The impact of current strategy using intracardiac echocardiography, lesion index, and minimum substrate ablation on clinical outcomes after catheter ablation procedure for atrial fibrillation
Abstract
Purpose
We developed the intracardiac echocardiography (ICE) technique to minimize radiation exposure and other recent technology during ablation procedure for atrial fibrillation (AF). The aim of this study was to validate the impact of the current strategy using the recent technology for AF ablation on outcomes after procedure.
Methods
We evaluated the safety and efficacy of the current strategy in consecutive set of patients undergoing first-time ablation for AF (N = 233) compared with the conventional strategy in earlier consecutive set of patients (N = 223). The current strategy included the technique of ICE to reduce radiation exposure, Ablation Index®-guided pulmonary veins isolation, and minimum substrate ablation targeting only for induced AF. Outcome measures were radiation exposure, procedure time, in-hospital adverse outcomes, and event-free survival from tachyarrhythmias.
Results
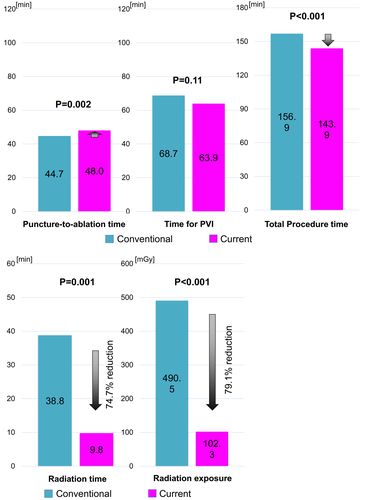
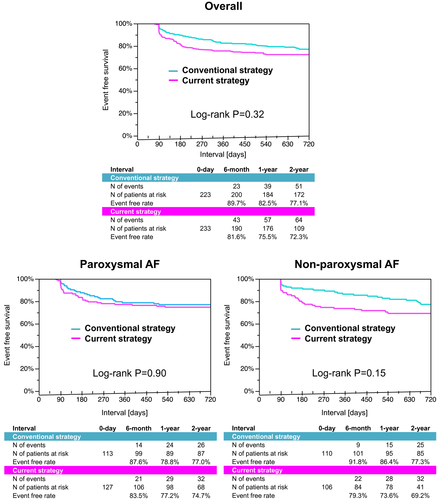
Puncture-to-ablation time was slightly, but significantly increased in the current strategy than in the conventional strategy (48.0 minutes vs 44.7 minutes, P = .002), although total procedure time was significantly decreased in the current strategy (143.9 minutes vs 156.9 minutes, P < .001). Likewise, radiation time and absorbed dose were significantly decreased in the current strategy (9.8 minutes vs 38.8 minutes, P < .001; 102.3 mGy vs 490.5 mGy, P < .001). The incidence of overall in-hospital adverse outcomes was 3.9% in the current strategy and each complication rate was comparable with the conventional protocol. The event-free survival from recurrent atrial tachyarrhythmias was not significantly different between two groups (72.3% vs 77.1% at 2-year, P = .32).
Conclusion
The current strategy using the recent technology with ICE, lesion index, and minimum substrate ablation was feasible and reduced total procedure time and radiation exposure. However, the arrhythmia-free survival could not be improved.
Abbreviations
-
- 3D
-
- 3-dimensional
-
- AF
-
- atrial fibrillation
-
- AT
-
- atrial tachycardia
-
- CFAE
-
- complex fractionated atrial electrogram
-
- ICE
-
- intracardiac echocardiography
-
- LA
-
- left atrium
-
- PVI
-
- pulmonary veins isolation
-
- PWI
-
- posterior wall isolation
-
- RA
-
- right atrium
-
- RF
-
- radiofrequency
-
- TSP
-
- transseptal puncture
-
- TVI
-
- tricuspid valve isthmus
1 INTRODUCTION
Catheter ablation for atrial fibrillation (AF) is the most popular electrophysiological procedure. During past decades, various types of electrodes, catheters, and 3-dimensional (3D) system have been developed and improved the quality of 3D imaging, mapping of arrhythmias, and ablation for the target site.1-6 Recently, SOUND STAR® (CARTO SOUND® system, Biosense Webster, Inc) has been developed as intracardiac echocardiography (ICE) for not only visualizing module but also 3D anatomic mapping.7, 8 The effective usage and the clinical impact of ICE on outcome measures have been reported.9, 10 Our institution also developed the technique using ICE (CARTO SOUND® system) and recent technology in CARTO system to minimize radiation exposure without contrast agents during the preparatory phase, such as placement of the catheter electrodes, transseptal approach to left atrium (LA), and electroanatomical mapping, during the ablation procedure. Furthermore, we started pulmonary veins isolation (PVI) under guidance with Ablation Index® (CARTO 3 V4; Biosense-Webster, Inc), which was developed as a new marker of lesion quality that incorporates contact force, application duration, and power delivery.11, 12 Furthermore, we began to perform minimum substrate ablation targeting only for induced and sustained AF in concern with an increase of iatrogenic AT after the procedure. This study was sought to evaluate the safety and efficacy of the current strategy including the technique of ICE, Ablation Index®-guided PVI, and minimum substrate ablation compared with the conventional strategy in daily clinical practice.
2 METHODS
2.1 Study population
Among 481 consecutive AF patients undergoing first-time catheter ablation procedure in our institution between April 2017 and June 2020, we enrolled 456 AF patients receiving first-time radiofrequency (RF) catheter ablation procedure in this study, excluding 10 patients who previously received MAZE procedure, 13 patients undergoing cryoballoon ablation procedure, and 2 patients using other ablation strategies. They received the conventional strategy before November 2018 (N = 223), and the current strategy using the technique to minimize radiation exposure with ICE and recent technology (SOUND STAR®/CARTO SOUND® system), middle-power application under the guidance with Ablation Index® (Biosense Webster, Inc), and minimum substrate ablation in addition to PVI after November 2018 (N = 233). Then, we evaluated the safety and efficacy of the current strategy compared with the conventional strategy.
Follow-up information was obtained by review of hospital chart and contact by letters and/or telephone call to the patient, relatives, and/or referring physicians. The study protocol was approved by the institutional review board in Mitsubishi Kyoto Hospital. Written informed consent for the catheter ablation procedure and follow-up was obtained from all patients.
2.2 Procedural protocol of catheter ablation
Antiarrhythmic drugs were discontinued before admission. As periprocedural management of anticoagulant therapy, warfarin was uninterrupted during the study period. Direct oral anticoagulants were interrupted with unfractionated heparin replacement on the day of the procedure before 2018, and uninterrupted thereafter. During the procedure, activated clotting time was kept above 300 seconds by unfractionated heparin. The procedure was performed under conscious sedation with propofol, and/or dexmedetomidine, and respiratory management with noninvasive positive pressure ventilation or laryngeal intubation.
In the current strategy, we used the technique using ICE and recent 3D technology in the CARTO system to minimize radiation exposure without contrast agents during the preparatory phase. We firstly got respiratory pattern by SOUND STAR® (CARTO SOUND® system; Biosense Webster, Inc), and ICE image of LA from the high right atrium (RA) was simply merged with 3D-CT image using the carina of right pulmonary veins as a reference point (Figure 1A). For strict respiratory gating, we set a strict range of the end-expiratory phase. Then, the local magnetic field in RA, which could show the location of a decapolar straight electrode in 3D-CT image of RA, was obtained by a 20-polar high-density catheter (PentaRay® 2-6-2 mm; Biosense Webster, Inc) without fluoroscopy (Figure 1B). Therefore, we could locate the electrode in coronary sinus under CARTO system, and fluoroscopic dose from insertion of sheath to this step was zero (Figure 1C). Then, we performed single tansseptal puncture (TSP) using ICE guidance and put a mark on the puncture site on 3D CT image using CARTO SOUND® system (Figure 1D). After the single TSP, we approached LA through the marked TSP site under 3D guidance by ablation catheter and ICE catheter. Totally, three sheaths were inserted into LA for the double circular catheter method during PVI. After the approach to LA, we performed CARTO SOUND® merge from LA and precisely merged ICE image with 3D-CT image (Figure 1E). After CARTO merge, bipolar voltage maps of LA with Fast Anatomical Mapping (FAM®; Biosense Webster, Inc) were manually acquired with PentaRay® catheter (Figure 1F). When patients demonstrated AF rhythm at the time of voltage mapping, we firstly measured complex fractionated atrial electrogram (CFAE) throughout LA during AF rhythm, and we also measured voltage mapping during sinus rhythm after sinus restoration by external defibrillator. Furthermore, we acquired FAM® of ablation catheter in sheath, which could demonstrate TSP line to easily re-approach to LA when necessary, and visualize esophageal electrode to adjust the position without fluoroscopic guidance (Figure 1G,H). After voltage mapping, extensive encircling PVI or single-ring posterior wall isolation (PWI) was performed by point-by-point ablation under the guidance of Ablation Index® using a 3.5-mm tip irrigation catheter (ThermoCool SmartTouch® catheter; Biosense Webster, Inc) with the double circular catheter method. Whether operators performed PVI or PWI was decided by themselves from anatomical position of pulmonary veins. Target Ablation Index® was set ≥550 except ≥600 at anterior carina and ≥400 at the posterior wall near the esophagus. RF energy was applied 35 W with appropriate contact force (>20 g), 40 W with low contact force (<20 g), and 25 W at the posterior wall near the esophagus (Figures 1-3). Subsequently, tricuspid valve isthmus (TVI) ablation was routinely performed regardless of the presence of typical atrial flutter with an 8-mm tip ablation catheter (Fantasista®; Japan Lifeline). After completion of the TVI block line, we checked dormant conduction of pulmonary veins with isoproterenol and adenosine triphosphate and additional applications were performed until the disappearance of dormant conduction when dormant conduction was provoked. Finally, induction test by burst pacing from the proximal coronary sinus electrodes at a cycle length of 200-300 ms with high-dose isoproterenol. We added substrate ablation (35W, drugging) targeting CFAE sites (interval confidence level ≥ 120) only when AF was easily induced and sustained beyond 5 minutes during induction test, and performed AT ablation only for sustained AT.
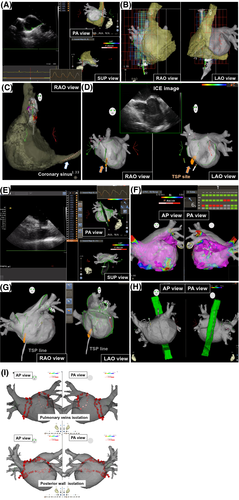
In the conventional strategy, electrodes were placed under fluoroscopic guidance. ICE was used only during simple mere of LA-CT image from RA, and single TSP. Through the puncture site, we inserted three sheaths into LA under fluoroscopic guidance. After the approach to LA, we performed CARTO merge with fluoroscopically defined landmark and surface. After CARTO merge, bipolar voltage maps of LA were acquired with PentaRay® catheter along in the same way as the current strategy. After voltage mapping, extensive encircling PVI or PWI were performed by point-by-point ablation using ThermoCool SmartTouch® with the double circular catheter method. The RF energy was applied 30W for 40 seconds except 35 W for 40 seconds at anterior carina and 25 W for 25 seconds at the posterior wall near the esophagus without Ablation Index®-guidance. Routine TVI ablation, dormant conduction test, and induction test were performed in the same way as the current strategy. Then we added substrate ablation targeting CFAE area and/or low voltage area (bipolar voltage < 0.5 mV, 35 W by 30 seconds) according to the severities in electroanatomic maps before PVI even when sustained AF was not induced by induction test. The selection of strategy substrate ablation was left to the discretion of operators. AT ablation was added only for sustained AT.
All procedures were performed under Trinias biplane system (version 6.2.4; SHIMADZU) and the frame rate was changed from 7 to 5 pps in the current strategy. The size of the field was 33 cm throughout the study period.
2.3 Post-procedural management after catheter ablation
After the first procedure, oral anticoagulant was continued for at least 3 months. Thereafter, discontinuation of oral anticoagulant in patients without atrial tachyarrhythmias recurrence was left to the discretion of the attending physician. After procedure, we administered flecainide or bepridil only within 1 month after procedure in non-paroxysmal AF patients to reduce early recurrence after procedure. After blanking period of 90 days, antiarrhythmic drugs were considered only when recurrent atrial tachyarrhythmias were detected. A 12-lead electrocardiogram was routinely measured at each clinical visit and 24-hour or 1-week Holter monitoring was recommended at 6-12 months. Additional 24-hour Holter monitoring and/or ambulatory electrocardiogram were recorded when patients had symptoms. When recurrent atrial tachyarrhythmias were detected after the blanking period of 3 months, the second procedure was recommended to the patient.
2.4 Definitions, outcome measures, and statistical analysis
Paroxysmal AF was defined as AF lasting <7 days at any timing. Outcome measures were radiation exposure, time for PVI, total procedure time, in-hospital adverse outcomes, and event-free survival from atrial tachyarrhythmias. In-hospital adverse outcomes included death, cardiac tamponade, atrio-esophageal fistula, systemic embolism, sepsis, pericarditis, phrenic nerve injury, gastric motility, and vascular complications. The recurrence of atrial tachyarrhythmias was defined as documented AF and/or atrial tachycardia lasting for >30 seconds or those requiring repeat ablation procedures with a blanking period of 90 days after the procedure. Each outcome measure was compared between the conventional strategy and the current strategy.
Categorical variables were presented as number and percentage and were compared with the chi-square test. Continuous variables were presented as mean with standard deviation and were compared using Student's t-test. Event-free survival from atrial tachyarrhythmias was estimated by the Kaplan-Meier method, and the differences were assessed by the log-rank test. Statistical analyses were performed using JMP 14 pro (SAS Institute Inc) software. All the analyses were two-tailed, and P < 0.05 was considered statistically significant.
3 RESULTS
3.1 Baseline characteristics
Mean age was 70 years old and the prevalence of female was about 30% (Table 1). About half of the study patients represented paroxysmal AF. Mean CHADS2 and CHA2DS2-VASc scores were 1.5 and 2.9, respectively. Most baseline characteristics, except vascular disease, did not significantly differ between the conventional strategy and the current strategy.
| Baseline characteristics |
Conventional strategy N = 223 |
Current strategy N = 233 |
P value |
|---|---|---|---|
| Age (years old) | 70.4 ± 9.8 | 70.4 ± 9.8 | .99 |
| Female | 75 (33.6%) | 80 (34.3%) | .87 |
| Body weight (kg) | 63.6 ± 12.9 | 63.8 ± 13.3 | .92 |
| Paroxysmal atrial fibrillation | 113 (50.1%) | 127 (54.5%) | .41 |
| Hypertension | 140 (62.8%) | 139 (59.7%) | .49 |
| Diabetes | 43 (19.3%) | 33 (14.2%) | .14 |
| Previous heart failure | 45 (20.2%) | 32 (13.7%) | .07 |
| Previous stroke | 22 (9.9%) | 18 (7.7%) | .42 |
| Vascular disease | 27 (12.1%) | 15 (6.4%) | .04 |
| CHADS2 score | 1.6 ± 1.3 | 1.4 ± 1.1 | .22 |
| CHA2DS2-VASc score | 2.9 ± 1.9 | 2.8 ± 1.8 | .30 |
Note
- Categorical variables are presented as number (percentage). Continuous variables are presented as mean ± SD.
3.2 Procedure detail between the conventional strategy and the current strategy
All patients were performed PVI or PWI, and the prevalence of these procedures did not significantly differ between two groups. The prevalence of substrate ablation including CFAE or low voltage area ablation was significantly low in the current strategy than in the conventional strategy (14.2% vs 24.2%, P = .006) (Table 2).
| Baseline characteristics |
Conventional strategy N = 223 |
Current strategy N = 233 |
P value |
|---|---|---|---|
| Procedure | |||
| Extensive encircling pulmonary veins isolation (PVI) | 186 (83.4%) | 199 (85.4%) | .56 |
| Single-ring posterior wall isolation (PWI) | 37 (16.6%) | 34 (14.6%) | .56 |
| Positive for dormant conduction | 8 (3.6%) | 16 (6.9%) | .11 |
| Supra vena cava isolation | 15 (6.7%) | 13 (5.6%) | .61 |
| Tricuspid valve isthmus ablation | 221 (99.1%) | 230 (98.7%) | .69 |
| Mitral valve isthmus ablation | 13 (5.8%) | 22 (9.4%) | .15 |
| Other atrial tachycardia ablation | 23 (10.3%) | 19 (8.2%) | .43 |
| Substrate ablation | 54 (24.2%) | 33 (14.2%) | .006 |
| Sustained atrial fibrillation by induction test | 24 (10.8%) | 33 (14.2%) | .27 |
| Time | |||
| Puncture-to-ablation time (min) | 44.7 ± 10.2 | 48.0 ± 12.3 | .002 |
| Time for PVI (min) | 68.7 ± 34.0 | 63.9 ± 29.5 | .11 |
| Total procedure time (min) | 156.9 ± 42.4 | 143.9 ± 37.6 | <.001 |
| Application | |||
| Total number of application sites | 92.2 ± 23.7 | 84.3 ± 23.1 | <.001 |
| Number of application during PVI/PWI | 68.2 ± 14.2 | 66.1 ± 16.3 | .14 |
| Total application duration (s) | 3172 ± 903 | 2454 ± 639 | <.001 |
| Ablation application during PVI/PWI (s) | 2267 ± 603 | 1727 ± 430 | <.001 |
| Radiation | |||
| Total radiation time (min) | 38.8 ± 36.0 | 9.8 ± 5.6 | <.001 |
| Radiation exposure (mGy) | 490.5 ± 325.3 | 102.3 ± 23.1 | .03 |
| Contrast | |||
| Contrast agents dose (mL) | 0.0 | 0.0 | 1.00 |
Note
- Categorical variables are presented as number (percentage). Continuous variables are presented as mean ± SD.
Puncture-to-ablation time was slightly, but significantly increased in the current strategy than in the conventional strategy (48.0 minutes vs 44.7 minutes, P = .002) (Figure 2), although total procedure time was significantly decreased in the current strategy (143.9 minutes vs 156.9 minutes, P < .001). Time for PVI was almost comparable between two groups (63.9 minutes vs 68.7 minutes, P = .11). Number of application during PVI between two groups was comparable (66.1 vs 68.2, P = .14), although the application duration during PVI was significantly lower in the current strategy (1727 seconds vs 2267 seconds, P < .001). Both total number of application sites and total application duration were also decreased in the current strategy.
Regarding radiation exposure, both radiation time and absorbed dose were significantly decreased in the current strategy (9.8 minutes vs 38.8 minutes, P < .001, 102.3 mGy vs 490.5 mGy, P < .001). In compliance with the protocol of the current study, contrast agents were not used for ablation procedure.
Learning curve of the current strategy was presented in Figure S1. Puncture to ablation time was slightly decreased in the first 10 cases, although significant learning curve was not observed in other outcomes.
3.3 Clinical outcome measures between the current and the conventional strategy
The incidence of overall in-hospital adverse outcomes after the procedure was 3.9% in the current strategy and 3.6% in the conventional strategy (P = .88). Cardiac tamponade occurred in totally three patients (0.9% vs 0.5%, P = .52), all of whom were successfully treated with pericardiocentesis without open surgical repair. The incidence of each complication was comparable between two groups (Table S1).
Follow-up duration was 2.2 ± 1.2 years and significantly shorter in the current strategy (1.6 ± 0.8 years vs 2.9 ± 1.3 years, P < .001). The event-free rate from recurrent atrial tachyarrhythmias after procedure did not significantly differ between 2 groups (72.3% vs 77.1% at 2-year, P = .32) (Figure 3). The comparable recurrence-free rate was preserved in the sensitivity analysis of AF types (74.7% vs 77.0% at 2-year, P = .90 in paroxysmal AF; 69.2% vs 77.3% at 2-year, P = .15 in non-paroxysmal AF).
4 DISCUSSION
The main findings of this study evaluating the safety and efficacy of the current strategy using the technique with CARTO SOUND®, Ablation Index®-guided PVI, and minimum substrate ablation in addition to PVI during catheter ablation for AF were the followings; (1) puncture-to-ablation time was slightly increased in the current strategy, but total procedure time was decreased; (2) the current strategy reduced majority of radiation exposure; (3) the incidence of in-hospital adverse outcomes and event-free survival from recurrent atrial tachyarrhythmias were almost unchanged.
Various ablation technologies in catheter ablation procedure for AF have developed during past decades after a report of AF origins in pulmonary veins by Haissaguerre et al in 1998.13 One of the important innovations was CT/MRI image integration into an electroanatomical mapping. The impact of the image integration on the freedom from arrhythmia recurrence after ablation procedure is controversial, although it could reduce radiation exposure.1, 2 Furthermore, ICE can guide TSP and precisely visualize real-time cardiac anatomy. Effective use of ICE image including fluoroless ablation technique improves the safety of ablation procedures and reduces unnecessary radiation exposure.7-10 Another important innovation was irrigated, contact force-sensing catheter, which was reported to increase freedom rate from arrhythmia recurrence.3-6 Recently, Ablation Index®, calculated by contact force, application duration, and power delivery, was developed, and has become a reliable marker of lesion quality for durable PVI.11, 12 Several studies reported Ablation Index®-guided high-power, short-duration ablation could shorten procedure time and reduce radiation exposure with low complication rate.14-16
We developed the technique using ICE image and recent technology CARTO system to reduce radiation exposure, and the current strategy included the technique, Ablation Index®-guided PVI, and minimum substrate ablation. We could demonstrate that the current strategy significantly shortened procedure time, which might be mostly caused by shortened time for substrate ablation, and reduced radiation exposure without safety concern and significant learning curve. Reducing patient radiation exposure during an ablation procedure is important because ablation procedure is sometimes required repeat procedures to eliminate arrhythmia for one patient. Furthermore, operators' radiation exposure is also not negligible.17 Operators wear radiation-protective equipment, neck guard, and eye glasses, although chronically exposed ionizing increases the risk of cardiovascular events and cancer.18-20 We should keep making efforts to reduce radiation exposure during ablation procedures.
Regarding freedom rate from tachyarrhythmias, the current strategy could not improve the arrhythmia-free survival after procedure compared with the conventional strategy. Ablation procedure technologies based on PVI including balloon ablation have gradually been mature. Therefore, Ablation Index®-guided PVI in the current strategy could not shorten the time for PVI, and reduce arrhythmia recurrence in paroxysmal AF patients. In non-paroxysmal AF, arrhythmia recurrence rate in the current strategy was statistically comparable within the conventional strategy, but Kaplan-Meier curves showed the relatively higher recurrence rate just after blanking period in the current strategy. In the current strategy, we added substrate ablation only when AF was sustained by induction test after PVI to avoid unnecessary substrate ablation. Although there is no established strategy beyond PVI for non-paroxysmal AF, substrate ablation for severe arrhythmogenicity contributing to maintenance of AF may be needed even if AF is not induced after PVI.
4.1 Limitations
The current study had several important limitations. First, this study was a single-center design and a relatively low number of the study population. However, the current strategy is unique and we consider the utility and the limitations could be properly evaluated in this study. Second, detailed data about time and radiation exposure during the respective components of the procedure were not available. Lastly, the difference in baseline characteristics, procedure period, and the follow-up duration might influence the difference of outcome measures because of the non-randomized nature of this study.
5 CONCLUSIONS
The current strategy using recent technology with ICE (CARTO SOUND®), Ablation Index®-guided PVI, and minimum substrate ablation during catheter ablation for AF was feasible, shortened total procedure time, and reduced radiation exposure. However, the arrhythmia-free survival after the procedure could not be improved.
ACKNOWLEDGMENT
We appreciated all the members of the cardiac catheterization laboratory in Mitsubishi Kyoto Hospital for their contribution to this study.
CONFLICTS OF INTEREST
Authors declare no conflict of interests for this article.
Open Research
DATA AVAILABILITY STATEMENT
All relevant data are within the manuscript.




