A decapolar 25-mm-diameter circular mapping catheter with improved detection of pulmonary vein potential disappearance in cryoballoon ablation
Abstract
Background
Disappearance of pulmonary vein (PV) potentials is an important indicator of successful PV isolation during cryoballoon ablation. Conventional octapolar 20-mm-diameter circular PV mapping catheters occasionally fail to identify persistent PV potentials. A new decapolar 25-mm-diameter circular PV mapping catheter has been introduced to improve the detection of PV potentials and their elimination. We compared the detection rates of PV potential disappearance between the decapolar and the octapolar PV mapping catheters.
Methods and Results
A total of 44 consecutive patients (175 PVs) undergoing initial cryoballoon ablation for atrial fibrillation were included. The decapolar catheter was used for 79 (45%) PVs in 20 (45%) patients. There was no difference in left atrial diameter or PV diameter in patients mapped with the two catheters. The decapolar mapping catheter demonstrated a significantly higher detection rate of PV potential disappearance than the octapolar catheter. The need for touch-up ablation was similar between the two groups (5% vs 8%, P = 0.39). When PV potentials were identified, the decapolar catheter detected significantly higher-amplitude PV potentials than the octapolar catheter. In multivariate analysis, use of the decapolar catheter was an independent predictor of the detection of PV potential disappearance.
Conclusions
The decapolar PV mapping catheter demonstrated improved detection of PV potential disappearance in cryoballoon PV isolation.
1 INTRODUCTION
Cryoballoon catheter ablation is a practical, alternative strategy to point-by-point radiofrequency catheter ablation for atrial fibrillation (AF).1-3 Elimination of abnormal left atrium (LA)–pulmonary vein (PV) conduction is the essential procedural endpoint of cryoballoon pulmonary vein isolation (PVI). However, previous studies have reported that the disappearance of LA–PV conduction was confirmed in only 47%-50% of PVs because of underrecording of PV potentials when using a conventional octapolar, 20-mm circular PV mapping catheter (Achieve™, Medtronic, Inc, Minneapolis MN).4, 5
A new decapolar, 25-mm circular PV mapping catheter (Achieve Advance™, Medtronic) is expected to record PV potentials more accurately because of improved electrode-tissue contact (Figure 1). An observational study reported that the new PV mapping catheter achieved real-time PV potential recording in 80% of PVs,6 however, this study was not a comparative study.
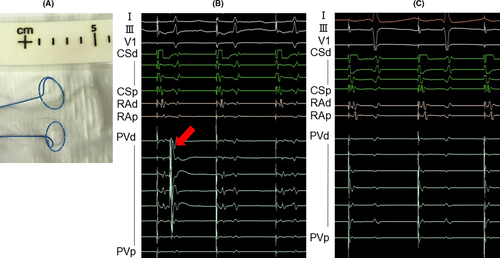
To our knowledge, the clinical benefit of the new PV mapping catheter compared to the conventional PV mapping catheter has not been well-clarified. The purpose of this study was to compare detection rates between the decapolar and octapolar PV mapping catheters.
2 METHODS
2.1 Patients
We performed a retrospective study of 44 consecutive patients who underwent an initial cryoballoon ablation of AF at our hospital in 2017. Exclusion criteria were age <20 years, left atrial thrombus, severe coronary artery disease slated for revascularization, and prior catheter ablation of AF.
We compared patients (n = 20) who underwent cryoballoon ablation using the decapolar PV mapping catheter from September to November 2017 with historical controls (n = 24) studied with the octapolar PV mapping catheter from July 2017 to August 2017. This study complied with the Declaration of Helsinki. Written informed consent for the ablation and participation in the study was obtained from all patients, and the protocol was approved by our Institutional Review Board.
2.2 Catheter ablation procedure
Electrophysiological studies and catheter ablation were performed by three experienced operators (M.M, T.K, Y.M) with the patient under intravenous sedation with dexmedetomidine and/or thamylal sodium. A 6-Fr decapolar electrode (BeeAT; Japan Lifeline, Tokyo, Japan) was inserted into the coronary sinus, while another 6-Fr decapolar electrode was placed in the right ventricle during left PVIs. Following a transseptal puncture of the fossa ovalis, a long sheath (FlexCath Advance™; Medtronic Inc) was introduced into the LA under intracardiac echocardiographic guidance. A cryoballoon catheter with a 28-mm balloon size (Arctic Front Advance™, Medtronic) was passed into the LA via the sheath. A PV mapping catheter was then inserted through the wire lumen of the cryoballoon catheter and advanced into a PV under fluoroscopic guidance and electroanatomical mapping (Carto [R] 3™; Biosense Webster, Inc., Diamond Bar CA, USA; or Ensite NavX™; St. Jude Medical, Inc., St. Paul MN). Next, the balloon was inflated and the balloon catheter was further advanced to the PV ostium as guided by the PV mapping catheter. After confirmation of PV occlusion by pulmonary venography, cryoablation commenced and continued for 180 seconds if confirmation of LA–PV disconnection or a PV temperature <−40°C could be achieved within the initial 60 seconds.7, 8 If these were not achieved during the initial 60 seconds, we stopped the freezing and changed the positions of the PV mapping catheter and the balloon catheter in order to reocclude the PV. If esophageal temperature fell to 20°C, the freezing was stopped and repeated after the temperature returned to baseline. To prevent phrenic nerve injury, 20-pole circular catheters (Inquiry Optima™, St. Jude Medical; or Lasso [R];Biosense Webster) were placed in the superior vena cava to pace the phrenic nerve during right PVIs. Diaphragmatic compound motor action potentials were also monitored, and freezing was stopped if diaphragmatic motion and/or the compound motor action potential of diaphragm decreased.
If the LA–PV conduction persisted after cryoballoon ablation, we performed an additional touch-up ablation using an open-irrigated linear ablation catheter with a 3.5-mm tip (Thermocool SmartTouch™; Biosense Webster or FlexAbility™; St. Jude Medical) via a FlexCath Advance™ sheath or a Swartz Braided SL0™ Transseptal Guiding Introducer Sheath (St. Jude Medical). Radiofrequency energy was applied for 30 seconds at each site using a maximum temperature of 42°C, and maximum power of 35 W. PVI was confirmed using the 20-pole circular catheter.9
2.3 Detection of PV potential disappearance
PV potentials were assessed just before the cryoballoon deployment. The band pass filter was set at 30-500 Hz. The mapping catheter was pulled back as proximally as possible near the distal hemisphere of the balloon. When the PV potentials were suspected to overlap with far-field signals from the neighboring tissues, such as the left atrial appendage, during sinus rhythm, a pacing maneuver was applied to separate PV potentials from far-field signals. The detection of PV potential disappearance was defined as when the LA–PV conduction was disconnected.4 When PV potential disappearance was detected, peak-to-peak amplitudes of PV potentials were measured.
2.4 Statistical analysis
Continuous data are expressed as the mean ± standard deviation or the median (interquartile range). Categorical data are presented as absolute values and percentages. Tests for significance were conducted using the unpaired Student's t test or the Mann-Whitney test for continuous variables, and the chi-squared test for categorical variables. Univariate and multivariate logistic regression analyses were used to determine the predictors that were associated with the detection of PV potential disappearance. Variables with a P < 0.05 in the univariate analysis were included in the multivariate analysis. All analyses were performed using commercial software (SPSS version 24.0.0.0™, SPSS, Inc., Chicago IL).
3 RESULTS
3.1 Patient characteristics
Patient characteristics are shown in Table 1. PVI was successfully completed in all patients. No severe complications such as stroke, cardiac tamponade, bradycardia requiring permanent pacemaker implantation, atrio-esophageal fistula, or persistent phrenic nerve paralysis were observed. Patients assessed with the decapolar mapping catheter had a higher prevalence of hypertension (P = 0.01) and a lower left ventricular ejection fraction (P = 0.04) than those assessed with the conventional mapping catheter.
| Variable | All (n = 44) | Decapolar (n = 20) | Octapolar (n = 24) | P-value |
|---|---|---|---|---|
| Age, y | 70 ± 9 | 70 ± 10 | 70 ± 9 | 0.94 |
| Female, n (%) | 23 (52) | 10 (50) | 13 (54) | 0.78 |
| Body mass index, kg/m2 | 23 ± 4.0 | 22 ± 3.9 | 24 ± 3.0 | 0.14 |
| Paroxysmal AF, n (%) | 40 (91) | 20 (100) | 20 (83) | 0.06 |
| CHA2DS2-VASc score | 2.5 ± 1.4 | 2.3 ± 1.3 | 2.7 ± 1.5 | 0.34 |
| Congestive heart failure, n (%) | 2 (5) | 0 (0) | 2 (8) | 0.19 |
| Hypertension, n (%) | 29 (66) | 9 (45) | 20 (83) | 0.01 |
| Diabetes mellitus, n (%) | 4 (9) | 0 (0) | 4 (17) | 0.06 |
| Hemodialysis, n (%) | 1 (2) | 1 (5) | 0 (0) | 0.27 |
| Pacemaker implantation, n (%) | 1 (2) | 0 (0) | 1 (4) | 0.36 |
| Class I antiarrhythmic agent, n (%) | 11 (25) | 5 (25) | 6 (25) | 1.00 |
| Class III antiarrhythmic agent, n (%) | 0 (0) | 0 (0) | 0 (0) | — |
| Echocardiography | ||||
| Left ventricular ejection fraction, % | 67 ± 7 | 64 ± 9 | 69 ± 5 | 0.04 |
| Left atrial diameter, mm | 38 ± 5 | 37 ± 6 | 39 ± 4 | 0.29 |
| Diameter of pulmonary vein ostium | ||||
| Left superior, mm | 20 ± 4 | 19 ± 5 | 20 ± 4 | 0.60 |
| Left inferior, mm | 18 ± 2 | 18 ± 3 | 18 ± 2 | 0.40 |
| Right superior, mm | 19 ± 3 | 19 ± 3 | 20 ± 4 | 0.33 |
| Right inferior, mm | 19 ± 4 | 19 ± 3 | 20 ± 4 | 0.61 |
3.2 Identification of PV potential disappearance
The decapolar PV mapping catheter more frequently detected PV potential disappearance than the octapolar catheter (Figure 2). Subgroup analysis revealed that the detection rate of PV potential disappearance was better with the decapolar than the octapolar catheter in the right superior PV (Figure 2). The decapolar catheter demonstrated amplitudes of PV potentials measuring twice the size of those measured by the octapolar mapping catheter, especially in the left superior and left inferior PVs (Figure 3).
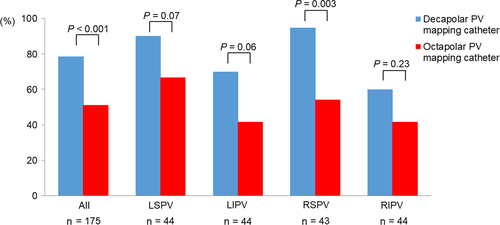
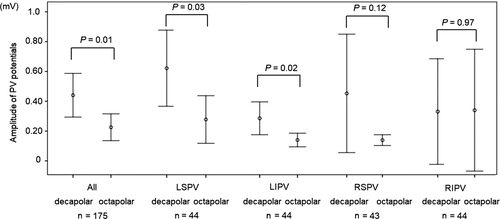
3.3 Comparisons of PVI procedures between the two mapping catheters
PVI procedure characteristics are shown in Table 2. The need for touch-up ablation, procedure time, and minimal balloon temperature were similar in the two groups.
| Variable | All (n = 44) | Decapolar (n = 20) | Octapolar (n = 24) | P value |
|---|---|---|---|---|
| Procedure time, min | 87 ± 28 | 85 ± 28 | 89 ± 28 | 0.64 |
| Fluoroscopy time, min | 24 ± 10 | 27 ± 13 | 22 ± 7 | 0.08 |
| Minimal temperature | ||||
| Left superior, °C | −48 ± 5 | −48 ± 4 | −48 ± 6 | 0.60 |
| Left inferior, °C | −42 ± 6 | −43 ± 6 | −41 ± 5 | 0.41 |
| Right superior, °C | −51 ± 6 | −50 ± 5 | −52 ± 6 | 0.39 |
| Right inferior, °C | −47 ± 6 | −46 ± 5 | −47 ± 6 | 0.29 |
| Cardiac rhythm during the freezing | ||||
| Sinus rhythm, n (%) | 118 (67) | 56 (71) | 62 (65) | 0.38 |
| Atrial fibrillation, n (%) | 43 (25) | 21 (27) | 22 (23) | 0.58 |
| Other atrial tachycardia, n (%) | 14 (8) | 2 (3) | 12 (13) | 0.02 |
| Number of cryoballoon applications, n | 1.0 (1.0-1.0) | 1.0 (1.0-2.0) | 1.0 (1.0-1.0) | 0.08 |
| Cryoballoon application time | ||||
| Left superior, s | 180 (180-208) | 186 (180-212) | 180 (180-180) | 0.10 |
| Left inferior, s | 180 (176-180) | 180 (180-199) | 180 (172-180) | 0.37 |
| Right superior, s | 180 (173-180) | 180 (158-240) | 180 (180-180) | 0.78 |
| Right inferior, s | 180 (180-180) | 180 (173-180) | 180 (167-180) | 0.84 |
| Touch-up ablations, n (%) | 12 (7) | 4 (5) | 8 (8) | 0.38 |
In patients with the decapolar catheter, the cause of cryoballoon ablation failure requiring touch-up ablation was narrow PV diameter (2 PVs) and an acute angle of the left inferior PV (2 PVs). In patients with the octapolar mapping catheter, touch-up ablation was required because of a large PV diameter as compared to the cryoballoon (4 PVs), an acute angle of the right inferior PV (2 PVs), and reconnection during the procedures (2 PVs).
The number of cryoballoon applications and fluoroscopy time tended to be higher in patients with the decapolar PV mapping catheter, and atrial tachycardia more frequently occurred with the octapolar PV mapping catheter.
3.4 Predictors of the detection of PV potential disappearance
Patient characteristics with or without detection of PV potential disappearance are shown in Table 3. In patients with successful detection, the decapolar PV mapping catheter was more frequently used and cardiac rhythm was more frequently sinus rhythm during the freezing.
| Variable | PV potential disappearance | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|
| Detected (n = 111) | Undetected (n = 64) | OR [95% CI] | P value | OR [95% CI] | P value | |
| Decapolar pulmonary vein mapping catheter, n (%) | 62 (56) | 17 (27) | 3.5 [1.8, 6.8] | <0.001 | 3.7 [1.8, 7.4] | <0.001 |
| Diameter of pulmonary vein ostium | ||||||
| Left superior, mm | 20 ± 4 | 19 ± 3 | 1.01 [0.9, 1.3] | 0.45 | — | — |
| Left inferior, mm | 18 ± 3 | 18 ± 2 | 1.1 [0.8, 1.3] | 0.66 | — | — |
| Right superior, mm | 20 ± 3 | 19 ± 4 | 1.1 [0.9, 1.3] | 0.46 | — | — |
| Right inferior, mm | 18 ± 3 | 20 ± 4 | 0.8 [0.7, 1.01] | 0.07 | — | — |
| Cardiac rhythm during the freezing | ||||||
| Sinus rhythm, n (%) | 81 (73) | 37 (58) | 2.0 [1.03, 3.8] | 0.04 | 1.9 [0.98, 3.9] | 0.06 |
| Atrial fibrillation, n (%) | 23 (21) | 20 (31) | 0.6 [0.3, 1.2] | 0.12 | — | — |
| Other atrial tachycardia, n (%) | 7 (6) | 7 (11) | 2.4 [0.2, 1.6] | 0.28 | — | — |
| Cryoballoon application site | ||||||
| Left superior, n (%) | 34 (31) | 10 (16) | 2.4 [1.1, 5.2] | 0.03 | 2.1 [0.9, 5.1] | 0.09 |
| Left inferior, n (%) | 24 (22) | 20 (31) | 0.6 [0.3, 1.2] | 0.16 | — | — |
| Right superior n (%) | 31 (28) | 12 (19) | 1.7 [0.8, 3.6] | 0.18 | — | — |
| Right inferior, n (%) | 22 (20) | 22 (34) | 0.5 [0.2, 0.9] | 0.03 | 0.5 [0.3, 1.2] | 0.13 |
- PV, pulmonary vein.
In multivariate analysis, decapolar catheter usage was an independent predictor of the detection of PV potential disappearance.
4 DISCUSSION
In this retrospective study, we investigated the association between the decapolar PV mapping catheter and the detection of PV potential disappearance in 44 patients undergoing cryoballoon AF ablation.
Our main findings were as follows: (1) The decapolar PV mapping catheter had a higher detection rate of PV potential disappearance than the octapolar PV mapping catheter. (2) There were no significant differences in frequency of the need for touch-up ablation and procedure time between two groups. (3) The decapolar catheter demonstrated 2× higher amplitudes of PV potentials than the octapolar catheter. (4) Decapolar catheter use was an independent predictor of the detection of PV potential disappearance. To our knowledge, this is the first clinical study to compare the efficacies of decapolar and octapolar PV mapping catheters during cryoballoon PVI.
4.1 Improved detection of PV-potential disappearance using the decapolar mapping catheter
The reasons for improved detection of PV potential disappearance in our study may be attributable to the more distinct and higher voltage amplitude electrograms recorded by the decapolar catheter than those by the octapolar device. Possible explanations for this include: (1) The decapolar PV mapping catheter has a larger diameter and more electrodes, so electrode-PV tissue contact is improved. (2) The decapolar catheter was difficult to insert into the distal PV because of its large diameter, resulting in placing the catheter at the PV ostium, where the greater muscle mass would allow more distinct electrograms to be recorded.4 The efficacy of using the decapolar catheter was more obvious in the left-sided PVs. The higher voltage amplitude of the electrograms should make it easier to distinguish PV potentials from left atrial appendage potentials.
The detection rates of PV potential disappearance using the decapolar catheter (79%) and the octapolar catheter (51%) are similar to those reported previously (80%6 and 47%-50%,4, 5 respectively), suggesting that the results would be applicable to various operators and patients.
4.2 Factors associated with detection of PV potential disappearance
Several factors, including cardiac rhythm during the freezing and anatomical characteristics of the PVs, may have influenced the recorded PV potentials and confirmation of LA–PV disconnection. Even after adjusting for these factors, decapolar PV mapping catheter use was the only independent predictor of the detection of PV-potential disappearance. The large size of the decapolar catheter might overcome other possibly associated factors.
4.3 Clinical implication of identifying PV potential disappearance
Improved identification of PV potential disappearance has several potential clinical benefits. (1) Ineffective cryoballoon application can be stopped in cases of persistent LA–PV conduction continuing over 60 seconds of balloon freezing, because a time to PVI of ≤60 seconds significantly predicted PVI durability.8 (2) Time to PVI has been reported as a marker for the estimation of AF recurrence after cryoballoon PVI.10 (3) Cryotherapy and procedure time are shortened when freezing duration is changed based on time to PVI.11
5 LIMITATIONS
Several limitations of our study warrant mention. First, amplitudes and detection of PV potential disappearance are manually measured, and a degree of operator dependence cannot be excluded. Second, there were some differences in patient characteristics between the two groups because of the difficulty of finding historical controls. Third, reasons for unsuccessful identification of PV potential disappearance, in addition to the difficulty of proving a negative, would include failure to detect a PV potential (mapping catheter failure) and failure to ablate an LA–PV connection despite clear PV potential recording (ablation catheter failure). These heterogeneous conditions made it difficult to determine a causal relationship. Fourth, with regard to the use of the decapolar PV mapping catheter in all cases, we consider that there is a disadvantage in cases with small PVs. In such cases, insertion of the mapping catheter into the PV is difficult and the mapping catheter tends to stick out from the PV. In addition, it is difficult for the larger catheters to select distal branches of the PV, limiting the choices for positioning the cryoballoon in the PV. An anatomical assessment for device selection is necessary before the procedure.
The small size of the study population is another problem, weakening the statistical analysis. The identical rates of touch-up ablation may be because of low statistical power.
6 CONCLUSIONS
The new decapolar PV mapping catheter improved the detection rate of PV potential disappearance compared with the octapolar catheter during cryoballoon PVI. Use of the decapolar PV mapping catheter may offer several clinical benefits.
ACKNOWLEDGMENT
We thank Libby Cone, MD, MA, from DMC Corp. (www.dmed.co.jp<http://www.dmed.co.jp/>) for editing drafts of this manuscript.
CONFLICTS OF INTEREST
Authors declare no conflict of interests for this article.




