Association of CYP2R1 rs10766197 with MS risk and disease progression
Significance: We investigated the association among vitamin D, SNPs in CYP2R1 and NADSYN1 genes, and MS. We found decreased 25-OH-vitamin-D3 concentrations in MS patients than in controls. The distribution of genotypic and allelic frequencies was not significantly different between patients and controls, except for rs10766197 CYP2R1. In particular, AA genotype had a higher frequency in MS male patients in comparison to male healthy controls. Moreover, A allele was associated with disease severity only in men patients. These findings suggest a role for CYP2R1 in MS and provide evidence for gender-specific mechanisms involved in MS risk and progression.
Abstract
Background
MS is a neurodegenerative autoimmune disease resulting from a complex interaction of genetic and environmental factors. Among these, vitamin D and genetic variants associated with vitamin D-metabolism gain great attention. The aim of our study was to assess five SNPs in NADSYN1 and CYP2R1 genes in relation to serum 25-OH-vitamin D3 levels in MS patients and controls.
Methods
25-OH-vitamin D3 levels and genotyping of CYP2R1- and NADSYN1-SNPs were investigated both in MS patients and in healthy controls.
Results
The analysis revealed lower 25-OH-vitamin D3 concentrations in MS patients than in controls and an association of rs10766197 CYP2R1 SNP with MS risk. After stratifying MS patients according to gender, we found that the minor allele A of rs10766197 had a higher frequency in men in comparison to women affected by MS. Additionally, the presence of allele A in men was associated with disease progression, assessed by EDSS and MSSS scores.
Conclusion
The findings of our study open new perspectives for a role of CYP2R1 in both risk and progression of MS, with sex-related differences.
1 INTRODUCTION
Vitamin D, a fat-soluble steroid hormone, is considered to be critically important for good bones and overall health throughout life. Vitamin D deficiency increases the risk of developing several bone diseases including rickets, osteomalacia, and osteoporosis, as well as various non-skeletal disorders, including cardiovascular diseases, autoimmune diseases, and some cancers. Among autoimmune diseases, a protective role of vitamin D on multiple sclerosis (MS) risk has been described since 1974 (Grant, 2008).
MS is a chronic neurological disease in which a complex interplay between inflammation, demyelination and neuroaxonal damage within the central nervous system (CNS) leads to clinical disability. Generally, MS patients have low vitamin D serum levels. Moreover, in vivo studies in murine model of MS (experimental autoimmune encephalomyelitis) as well as in vitro analyses in MS patients, revealed an immunomodulatory effect of vitamin D (Correale, Ysrraelit, & Gaitán, 2009; Smolders et al., 2009). Finally, murine studies have identified a role of vitamin D in de/remyelination (Nystad, et al., 2014). These evidences support a possible pathogenic effect of hypovitaminosis D in MS risk.
In humans, vitamin D is mainly synthesized in the skin after ultraviolet (UV) sun radiation exposure and, only a small amount, is obtained through the diet because most common natural foods have a very low vitamin D content (Bivona et al., 2016). In addition, vitamin D can be obtained from vitamin D supplements, multivitamin tablets, or fortified food products. Thus, personal, social and cultural factors are important determinants of vitamin D status via their effects on sun exposure and diet.
An inter-individual variability in vitamin D status has been reported; approximately 25% can be explained by external factors including exposure to sunlight (geographical latitude and season of measurement) and estimated vitamin D intake. Genetic factors represent the most relevant contributors, as initially suggested by twin and family-based studies, accounting for 23–80% of vitamin D variation (Snellman et al., 2009; Engleman et al., 2013). In the last decade, genome wide-association study (GWAS) and an increasing number of candidate gene studies have identified genes involved in synthesis, metabolism and transport of vitamin D associated with vitamin D status (Figure 1). Previously, we assessed the influence of vitamin D receptor (VDR), group-specific component (GC), and cytochrome P450 27B1 (CYP27B1) on 25(OH) D levels in MS patients (Agnello et al., 2016; Agnello et al., 2017). Recently, two independent GWAS, based on participants from European ancestry, identified genetic variations in a novel locus DHCR7/NADSYN1 and in CYP2R1 gene associated with lower vitamin D levels (Ahn et al., 2010; Wang et al., 2010).
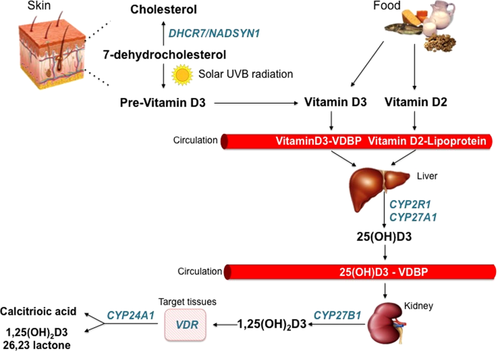
Metabolism of Vitamin D: both endogenous (vitamin D3) and exogenous (vitamin D3-D2) vitamin D circulates bound to VDBP (vitamin D binding protein) or lipoprotein. In the liver, vitamin D is hydroxylated into 25(OH)D by CYP2R1 or CYP27A1. In the kidney, the latter is hydroxylated into 1,25(OH)2D by CYP27B1. 1,25(OH)2D is the biologically active form of vitamin D and exerts its pleiotropic effect through the interaction with vitamin d receptor (VDR) in the target tissues. CYP24A1 catalyzes the conversion of 25(OH)D and 1,25(OH)2D into 24-hydroxylated products (calcitroic acid and 1,25(OH)2D 26,23 lactone), which constitutes the result of vitamin D degradation [Color figure can be viewed at wileyonlinelibrary.com]
CYP2R1 and NADSYN1 represent the major enzymes “upstream” of 25-OH-vitamin D3. CYP2R1, a member of the cytochrome P450 superfamily, is a key vitamin D-25 hydroxylase that catalyzes the hepatic hydroxylation of vitamin D at the 25-C position to form 25-OH-vitamin D3 (calcidiol), the most abundant form of circulating vitamin D and an indicator of vitamin D status. NADSYN1 (nicotinamide adenine dinucleotide synthetase 1) is a glutamine-dependent enzyme, which catalyzes the final step in the biosynthesis of NAD+, a coenzyme in metabolic redox reactions, a precursor for several cell signalling molecules and a substrate for protein posttranslational modifications. NADSYN1 is located on chromosome 11 (11q13.4) close to the dehydrocholesterol reductase (DHCR-7) gene, which encodes 7-dehydrocholesterol reductase, an enzyme involved in the conversion of 7-dehydrocholesterol in cholesterol in the skin, reducing the amount of substrate available for 25-OH-vitamin D3 synthesis (Foucan et al., 2013). UV radiation from sun converts 7-dehydrocholesterol into pre-vitaminD3, which immediately undergoes a thermal isomerization into vitamin D3, a precursor of 25-OH-vitamin D3 (Figure 1). A recent GWAS revealed that variants near genes involved in cholesterol synthesis influence the vitamin D status (Wang et al., 2010).
The aim of this study was to extend our previous findings on the association between vitamin D-related genes and MS risk, assessing five single nucleotide polymorphisms (SNPs) in NADSYN1 and CYP2R1 genes in relation to 25-OH-vitamin D3 levels in a cohort consisting of MS patients and control subjects.
2 MATERIAL AND METHODS
2.1 Patients and controls
This retrospective case-control study included a total of 235 subjects from Western Sicily, 105 cases with sclerosis multiple and 130 controls. Cases were recruited from June 2103 to December 2014, at the Department of Neurology, University Hospital in Palermo. Controls were blood donors matched by age, gender and ethnicity, recruited from April 2015 to July 2016, at the Unit of Transfusion Medicine of Villa Sofia-Cervello Hospital in Palermo. The local medical ethics committee approved the protocol and all subjects agreed to participate after informed consent. Diagnosis of MS was made by an experienced neurologist and based on a previous history of disease, physical examination, cerebrospinal fluid analysis, and magnetic resonance imaging (MRI) findings according to revised McDonald criteria (Polman et al., 2011). The neurological status of patients was assessed using Kurtzke's Expanded Disability Status Scale (EDSS). The progression of disability was assessed using the Multiple Sclerosis Severity Score (MSSS; Roxburgh et al., 2005). The annualized relapse rate (ARR) was calculated in the year prior the genotyping.
The study group consisted of 25 men and 80 women, mean age 39 ± 10 years. The overall mean of disease duration was 12 ± 9.6 years, EDSS score 3.15 ± 2.1, MSSS score 3.89 ± 2.6, and ARR score 1.13 ± 0.97.
The control group consisted of 69 men and 61 women with a mean age of 44 ± 9.9 years.
Clinical characteristics of MS patients and controls stratified according to sex are reported in Table 1.
| Patients (n=105) | Controls (n=130) | |
|---|---|---|
| Age (years) | 39.8 ± 9.9 | 44 ± 9.9a |
| Sex, n (male/female) | 25/80 | 69/61 |
| Disease duration (years) | 11.6.±9.8 | – |
| Age of MS onset (years) | 28.0 ± 7.9 | – |
| MS-type (n) RR/SP/PP | 90/14/1 | – |
| EDSS | 3.0 ± 2.2 | – |
| MSSS | 3.8 ± 2.7 | – |
| ARR | 1.25 ± 0.96 | – |
| 25-OH-vitamin D3 | 21.8 ± 7.2 | 39.1 ± 9.3a |
- Data are shown as: mean±SD.
- a p<0.05
2.2 Molecular analysis
Whole blood in EDTA was stored at 4 ºC for subsequent DNA extraction. Genomic DNA was extracted from 200 µl of whole peripheral blood using a commercial Kit (Qiagen) according to manufacturer's instructions. The DNA quality was evaluated by electrophoresis in a 0.8% agarose gel, quantified by using absorbance spectrophotometric analysis and stored at -20 °C for subsequent analysis.
All samples were genotyped for rs10766197 and rs10741657 of CYP2R1 gene, and for rs38292251, rs7944926 and rs12785878 of NADSYN1 gene using Real-Time allelic discrimination Taq-Man assay (Applied Biosystems). Characteristics of genetic variations in CYP2R1 and NADSYN1 genes are reported in Table 2.
| Gene | Chrom region | SNP | Ancestral Allele | Allele Subst | SNP Location |
|---|---|---|---|---|---|
| CYP2R1 | 11p15.2 | rs10741657 | G | A | Promoter region |
| rs10766197 | G | A | Promoter region | ||
| NADSYN1 | 11q13.4 | rs3829251 | G | A | Intron |
| rs7944926 | G | A | Intron | ||
| rs12785878 | G | T | Intron |
- Abbreviations: Chrom reg, Chromosomal region; Subst, substitution; SNP, single allele nucleotide.
The genotyping was performed using a 7500 real-time PCR system. All PCR reactions mixtures contained 1 µl di DNA, 5 µl TaqMan Genotyping Master Mix, 0.25 µl genotyping Assay mix containing primers and FAM or VIC labelled probes and distilled water for a final volume of 20 µl. The Real Time PCR conditions were: initially 60 °C for 30 seconds and then 95 °C for 10 min, and subsequently 40 cycles of amplification (95 °C for 15 seconds and 60 °C for 1 min), and then 60 °C for 30 seconds (Applied Biosystems).
2.3 Biochemical analysis
25-OH-vitamin D3 concentrations were assessed on serum. Serum tubes were centrifuged and serum was stored a -20 ºC up to the analysis.
Serum 25-OH-vitamin D3 levels were determined by a high-performance liquid chromatography (HPLC) using a Chromosystem reagent kit (Chromsystems Instruments & Chemicals) and a chromatographic system equipped with a Waters 1525 Binary HPLC pump connected to a photo diode array detector; detection was carried out at 265 nm. Chromatographic separation was performed as follows: C18 analytical column, column temperature 25 C, flow rate 0.7 ml min-1, wavelength 265 nm, and sample injection volume 50 µl. Chromatographic separation was performed with isocratic elution with retention time of 4.2 min. In accordance with the kit's instructions, 25-OH-vitamin D3 serum levels < 15 μ5vel from 15-30 μ5-3 and >30 μ303 were considered the threshold values for identifying deficiency, insufficiency and normality of vitamin D levels, respectively.
2.4 Statistical analysis
Using SPSS software (version 13.0), statistical analysis was performed. For genetic association analyses, all genotypes were tested for Hardy-Weinberg equilibrium using χs test. Allelic and genotypic frequencies of SNPs were compared between MS patients and control group by Fisher's exact test, estimating odds ratio (OR) with 95% confidence interval (CI). Trend analysis was performed by extended Mantel Haenszel procedure. All quantitative results are expressed as the mean ± standard deviation. The Kolmogorov-Smirnov test was used for testing normality of distribution of quantitative variable. The association between variables was assessed using the test for categorical variables and the Mann-Whitney test for continuous variables. Multiple logistic regression models were applied by using dominant (major allele homozygotes versus heterozygotes plus minor allele homozygotes) and recessive (major allele homozygotes plus heterozygotes versus minor allele homozygotes) models. A p-value < 0.05 was considered statistically significant.
3 RESULTS
3.1 25-OH-vitamin D3 measurements
Mean serum values of 25-OH-vitamin D3 were 21.8 ± 7.2 µg/L in MS patients and 39 ± 9.3 µg/L in controls (p < 0.001). Vitamin D insufficiency was prevalent in MS patients (58%), 26% had vitamin D deficiency and only 15% had normal vitamin D levels. When stratifying MS patients according to gender and age, we found slightly decreased 25-OH-vitamin D3 levels in men with respect to women (20.1 ± 6.2 µg/L vs. 22.3 ± 7.3 µg/L, respectively; p > 0.05) but no age-related differences.
3.2 Genotyping results for CYP2R1
The genotypic distribution of both rs10741657 and rs10766197 SNPs of CYP2R1 gene was found to be in Hardy-Weinberg equilibrium in MS patients as well as in controls. When comparing genotype distributions and allele frequencies of the two SNPs selected between cases and controls, significant differences were observed only for rs10766197 (Table 3).
| CYP2R1 SNP | Genotype/Allele | MS patients, n (%) | Controls n (%) | OR (95% CI) | p Value |
|---|---|---|---|---|---|
| rs10766197 | GG | 16 (15) | 41 (32) | 1.00 (Ref) | |
| GA | 48 (46) | 56 (43) | 2.19 (1.09–4.39) | 0.03 | |
| AA | 41 (39) | 33 (25) | 3.18 (1.52–6.65) | 0.002 | |
| G | 80 (38) | 138 (53) | 1.00 (Ref) | ||
| A | 130 (62) | 122 (47) | 1.83 (1.26–2.66) | 0.001 | |
| rs10741657 | GG | 62 (59) | 65 (50) | 1.00 (Ref) | |
| GA | 38 (36) | 52 (40) | 0.76 (0.44–1.32) | 0.40 | |
| AA | 5 (5) | 13 (10) | 0.40 (0.13–1.19) | 0.10 | |
| G | 162 (77) | 182 (70) | 1.00 (Ref) | ||
| A | 48 (33) | 78 (30) | 0.69 (0.45–1.04) | 0.09 |
The frequency of the rs10766197 minor allele (A) was significantly higher in MS patients than in controls (62% vs 47%, p = 0.001; Table 3). The frequency of GA genotype (heterozygous minor allele carriers) was 46% in MS patients vs. 43% in controls (OR 2.19, 95% CI 1.09-4.39, p = 0.03) and the frequency of AA genotype (homozygous minor allele carriers) was 39% in MS patients vs 25% in controls (OR 3.18, 95% CI 1.52-6.65, p = 0.002; Table 3), revealing a moderate association of allele A to MS. Moreover, when performing logistic regression analysis of dominant and recessive models between MS patients and controls, we found that the rs10766197 in the CYP2R1 gene was significantly associated with an increased risk of MS under the dominant and recessive model (OR 2.5, 95% CI 1.3-4.7, p = 0.003 and OR 1.88, 95% CI 1.07-3.2, p = 0.003, respectively).
The analysis of the rs10766197 distribution in MS patients, stratified according to the 25-OH-vitamin D3 concentrations, revealed that patients who carried the genotype AA presented a trend of lower levels of 25-OH-vitamin D3 in comparison to those with genotype GG or GA, although not statistically significant: GG: 22.3 ± 6.8 µg/L, GA: 22.2 ± 8 µg/L and AA: 19.2 ± 4.3 µg/L, p > 0.05.
After stratifying the rs10766197 genotype distribution according to gender, we observed significant differences in men but not in women. In particular, the frequency of AA genotype was significantly increased in men affected by MS with respect to men of controls (32% vs. 19%, respectively). Logistic regression analysis adjusted for sex confirmed that the AA genotype frequency was significantly increased in MS male patients in comparison to male controls (Table 4).
| Male | Female | |||||
|---|---|---|---|---|---|---|
| Genotype | GG | GA | AA | GG | GA | AA |
| MS patients, % | 16 | 32 | 52 | 15 | 50 | 35 |
| Controls, % | 37 | 44 | 19 | 26 | 42 | 32 |
| Odd ratio significance | ||||||
| OR | 0.34 | 2.9 | 5.0 | 0.50 | 1.97 | 1.13 |
| 95%CI | 0.10-1.11 | 0.89-9.47 | 1.83-13.6 | 0.21-1.17 | 0.85-4.55 | 0.55-2.28 |
| P value | 0.07 | 0.08 | 0.002 | 0.13 | 0.14 | 0.73 |
When we performed a logistic regression analysis adjusted for gender, the significant differences of AA genotype frequency between patients and control group were confirmed (OR: 5,95% CI 1.83-13.6, p = 0.002). The analysis of the effect of rs10766197 on age of disease onset, EDSS, MSSS and ARR did not reveal any effect of the SNP on disease course. Interestingly, when considering MS patients carrying A allele (AA and AG) and stratifying them according to gender, we observed significantly higher MSSS and EDSS scores in males compared to females (Figure 2).
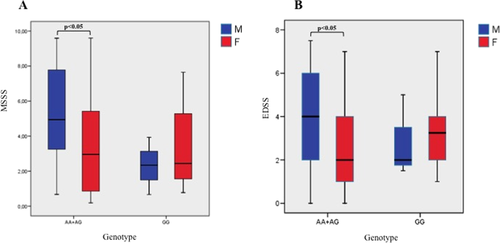
Distribution of MSSS (A) and EDSS (B) scores in men and in women affected by MS [Color figure can be viewed at wileyonlinelibrary.com]
The rs10741657 was not associated with any clinical or biochemical parameters in MS cases (data not shown).
3.3 Genotyping results for NADSYN1
The genotypic distribution of NADSYN1 polymorphisms was found to be in Hardy-Weinberg equilibrium both in MS patients and in controls. The genotypic and allelic frequencies distribution of the polymorphisms was not found to be significantly different between MS patients and controls (Table 5). Also, none of the three polymorphisms were found to be associated with any of the studied clinical or biochemical parameters in MS cases.
| NADSYN1 SNP | Genotype/Allele | MS patients, n (%) | Controls n (%) | OR (95% CI) | pValue |
|---|---|---|---|---|---|
| rs3829251 | GG | 79 (75) | 85 (65) | 1.00 (Ref) | |
| GA | 26 (25) | 43 (34) | 0.65 (0.36–1.15) | 0.15 | |
| AA | 0 | 2 (1) | 0.21 (0.01–4.55) | 0.49 | |
| G | 184 (87) | 213 (69) | 1.00 (Ref) | ||
| A | 26 (13) | 47 (31) | 0.64 (0.38–1.07) | 0.09 | |
| rs7944926 | GG | 47 (45) | 61 (47) | 1.00 (Ref) | |
| GA | 53 (50) | 56 (43) | 1.20 (0.70–2.05) | 0.50 | |
| AA | 5 (5) | 13 (10) | 0.49 (0.16–1.49) | 0.30 | |
| G | 147 (47) | 177 (56) | 1.00 (Ref) | ||
| A | 63 (30) | 83 (44) | 0.92 (0.62–1.35) | 0.68 | |
| rs12785878 | GG | 5 (5) | 13 (10) | 1.00 (Ref) | |
| GT | 52 (49) | 57 (44) | 2.37 (0.79–7.11) | 0.13 | |
| TT | 48 (46) | 60 (46) | 2.08 (0.69–6.24) | 0.20 | |
| G | 62 (30) | 82 (40) | 1.00 (Ref) | ||
| T | 148 (70) | 177 (60) | 0.90 (0.60–1.34) | 0.68 |
4 DISCUSSION
We have selected five SNPs in CYP2R1 and NADSYN1 genes based on their previous association with 25-OH-vitamin D3 levels identified in populations with different ethnic background (Wang et al., 2010; Ramos-Lopez, Bruck, Jansen, Herwig, & Badenhoop, 2007; Nissen et al., 2014). Moreover, both SNPs in CYP2R1 gene (rs10741657 and rs10766197) have been significantly associated with lower 25-OH-vitamin D3 concentrations in asthmatic and diabetic (diabetes type 1) patients (Ramos-Lopez, Bruck, Jansen, Herwig, & Badenhoop, 2007; Wjst et al., 2006). Both disorders and MS are hallmarked by an autoimmune pathogenesis.
In our study, when exploring the association among 25-OH-vitamin D3 serum levels, SNPs in CYP2R1 and NADSYN1 genes and MS in a group of Sicilian patients and healthy individuals, we found lower levels of 25-OH-vitamin D3 in MS patients than in controls and an association between the minor allele (A) of CYP2R1 rs10766197 and MS risk. Moreover, a logistic regression adjusted for gender showed a significant difference of AA genotype frequency in MS male patients and in male controls. Additionally, A allele (AA and AG) was associated with disease severity, assessed by EDSS and MSSS scores, in MS male patients. Finally, the rs10741657 of CYP2R1 as well as all studied SNPs of NADSYN1 gene did not show any different genotypic or allelic frequency distribution between MS patients and controls and were not associated with any of the clinical or biochemical parameters in MS patients.
To date, this is the first study that provides the evidence of a potential influence of CYP2R1 rs10766197 on MS risk. Recently, Laursen et al found a significant association of the CYP2R1 rs10741657 with MS risk in a cross-sectional study performed on Danish population (Laursen et al., 2015). Similarly, they found no association between NADSYN1 genetic variants, 25-OH-vitamin D3 levels and MS risk. Conversely, Alloza et al. in a large cohort of Spanish MS patients identified a moderately significant association of NADSYN1 rs12785878 with MS risk (Alloza et al., 2012).
Interestingly, the most relevant finding of the present study allows us to propose that rs10766197 CYP2R1 SNP is associated with MS, especially in men, and, only in men, it is associated with disease progression.
It is well documented that MS, as well as most autoimmune diseases, is characterized by a sexual dimorphism influencing both incidence and severity of the disease. In general, a higher risk of developing MS in women, with a female to male ratio of 2:1–3:1 and a “maternal parent-of-origin” effect in MS susceptibility have been reported; on the other hand, a more rapid accrual of disability and progression of EDSS and an overall worse course of the disease has been documented in men affected by MS (Bove & Chitnis, 2012; Golden, & Voskuhl, 2017; Sadovnick, 2013).
Several hypotheses have been advanced to explain the sex-related differences of MS. First of all, a role for sex hormones has been proposed. Evidence revealed that estrogens have an effect on immune system function (Ortona et al., 2016). Additionally, a synergy between 17-βestradiol (E2) and vitamin D3 has been observed in experimental autoimmune encephalomyelitis (EAE), the best animal model of MS (Nashold, Spach, Spanier, & Hayes, 2009). In particular, vitamin D3 may stimulate local E2 synthesis within the brain by increasing the expression of the CYP19A1, which encodes the enzyme that catalyze the conversion of androgens to estrogens in glial cells (Krishnan et al., 2010). E2, in turn, is an important regulator of vitamin D3 metabolism, enhancing its immunosuppressive effects (Correale, Balbuena Aguirre, & Farez, 2013). Correale et al., investigating the relation between 1,25-(OH)2D3 and female hormones in MS patients, found that 1,25-(OH)2D3 had significantly stronger immunomodulatory effects in female than in male MS patients (Correale, Ysrraelit, & Gaitán, 2010). Animal studies revealed that E2 could stimulate 1,25-(OH)2D3 accumulation in females both suppressing CYP24A1 transcript and enhancing VDR gene expression in the CNS (Nashold, Spach, Spanier, & Hayes, 2009). In addition to their role in the immune system, both estrogens and 1,25-(OH)2D3 have important neuroprotective effects on the CNS. Thus, estrogens and vitamin D3 may act synergistically to reduce disease risk and severity in women (Correale, Balbuena Aguirre, & Farez, 2013; Disanto, Handel, & Ramagopalan, 2011). Consequently, men affected by MS carriers of SNPs associated with decreased vitamin D levels are more susceptible to rapid disease progression than women affected by MS with the same SNPs because estrogens exert a protective effect. In our study, we found that MS males carrying allele A of rs10766197 CYP2R1 SNP had significantly higher EDSS and MSSS scores and lower 25-OH-vitamin D3 serum levels in comparison to MS females. However, 25-OH-vitamin D3 serum levels were not significantly different between men and women probably because of small sample size with a low number of MS male. Several Authors showed that subjects carrying the AA genotype of rs10766197 CYP2R1 SNP have lower vitamin D levels compared to subjects carrying the GG genotype, after adjustment for potential predictors, including age, season and sex (Arabi et al., 2017; Barry et al., 2014).
Another hypothesis suggests that sex-specific epigenetic mechanisms may have a key role in determining the susceptibility and pathogenesis of MS because none of the predisposing genes are located in the X chromosome (Burrell, Handel, Ramagopalan, Ebers, & Morahan, 2011). The suggestion of the potential involvement of epigenetic in MS comes from studies on HLA alleles transmission. In particular, HLA-DRB5*0101/HLA-DRB1*1501/HLA-DQA*0102/HLA-DQB1*0602 haplotype has been found to be more common in female than male patients and, in families with two generations of MS, it showed a higher frequency in females of the latest generation (Chao et al., 2009; Chao, 2011; Hensiek et al., 2002). Recently, some studies identified different HLA-DR alleles associated with high-risk or protective influences on MS (Nolan et al., 2012; Chao, Lincoln, Dyment, Ramagopalan, & Ebers, 2011). However, further studies are mandatory to understand how genetic, epigenetic, hormonal and environmental factors interact in the different development of MS in men and in women.
The strength of our findings is the ethnic homogeneity of our populations considering that ethnic differences in vitamin D status and genetic background have long been recognised. Indeed, ancestry of population as well as the region in which the population is located represent important determinants of both genetic background and lifestyle, which in turn influences vitamin D levels. However, this study was performed on a small sample size and our findings should be confirmed in a larger population of MS patients.
5 CONCLUSIONS
Our study has revealed a new susceptibility genetic variant, CYP2R1 rs10766197, for MS that has not been reported before.
CONFLICT OF INTEREST STATEMENT
The Authors have no conflicts of interest regarding the publication of this article
AUTHOR CONTRIBUTION
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study and concept design: C.S., L.A. and M.C. Acquisition of data: C.S., L.A. and B.L.S. Analysis and interpretation of data: C.S., L.A. and M.C. Drafting of the manuscript: C.S. and L.A. Critical revision of the manuscript for important intellectual content: C.S., L.A. and M.C. Statistical analysis: C.S. and C.B. Administrative, technical, and material support: P.R., G.S., R.S. and G.B. Study supervision: M.C.