tricuspid biological valve replacement and surgical lead extraction. The SelectSecure lead was easily removed using gentle traction. There was no effect on conduction disturbances in the left bundle branch associated with lead removal (Figure 2). Because atrial fibrillation persisted both pre- and postoperatively and no bradycardia was noted, a new pacemaker was not reimplanted.

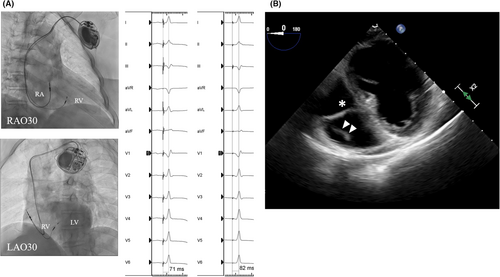
A second case is a 72-year-old woman who underwent LBBAP with a SelectSecure lead for a complete atrioventricular block. The patient had a history of rheumatoid arthritis. Etanercept (15 mg) was prescribed once weekly. She underwent LBBAP with SelectSecure lead and the details of the implantation procedure are shown in Figure 3A. After implantation of 19 months, she presented to our institution with a high-grade fever of 38°C and swelling of the pacemaker pocket. TEE demonstrated a 10 mm large, mobile tricuspid valve vegetation associated with the pacemaker lead (Figure 3B). The size of the vegetation was not so large (<20 mm) and we determined that transvenous lead extraction was feasible. The patient underwent transvenous lead extraction via the subclavian approach on day 4. If lead extraction was difficult with simple traction, mechanical extraction tools were planned to be used. Fortunately, the SelectSecure lead was extracted easily by manual traction with counterclockwise rotation (Video S1). Antibiotic treatment was continued with temporary pacing. After 3 days of lead extraction, blood cultures became negative. A leadless pacemaker was implanted 20 days after lead extraction.
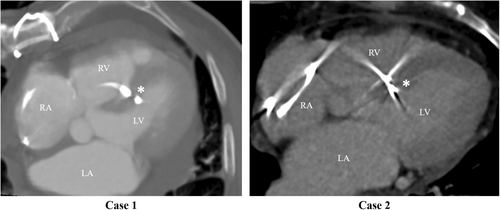
A CT scan before lead extraction demonstrated that the ventricular leads were located deep inside the interventricular septum (Figure 4). Due to the nature of the LBBAP procedure, the possibility that the tip of the lead may protrude into the left ventricle cannot be ruled out. In both cases, transesophageal echocardiography and contrast-enhanced CT of the entire body revealed neither left-side vegetation nor left-side embolism, that is, cerebral, splenic, or renal infarction. A ventricular septal defect was not evident on postprocedural echocardiography in both cases.
Herein, we report two cases of infected SelectSecure lead extraction in patients with LBBAP. The leads had been implanted for 11 months in Case 1 and 19 months in Case 2. Complete procedural success was achieved using simple traction even though the ventricular leads were located deep inside the interventricular septum in both cases. To date, there have been two case reports of SelectSecure lead extraction of LBBAP.1, 2 In these case reports, the leads were extracted with manual traction, and the ventricular septal defect was not observed after lead removal, which was in line with our cases.
We speculate on the following reasons why the adhesions of the leads were so light that they could be easily removed. First, because the SelectSecure lead was thinner than the conventional lead, there was less adhesion between the lead and the surrounding tissue. Shepherd et al. reported that a greater number of SelectSecure lead could be extracted with simple manual traction alone compared with the conventional lead.3 Second point is the unique implantation technique that implants the lead deep into the interventricular septum when performing LBBAP. Jastrzebski et al. reported lead behaviors when performing LBBAP.4 Screwdriver effect, lead progresses without entangling surrounding myocardial tissue each time the lead is turned. In the case of a successful LBBAP, there is a possibility that the lead tip is only pinched in the myocardium and is not firmly fixed by involving the surrounding tissue, and the lead tip is lightly fixed. Third, lead-dwelling time was relatively short. Lead adhesions become more severe with longer dwelling periods. Due to these reasons, we consider that the extraction of the SelectSecure lead from deep inside the interventricular septum was easier than expected.
In conclusion, we report two cases of SelectSecure lead extraction used for LBBAP. Even though the leads were inserted deep into the ventricular septum, they were extracted easily using manual traction, and the ventricular septal defect was not observed after lead removal.
CONFLICT OF INTEREST STATEMENT
There is no conflict of interest for any of the authors.
ETHICS STATEMENT
The study complied with the Declaration of Helsinki and informed consent has been obtained from the participant.
PATIENT CONSENT STATEMENT
The patient consent statement was taken.




