Physiological aging at striatal synapses
Abstract
Mike Levine's body of work guides thinking on how the basal ganglia process information to create coordinated movements and skill learning throughout the life span and in disease. This special issue is a nod to Mike's career and a well-deserved gesture by the neuroscience community thanking him for the impact he has made on many people's careers and the field of basal ganglia physiology. This paper reviews how aging impacts basal ganglia processing with a focus on single cell and synaptic physiology. This review begins with the work Mike did with his collaborators Nat Buchwald, Chester Hull and Jay Schneider. These early studies paved the way for subsequent studies on changes in synaptic processing that occur with aging in the basal ganglia. The primary focus of this review is aging at corticostriatal synapses. Corticostriatal synapses show reduced expression of both short-term and long-term synaptic potentiation. The roles of age-related changes in calcium homeostasis, vesicle cycling, dopamine modulation, and NMDA receptor function in aging's effect on synaptic plasticity are discussed. The article ends with a review of mitochondrial aging theory as it applies to age-induced changes in corticostriatal synaptic function.
Significance
Aging is associated with losses in skilled movement, automaticity, and coordination that contribute to declines in the quality of life and may underlie the link between aging and basal ganglia disease. The basal ganglia are an interconnected part of the brain that communicates with all regions of the cerebral cortex to generate a program for executing the best sequence of muscle contractions for executing complex movements. Age is the greatest risk factor for diseases affecting the basal ganglia. Diseases like stroke and Parkinson's disease directly impact basal ganglia function. Huntington's disease is an inherited disease of the basal ganglia, where age plays an important role in determining the expression of symptoms. Knowledge of how aging impacts signal processing in the basal ganglia provides insights into aging and disease-related changes in mobility and coordination. These studies also provide a means for measuring interventions that may slow declines seen with aging and disease.
1 STRIATAL SYNAPTIC PHYSIOLOGY
I joined Mike's laboratory as a postdoctoral fellow in 1985 when a majority of mammalian electrophysiologists directed their attention to the hippocampus. A relatively small group of pioneering scientists made it their career to study basal ganglia electrophysiology to gain insight into how striatal synapses behave in normal and pathological states. Studying synaptic physiology in the striatum was a daunting task. The striatum lacks lamination in both its cellular landscape and in its inputs. Medium-sized spiny projection neurons (MSNs) make up the majority of neurons in the striatum; however, a number of different types of interneurons can be found interspersed among the MSNs. The striatum is a large subcortical area that receives very different information in its medial to lateral, rostral to caudal, and dorsal to ventral dimensions (Partridge, Tang, & Lovinger, 2000; Smith, Musleh, Akopian, Buckwalter, & Walsh, 2001). This anatomical variation translates into physiological differences that had to be worked out and considered when interpreting synaptic function in the striatum (Partridge et al., 2000; Smith et al., 2001). Early in vitro analysis of plasticity at excitatory glutamatergic synapses was also complicated by potential contribution of thalamostriatal inputs (Ding, Peterson, & Surmeier, 2008). Striatal synaptic architecture is not uniform in terms of glutamatergic inputs, modulatory influences by cholinergic interneurons, in dopamine release from nigrostriatal terminals that remain intact, and with respect to whether recordings are made from direct or indirect pathways of the basal ganglia (Cepeda et al., 2008; Freeze, Kravitz, Hammack, Berke, & Kreitzer, 2013; Graybiel, 2005; Kawaguchi, Wilson, & Emson, 1990). Interpretation is complicated even further by differences that exist even when comparing strains within the same species (Akopian et al., 2008; Siviy et al., 2015).
There were two major laboratories in the United States dedicated to gaining understanding into striatal physiological function in the 1980s: the laboratory of Nathaniel Buchwald, Chester Hull and Michael Levine and the laboratory of Steve Kitai (Kita, Kita, & Kitai, 1984; Levine, 1988; Levine et al., 1986; Levine, Lloyd, Hull, Fisher, & Buchwald, 1987; Levine, Schneider, Lloyd, Hull, & Buchwald, 1987; Vandermaelen & Kitai, 1980; Wilson, Chang, & Kitai, 1982). These laboratories produced generations of striatal physiologists including Charlie Wilson and Jim Surmeier from Steve Kitai's laboratory and Jay Schneider, Carlos Cepeda, Casey Cromwell, and myself from Michael Levine's laboratory. It was an exciting time that sprung a number of new striatal electrophysiology laboratories including Anatol Kreitzer's, James Tepper's, David Lovinger's, Tony Grace's, and Michael Zigmond's. International laboratories devoted to striatal synaptic physiology emerged at this time as well including Jorge Aceves’ laboratory with his collaborators Jose Bargas and Elvira Galarraga, Ulrich Misgeld's laboratory, Stéphane Charpier, and Giorgio Bernardi's laboratory which included Nicola Mercuri, Paolo Calabresi, and Alessandro Stefani. This is just a short list of the many key investigators studying striatal electrophysiology during the era of discovery in the basal ganglia. This period laid the groundwork for major advances in the understanding of how the basal ganglia work to produce coordinated motor behavior.
2 AGING AT CORTICOSTRIATAL SYNAPSES
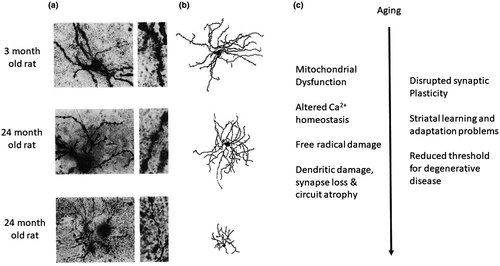
My discussion of physiological aging in the striatum appropriately begins with Mike Levine's pioneering studies comparing synaptic responsiveness between young (1–3 years of aged) and aged (11–14 years of age) striatal neurons in cats (Levine, Lloyd, et al., 1987; Levine, Schneider, et al., 1987). These studies were performed in vivo and the aging comparison looked at both corticostriatal and nigrostriatal synaptic inputs. Extracellular single-unit recordings and population analysis revealed a decrease in the percentage of neurons showing an initial excitatory drive for both corticostriatal and nigrostriatal inputs. The cat was an excellent basal ganglia model system because of its dexterity, complex locomotor behavior, automaticity, and skill learning. In vivo striatal recordings performed by Levine, Lloyd, et al. (1987) featured rapid excitation followed by a prolonged period of reduced activity, ending with rebound excitation with either corticostriatal or nigrostriatal stimulation. This study demonstrated that aging reduced the initial excitation as well as less rebound excitation following the characteristic inhibition that followed the initial synaptic excitation. A companion paper published a few months later eliminated potential age-related differences in the stimulating electrode's ability to activate corticostriatal afferents by showing that aging also reduced the responsiveness of striatal neurons to whisker stimulation in cats (Levine, Schneider, et al., 1987). The number of neurons responding to direct whisker stimulation decreased as did the size of somatosensory receptive fields, suggesting aging in the cat was causing a central decrease in the strength of corticostriatal inputs. These studies were legendary not only for the findings, but also for the monumental accomplishment of recording from hundreds of neurons using an in vivo recording method that required surgery, chronic access ports, and dealing with respiratory movements. Levine, Lloyd, et al. (1987), Levine, Schneider, et al. (1987) also showed an overall age-related decrease in the generation of spontaneous action potentials in the neurons sampled. A parallel study examined single-cell morphology using the Golgi method in age-matched cats, and found that aging decreased the number of dendritic spines, as would be expected with synapse loss in aging (Levine, 1988; Figure 1). This finding was corroborated in an ultrastructural study using electron microscopy (Levine et al., 1988).
Source: Adapted from Dunia et al. (1996).
The use of cats as a model system for studying movement, skill learning, and automaticity was replaced by the rat model in the 1980s (Bargas, Surmeier, & Kitai, 1991; Surmeier, Bargas, & Kitai, 1988, 1989; Vandermaelen & Kitai, 1980). Carlos Cepeda from Mike Levine's lab performed in vivo intracellular recordings from anesthetized rats to begin to tease apart synaptic properties from membrane properties in the age-related decreases in synaptic excitation seen in Mike's previous studies from aged cats (Cepeda, Walsh, Hull, Buchwald, & Levine, 1989). This study corroborated the decline in corticostriatal excitability seen in cats. Intracellular recordings were able to show decreased frequency and size of spontaneous synaptic potentials in aged MSNs, and an increase in the stimulation intensity required to evoke both synaptic potentials and synapse-triggered action potentials. Intracellular injection of depolarizing current also demonstrated reduced intrinsic ability of aged rat striatal neurons to elicit action potentials. This study demonstrated a potentially reduced role in the striatum's ability to modulate motor function through a combination of reduced intrinsic and synaptic excitation.
3 NEW APPROACHES TO STUDYING SYNAPTIC PHYSIOLOGY IN THE STRIATUM
Jim Surmeier, Jose Bargas, Jim Tepper, Charlie Wilson, and Steve Kitai and their many collaborators recognized the need to perform rigorous analysis of ionic currents intrinsic to striatal MSNs to get at more mechanistic questions using the rat model, and their early work changed the approach to studying striatal single-cell physiology moving forward. They developed stable in vivo recording techniques in the rat, striatal cell culture models, and in vitro brain slice techniques that allowed striatal scientists to address issues like the well-developed K+ A-current that dramatically influences striatal neuron firing behavior (Bargas et al., 1991; Kita et al., 1984; Surmeier et al., 1988, 1989). Jim Surmeier's lab took the analysis one step further when he applied whole-cell voltage clamp techniques to define and quantify properties of striatal neuron ionic currents (Plotkin, Day, & Surmeier, 2011; Stefani, Surmeier, & Kitai, 1990). Striatal neurons were defined in this way for the first time, but a limitation early on was that studies had to be performed in neonatal neurons with limited dendritic development to impose any kind of voltage control when analyzing ionic currents (Akopian & Walsh, 2006; Plotkin et al., 2011). This limitation along with more developed glia and fragile membranes seen in striatal neurons from aged animals made it clear that the area of aging was going to have to rely on intracellular recording techniques to further the field of electrophysiological aging at corticostriatal synapses.
Age-related decreases in nigrostriatal excitation are likely a consequence of the well documented age-related loss of nigrostriatal synapses, which also helps to explain why age is the biggest risk factor for Parkinson's disease (Scherman et al., 1987). Studies on long-term plasticity of corticostriatal inputs demonstrated a clear modulatory role played by dopamine and that an age-related loss of dopamine in the striatum contributes to altered plasticity seen in aging (Akopian & Walsh, 2006). This age-related decline in synaptic excitation of striatal neurons can be extrapolated to play a role in age-related declines in basal ganglia-dependent cognition and motor function (Bäckman, Nyberg, Lindenberger, Li, & Farde, 2006).
4 LONG-TERM SYNAPTIC PLASTICITY AT CORTICOSTRIATAL SYNAPSES
My laboratory and Paolo Calabresi's laboratory began to explore the expression of short- and long-term synaptic plasticity at corticostriatal synapses in the early 1990s (Akopian et al, 2000; Akopian and Walsh, 2007; Calabresi, Maj, Pisani, Mercuri, & Bernardi, 1992, b; c; Walsh, 1993; Walsh and Dunia, 1993). MSNs make up approximately 95% of neurons in the striatum and, thus the odds were high that recordings were made from MSNs (later verified in other laboratories using whole-cell RNA analysis and/or targeting of neurons fluorescently labeled using transgenic mouse models). As mentioned earlier, unlike in cortical brain areas, challenges existed in methods for induction and interpretation of striatal plasticity because of the complexity of striatal cellular distribution and synaptic architecture over multiple domains. In vitro brain slices taken from young adult rats demonstrated tetanic stimulation of the dorsal striatum or the overlying cortex produced variable short- and long-term synaptic plasticity (Akopian & Walsh, 2006; Calabresi et al., 1992, 1992; Walsh, 1993). Many groups subsequently joined the investigation to determine how differences in dopamine modulation of activated corticostriatal synapses played a major role in the kind of plasticity being expressed at corticostriatal synapses (depression or potentiation) (Kreitzer & Malenka, 2005, 2007, 2008; Nelson & Kreitzer, 2014; Wang et al., 2006). It became clear that D2 dopamine receptor activation tipped the scale toward long-term synaptic depression (LTD), and D1 dopamine receptor activation facilitated the expression of NMDA receptor-dependent long-term synaptic potentiation (LTP)(Akopian et al., 2008; Calabresi et al., 1992, 1992; Centonze et al., 1999; Kerr & Wickens, 2001; Lovinger, 2010; Villar & Walsh, 1999; Wickens, Begg, & Arbuthnott, 1996). The mechanism of dopamine modulation was found to be much more complex than a simple direct action on MSNs. It was found that D2 dopamine receptor activation of cholinergic interneurons played a major role by indirectly modulating glutamate release from corticostriatal synapses. D2 dopamine receptor activation of cholinergic interneurons caused them to release endocannabinoids that acted presynaptically at corticostriatal terminals to reduce the release of glutamate (Kreitzer & Malenka, 2005, 2007, 2008; Nelson & Kreitzer, 2014; Wang et al., 2006). The field of corticostriatal long-term synaptic plasticity is enormous and deserving of an entire series of review articles on itself, but this background lays the groundwork for interpreting how aging impacts plasticity.
5 AGING AND CORTICOSTRIATAL SYNAPTIC PLASTICITY
The body of knowledge on the mechanism of corticostriatal long-term plasticity became even more molecular with the use of mouse models because it offered the opportunity to combine molecular genetics with physiology through the development of transgenic animals. The mouse, however, was not the model system for studying aging. The gold standard for studying aging, not only in the brain, but for all mammalian biology was the male Fischer 344 rat, due in part to the large aging colony made available to the research world by the National Institute of Aging (Weindruch & Masoro, 1991). The Fischer 344 rat was not without problems, especially with respect to kidney disease, but it still dominated research as a model for aging. Outbred strains between the Fischer 344 and the Brown Norway rat came later, but the majority of research was done on the Fischer 344. My laboratory began investigating corticostriatal aging in the male Fischer 344 rat by studying simple presynaptic forms of plasticity that relied on calcium handling in presynaptic terminals. This direction was selected in light of many studies that found aging disrupted calcium homeostasis and that this change decreases the expression of calcium-dependent forms of plasticity and cell viability in the hippocampus (Disterhoft & Oh, 2006; Gibson & Peterson, 1987; Kumar & Foster, 2005; Landfield, 1987; Mattson, 2007; Oh, Oliveira, Waters, & Disterhoft, 2013; Thibault & Landfield, 1996). We found that aging resulted in a progressive loss in the ability of corticostriatal synapses to express paired-pulse potentiation produced by short interstimulus intervals, less than 50 ms, as well as in the expression of post-tetanic potentiation (Ou and Walsh, 1997; Ou et al, 1997). We also found that paired-pulse synaptic depression elicited at pairing intervals of 200 ms–1 s increased with aging (Akopian & Walsh, 2006). Paired-pulse plasticity and post-tetanic potentiation are short-term forms of synaptic plasticity determined by a combination of calcium handling in the presynaptic terminal and the availability of different pools of neurotransmitter containing vesicles (Regehr, 2012). We also found that post-tetanic plasticity at corticostriatal synapses involved a postsynaptic participation of NMDA receptors and voltage-dependent calcium channels (Akopian & Walsh, 2002). Aging of MSNs was also associated with a major decline in the expression of dendritic calcium-mediated potentials in populations of aged neurons experiencing regression of dendrites and dendritic spines (Dunia et al., 1996) (Figure 1). It is likely that NMDA receptor contributions to short-term plasticity at corticostriatal synapses also declines with aging since evidence of declining numbers of NMDA receptors exist in the aged striatum (Lin, 2006; Magnusson, Brim & Das, 2010).
Our laboratory next looked into the impact aging has on long-term forms of synaptic plasticity at corticostriatal synapses. Studies from the hippocampus showed nifedipine block of L-type Ca2+ channels reversed age-related changes in synaptic plasticity, but we found the same manipulation had no effect on age-related changes in corticostriatal long-term synaptic plasticity (Akopian & Walsh, 2006; Kumar, Bodhinathan, & Foster, 2009; Norris, Halpain, & Foster, 1998; Walsh et al, 1994). We did find, however, that aging at corticostriatal synapses resulted in reduced expression of NMDA receptor-dependent LTP (Akopian & Walsh, 2006). NMDA receptor-dependent LTP at corticostriatal synapses is enabled by dopamine release and activation of D1 dopamine receptors, while corticostriatal LTD is D2-dopamine receptor-dependent (Akopian & Walsh, 2006). The age-related decline we found in NMDA receptor-dependent corticostriatal LTP was D1-dopamine receptor-dependent as well (Akopian & Walsh, 2006). Interestingly, we did not find an age-related decline in the expression of corticostriatal LTD in spite of the many reports documenting age-related decline in D2 dopamine receptor expression in the striatum (Merchant, Dobie, & Dorsa, 1993; Volkow et al., 1996).
6 THE MITOCHONDRIAL AGING THEORY
A dominant theory in the field of aging is that aging is caused, in part, by mitochondrial dysfunction (Barja, 2014; Reddy & Reddy, 2011). Mitochondrial impairment leads to increased free radical production, diminished ATP production, and altered calcium homeostasis and apoptosis (Barja, 2014). Acute application of H2O2 to young rat brain slices did reduce corticostriatal synaptic potentiation, much like that seen with aging. Application of the vitamin E-based free radical quencher trolox reversed the H2O2-mediated effect, but it had no effect on age differences in the expression of synaptic plasticity at corticostriatal synapses (Akopian & Walsh, 2006). We next tested the hypothesis that in vivo chronic exposure to the mitochondrial complex II inhibitor 3-nitropropionic acid (3-NP) in 1–2-month-old rats could cause enduring changes in the expression of synaptic plasticity at corticostriatal synapses. We found that long-term survival (1–3 months after the 3-NP injection) produced changes in short- and long-term corticostriatal synaptic plasticity reminiscent of that seen in aging (Akopian, Crawford, Petzinger, Jakowec, & Walsh, 2012). The dose of 3-NP used in this study was low enough to not cause lesions in the striatum (Akopian et al., 2012). A companion study from our group showed 3-NP dramatically increased all forms of synaptic potentiation for the first 24 hr after 3-NP exposure, suggesting early excitotoxicity may play a role in the lasting “age-like” changes seen at corticostriatal synapses months later (Akopian et al., 2008, 2012). These studies, in addition to others, are suggestive of a synaptic mechanism contributing to pathology seen with ischemia, in HD models generated by mitochondrial toxins, and in multiple transgenic HD mouse models (Cummings, Cepeda, & Levine, 2010; Simpson & Isacson, 1993; Toner and Stamford, 1999). A common thread for these striatal diseases and with aging is that dysfunctional, oxidative metabolism and Ca2+ handling are likely contributors and thus aging may reduce the threshold for how these mechanisms create pathology in disease (Figure 1) (Bossy-Wetzel, Petrilli, & Knott, 2008; Farshbaf & Ghaedi, 2017; Hamilton, Pellman, Brustovetsky, Harris, & Brustovetsky, 2016; Lin & Beal, 2006; Reddy & Reddy, 2011; Reynolds, Carter, & Morton, 1998).
7 CHALLENGES AND FUTURE DIRECTIONS FOR AGING AND AGE-RELATED RESEARCH IN THE BASAL GANGLIA
Advances in science and medicine have reduced the risk and severity of infectious diseases and slowed the course of chronic diseases like type-2 diabetes to promote unprecedented increases in the life span. The growing numbers of older people means those diseases where age is the biggest risk factor are becoming a more prominent feature of medical practice. Diseases of aging characteristically show a slow, unrelenting morbidity that has serious cost attached to it for the individual and for society as a whole.
Many scientists believe the key to treat many age-related diseases lies in strategies that slow aging. Aging shares many molecular mechanisms with chronic diseases of aging, which makes aging an attractive specialty of study for understanding mechanisms of disease and for the development of new treatment strategies (Niccoli & Partridge, 2012). Aging studies, however, are hamstrung by the cost of performing the study. The field of aging research attempted to minimize these costs by turning to short-lived species like yeast, drosophila, and Caenorhabditis elegans (C. elegans) for the discovery of pathways to increase or decrease the life span. The cost of performing research is still an impasse, however, for research to examine pathways discovered in these model organisms and their interactions with disease in mammals.
Rodent model aging studies attempt to cut costs by using cross-sectional comparisons between young and aged groups, but still the cost per animals is substantial, and cross-sectional studies deal with problems inherent to using inbred strains, and variables like infections, inflammation, exposure to antibiotics, head trauma, and even sociality that can influence biological outcomes. The paucity in electrophysiological analyses of aging also reflects the reality that aged tissue and young tissue do not survive the trauma of processing equally as aged neurons are more fragile and unstable during single-cell recordings. Glial cells are more numerous and more elaborate in aged brain tissue, which limits the success of using whole-cell recording techniques in aged animals. Voltage clamp findings are also limited by the lack of space clamp inherent to neurons with extensive dendrites. Transgenic mouse models are the most frequently used tool to look at the impact of genes identified in family lines of age-related diseases like Parkinson's and Huntington's diseases, but again, cost is an impasse to creating aging colonies that would allow investigators to look at how synaptic physiology is impacted in these transgenic models over the life course (Joshi et al, 2009). The financial hurdle seen in rodent models for studying the interaction of aging and disease is intensified when studying human populations and consequently, a number of large pharmaceutical companies have decided to end research into the development of treatments of Alzheimer's and Parkinson's diseases (Dwyer, 2018). The solution to these problems lies in creating greater awareness in the neuroscience community and the general public about how important studies connecting aging to disease are. Increasing awareness would then, hopefully, increase budgets and the number of funding opportunities for researching aging and age-related diseases.
ACKNOWLEDGMENTS
This article is in recognition of Michael Levine's many scientific accomplishments and his influence on how neuroscience views the functioning of the basal ganglia. Mike and I would also like to take this opportunity to recognize two other scientists who worked in both of our laboratories. Gloria Allen retired as a skilled histologist in our laboratories to process cells labeled during our physiological recordings. Dr. Garnik Akopian worked in my laboratory for close to 20 years and in Mike's laboratory for 2 years when he suddenly passed. His electrophysiological skills, determination, and honesty were unrivaled and his contributions to the field of basal ganglia electrophysiology are many.
CONFLICT OF INTEREST
There is no conflict of interest.
AUTHOR CONTRIBUTIONS
All authors had full access to the data and take responsibility for the integrity and accuracy of the analyses. J.P.W. and G.A. performed the experiments and analyzed the data. The work was directed by J.P.W. All authors collaborated on writing and editing the manuscript.