Lithium improves cell viability in psychosine-treated MO3.13 human oligodendrocyte cell line via autophagy activation
SIGNIFICANCE Psychosine (PSY) is a cytotoxic sphingolipid, which leads to widespread degeneration of oligodendrocytes and Schwann cells, causing demyelination in globoid cell leukodystrophy (GLD). Here, we report on autophagy in the human oligodendrocyte cell line MO3.13 treated with PSY and exploitation of lithium as autophagy modulator to rescue cell viability. Our data provide novel information on the intracellular pathways activated during PSY-induced toxicity and suggest the autophagy pathway as a promising novel therapeutic target for ameliorating the GLD phenotype.
Abstract
Globoid cell leukodystrophy (GLD) is a rare, rapidly progressing childhood leukodystrophy triggered by deficit of the lysosomal enzyme galactosylceramidase (GALC) and characterized by the accumulation of galactosylsphingosine (psychosine; PSY) in the nervous system. PSY is a cytotoxic sphingolipid, which leads to widespread degeneration of oligodendrocytes and Schwann cells, causing demyelination. Here we report on autophagy in the human oligodendrocyte cell line MO3.13 treated with PSY and exploitation of Li as an autophagy modulator to rescue cell viability. We demonstrate that PSY causes upregulation of the autophagic flux at the level of autophagosome and autolysosome formation and LC3-II expression. We show that pretreatment with Li, a drug clinically used to treat bipolar disorders, can further stimulate autophagy, improving cell tolerance to PSY. This Li protective effect is found not to be linked to reduction of PSY-induced oxidative stress and might not stem from a reduction of PSY accumulation. These data provide novel information on the intracellular pathways activated during PSY-induced toxicity and suggest the autophagy pathway as a promising novel therapeutic target for ameliorating the GLD phenotype. © 2016 Wiley Periodicals, Inc.
Globoid cell leukodystrophy (GLD, or Krabbe's disease) is a rare, rapidly progressing childhood leukodystrophy triggered by deficit of the lysosomal enzyme galactosylceramidase (GALC; Wenger, 2011), and included in the large group of the lysosomal storage disorders (LSDs). The physiopathological hallmarks of GLD are progressive demyelination, reactive astrocytosis, and microgliosis (Wenger et al., 1997).
GALC degrades galactosylceramide (GLC), a major component of myelin, and other terminal β-galactose-containing sphingolipids, including galactosylsphingosine (PSY). Unlike other sphingolipid storage diseases, abnormal accumulation of the primary substrate of the deficient enzyme does not occur because GLC can also be degraded by GM1 ganglioside β-galactosidase (Kobayashi et al., 1985; Suzuki, 2003). However, PSY cannot be hydrolyzed by this enzyme and progressively accumulates in the nervous system of GLD patients (Vanier and Svennerholm, 1976; Svennerholm et al., 1980). PSY accumulation is believed to be the main cause of the widespread degeneration of oligodendrocytes and Schwann cells, causing demyelination in GLD (Giri et al., 2006). It has indeed been demonstrated that PSY accumulates in cell membrane raft microdomains, disrupting their architecture (White, 2009; Hawkins-Salsbury, 2013), and elevates reactive oxygen species (ROS) production and calcium inflow, triggering apoptotic cell death (Voccoli et al., 2014). For a comprehensive overview of the molecular pathways affected by PSY, refer to the recent review published by Graziano and Cardile (2015).
Autophagy is a lysosomal degradation pathway that controls cytoplasm homeostasis by eliminating protein aggregates and damaged organelles, carbohydrates, and lipids and that allows the cell to adapt its metabolism and meet its energy needs (Ravikumar, 2010). Autophagy starts with the formation of double-membrane-layer vesicles called autophagosomes from cup-shaped structures known as phagophores. This process requires many autophagy-related (ATG) proteins that are hierarchically recruited at the assembly site as well as class III phosphoinositide 3-kinases (PI3Ks) and soluble N-ethylmaleimide-sensitive factor attachment protein (SNAP) receptors (SNAREs). The ATG8 or its mammalian homologous microtubule-associated protein 1 light-chain 3 (LC3) has important multiple roles. It is synthesized as a precursor and processed, resulting in the cytosolic isoform LC3-I. LC3-I can be then conjugated to phosphatidylethanolamine to form LC3-II, which is specifically targeted to the autophagosome membrane. LC3 helps in phagophore edge folding and binds adaptor proteins (such as the ubiquitin-binding protein p62) that, in turn, can regulate selective autophagy of different cellular or molecular structures. Recent data suggest that autophagosomes originate from endoplasmic reticulum (ER) membranes and then are spread out in the cytosol to sequester the cytoplasmic material to be disassembled (Shibutani et al., 2014); they must be then transported along microtubules to reach lysosomes, which typically collect at the microtubule organizing centers. Here autophagosomes fuse with lysosomes, forming the so-called autolysosomes (Klionsky et al., 2014) and allowing the final stage of autophagy in which the cargo is exposed to the acidic lysosomal environment for degradation and recycling of nutrients and membrane components.
Autophagy is stimulated in physiological states such as starvation and can be affected in various pathological conditions, such as metabolic diseases, neurodegenerative disorders, infectious diseases, and cancer (Kroemer, 2015). Studies on LSDs (Settembre et al., 2008a, 2008b; Lieberman et al., 2012) suggest that defective autophagy might be a general feature of this group of diseases. Lieberman et al. (2012) provided an overview of the findings that have been obtained through analysis of the autophagic pathway in LSDs, showing that many of them share common features, hallmarks of impaired autophagy: increased autophagic vesicle formation, accumulation and defective degradation, increased polyubiquitinated proteins, increased dysfunctional mitochondria, and increased p62 expression. However, to the best of our knowledge, autophagy has never been analyzed in GLD except for a study performed by Ribbens et al. (2014). They presented a new GALC-deficient murine oligodendrocyte line, which showed LC3-enhanced lysosomal localization and increased expression under treatment with PSY with respect to wild-type (WT) cells.
Here we report on autophagy in the human oligodendrocyte cell line MO3.13 treated with PSY and exploited lithium (Li) as an autophagy modulator. Li is a drug clinically used to treat bipolar disorders that was recently demonstrated to be an autophagy modulator via a mammalian target of rapamycin (mTOR)-independent pathway and effective in the clearance of accumulating molecules in neurodegenerative diseases (e.g., the mutant Huntingtin in Huntington's disease or mutated tau protein in tauophaties; Motoi et al., 2014). Li was administered to MO3.13 cells treated with PSY, and autophagy activation was evaluated. We studied LC3 localization by LC3-YFP and LC3-RFP-GFP transfection and confocal fluorescence microscopy and p62 and LC3-I/-II expression by Western blotting. The effects of Li on PSY accumulation (by high-pressure liquid chromatography/tandem mass spectrometry [HPLC/MS] methods), ROS production (by fluorescence staining and flow cytometry), and PSY-induced apoptosis (by flow cytometry and microplate fluorimetry) were finally measured.
MATERIALS AND METHODS
Cell Culture and Treatments
Human oligodendrocyte MO3.13 cells (Tebu Bio, Le-Perray-en-Yvelines, France; catalog No. CLU301-P) were maintained at 37 °C in a humidified atmosphere containing 5% CO2 in high glucose Dulbecco's Modified Eagle Medium (DMEM) supplemented with 2 mM L-glutamine, 1% penicillin/streptomycin, and 10% heat-inactivated fetal bovine serum (FBS); all products were from GIBCO-Life Technologies (Carlsbad, CA). MO3.13 cells were seeded at 50,000 cells/cm2, 24 hr after plating cells were washed twice with phosphate-buffered saline (PBS) and then cultured in 0.2% FBS medium (DMEM supplemented with 0.2% FBS, 2 mM L-glutamine, 1% penicillin/streptomycin). For experiments, MO3.13 cells were cultured in 0.2% FBS medium (control) and treated with PSY 10 μM or pretreated with Li acetate (Li) 1 mM or 2 mM (for 30 min) and then with PSY (Li1 + PSY and Li2 + PSY, respectively), for 24 hr. For selected experiments, cells were also treated with prolyl endopeptidase inhibitor 2 (PEI) 10–250 μM for 30 min before PSY administration (PEI + PSY), Li 0.5–100 mM in 0.2% FBS medium for 24 hr, or bafilomycin (BAF) 200 nM (4 hr before cell lysis) after PSY or Li + PSY treatments. PSY (Sigma Aldrich; St. Louis, MO), PEI (Santa Cruz Biotechnology, Dallas, TX), and BAF (Sigma Aldrich) were dissolved in dimethylsulfoxide (DMSO), whereas Li was diluted in Milli-Q water; control cultures received the same quantity of vehicle (DMSO or/and Milli-Q water), which never exceeded 0.6% v/v.
Transfections
For autophagosome and autolysosome formation analysis, cells were plated into 35-mm IBIDI microdishes (Ibidi, Martinsried, Germany) and 12 hr later transfected with LC3-YFP or LC3-RFP-GFP, respectively. LC3-YFP construct contains the LC3 gene fused to the yellow fluorescent protein (YFP) gene (kindly supplied by Dr. Mario Costa; Masiero et al., 2009), whereas the LC3-RFP-GFP (ptfLC3) tandem construct contains the LC3 gene fused to both red and green fluorescent proteins (RFP and GFP; ptfLC3 was a gift from Tamotsu Yoshimori; Addgene plasmid No. 21074). The constructs were transformed in Dh5-Alpha competent cells (Thermo-Fisher; Waltham, MA), grown overnight in Luria Bertani (LB) broth, and extracted with a Qiagen Plasmid Kit (Qiagen, Hilden, Germany), according to kit instructions. Plasmid DNA concentration and purity were measured with a biophotometer, followed by storage at –20 °C. For each sample dish, the transfection solution was prepared by adding 1 μg plasmid DNA and 2 μl Lipofectamine 2000 (Life Technologies) in 100 μl Optimem medium (Life Technologies). After 20 min of incubation at room temperature (RT; for liposome reaction) the transfection solution was added to the cells. Twenty-four hours after transfection, MO3.13 cells were treated as previously reported (control, PSY, Li1 + PSY, and Li2 + PSY for the LC3-YFP transfected cells; control, PSY, and PSY + BAF for the LC3-RFP-GFP transfected ones) and finally imaged by fluorescence confocal microscopy.
Confocal Imaging and Autophagosome/Autolysosome Analysis
Twenty-four hours after treatments, MO3.13 cells were tested for LC3-YFP or LC3-RFP-GFP puncta formation by live-cell confocal imaging. Confocal images were acquired with a laser scanning confocal microscope TCS SP2 (Leica Microsystems, Hilden, Germany) equipped with a cell incubator (37 °C and 5% CO2), a 63× oil objective, and an argon (488 and 514 nm) laser. Each reported confocal image was obtained from a z-series (stack depth was within 10 μm; steps 0.5–1 μm; each image was averaged three times). The resulting z-stack was processed in ImageJ (NIH; RRID:SCR_003070) into a single image by using “z-project” and “Max intensity” options. For a quantitative analysis of autophagosome and autolysosome formation, every image has been thresholded using “threshold”; after this, binary images were inverted, and the number of particles was analyzed by the “Analyze Particles” plugin, by setting “size (pixel2)” = from 2 to infinity, and “circularity” = from 0.00 to 1.00. A region of interest (ROI) for every analyzed cell was made in brightfield images, and the particles were then analyzed for every single cell (ROI).
Western Blotting
For Western blotting experiments, MO3.13 cells were cultured on standard 60-mm dishes, treated as previously described, and then lysed on ice with RIPA buffer (Sigma-Aldrich) containing protease and phosphatase inhibitors cocktail (cOmplete and PhosSTOP; Roche Diagnostics, Basel, Switzerland). Cell lysates were sonicated (for 4 sec at 12 μm of intensity) and after centrifugation (15,000g for 25 min, 4 °C) tested for protein concentration by micro-BCA protein assay kit (Thermo Scientific Pierce). The samples were boiled in Laemli buffer containing β-mercaptoethanol (5% final concentration) for 5 min and centrifuged at room temperature, and the supernatants were finally used for gel electrophoresis (SDS-PAGE) or kept at –80 °C until use. Samples (40 μg) were resolved by SDS-PAGE using Gel Criterion XT-Precasted polyacrylamide gel 4–12% Bis-Tris (Bio-Rad, Hercules, CA) and subsequently transferred to nitrocellulose membranes, as was done by Tonazzini et al. (2016). Immunodetection was performed with the following antibodies against: LC3B (Abcam Cambridge, United Kingdom; catalog No. ab48394; RRID:AB_881433); p62 (Abcam; catalog No. ab56416; RRID:AB_945626), actin (Sigma-Aldrich; catalog No. A-3853; RRID:AB_262137), and tubulin (Sigma-Aldrich; catalog No. T6074; RRID:AB_477582; see Table 1). On the following day, blots were incubated with the corresponding peroxidase-linked secondary antibodies (goat anti-rabbit or mouse IgG-HRP conjugate; Bio-Rad, catalog No. 170-6516; RRID:AB_11125547; and catalog No. 170-6515; RRID:AB_11125142; dilution 1:2,500), and after incubation membranes were developed with Clarity enhanced chemiluminescent substrates (Bio-Rad). The chemiluminescent signal was acquired with an ImageQUANT LAS400 scanner (GE Healthcare Life Sciences, Uppsala, Sweden), and the density of immunoreactive bands was quantified in ImageJ. The results were normalized to the actin (LC3) or tubulin (p62) content and averaged for each repetition toward the control or PSY conditions. The signals of LC3 and P62 were detected in different blots to avoid artifacts.
| Antigen | Description of immunogen | Source; host species; catalog No.; clone or lot No.; RRID | Concentration (μg/ml) |
|---|---|---|---|
| LC3-B | Synthetic peptide corresponding to human LC3B made to an N-terminal portion of the human LC3 protein sequence (between residues 1 and 100) | Abcam, Cambridge, United Kingdom; rabbit/polyclonal; ab48394; RRID: AB_881433 | 1 |
| P62 | Recombinant full-length protein corresponding to amino acids 1–441 of human SQSTM1/ p62 | Abcam; mouse/monoclonal; ab56416; RRID: AB_945626 | 0.6 |
| Actin | Synthetic actin C-terminal peptide Ser-Gly-Pro-Ser-Ile-Val-His-Arg-Lys-Cys-Phe attached to a multiple antigen peptide (MAP) backbone | Sigma-Aldrich, St. Louis, MO; mouse/monoclonal; A-3853; RRID:AB_262137 | 0.5 |
| α-Tubulin | Sarkosyl-resistant filaments from Strongylocentrotus purpuratus (sea urchin) sperm axonemes; recognizes an epitope located at the C-terminal end of the α-tubulin isoform in a variety of organisms | Sigma-Aldrich; mouse/monoclonal; T6074; RRID:AB_477582 | 0.5 |
Antibody Characterization
Table 1 lists the primary antibodies, with their immunological features, commercial sources, and dilutions. The LC3B antibody recognized the expected full-length and cleaved forms of LC3-II protein (15 and 17 kDa, respectively), and the p62 antibody recognized a 62-kDa band, identical to that from previous reports (Ramírez-Peinado et al., 2013, and Vazquez-Martin et al., 2009, respectively). To avoid artifacts from different transfer rates at different heights of the polyacrylamide gel, LC3 and p62 band intensities have been normalized to different control proteins, actin (LC3) and α-tubulin (p62). Actin and α-tubulin antibody detected 42- and a 55-kDa bands, respectively (Nelson et al., 2012; Tresse et al., 2010).
Lipid Extraction
For lipid extraction, MO3.13 cells were plated on standard 24-well plates, treated as previously reported, washed with cold PBS, and lysed on ice with 80 μl/well of RIPA (Sigma-Aldrich), containing a protease and phosphatase inhibitors cocktail. Each condition was used in triplicate, and a control well (without cells) was prepared for each treatment. For each sample, a 150-μl mixture was prepared combining 75 μl of the cell lysate, 12 μl N,N-dimethylsphingosine, 1,250 μM (N,N-DMS, the selected internal standard for LC/MS-MS; Zanfini et al., 2013; Sigma-Aldrich), and 63 μl MilliQ water. Then the mixtjre was divided into three parts (50 μl each), and 500 μl of chloroform/methanol solution (2:1) was added to each part. Samples were vortexed, left at RT for 10 min, and supplemented with 100 μl NaCl 0.9% p/v in Milli-Q water. The biphasic mixture was centrifuged at 800g for 30 min, and the lower layer was collected and evaporated to dryness under vacuum at 30 °C. The residue was dissolved in 50 μl methanol/formic acid 100/0.1 and processed for high-performance liquid chromatography and tandem mass spectrometry (HPLC/MS).
HPLC-MS Analysis
HPLC-MS quantitation was performed on a Shimadzu Nexera UHPLC chromatograph interfaced with an AbSciex 3200 QTRAP mass spectrometer (AB SCIEX, Toronto, Ontario, Canada). HPLC analyses were performed on a Vydac C4 1 × 250 mm (particle size 4 μm), using water/methanol/isopropanol/formic acid 40/55/5/0.1 (A) and methanol/isopropanol/formic acid 95/5/0.1 (B) as mobile phases at 0.1 ml/min flow. Runs were performed under a 45-min linear gradient from 0 to 100% of solvent B, followed by a 5-min purge step at 100% of B and by a 10-min re-equilibration step to the starting conditions.
MRM analyses were performed under the following conditions: ion spray voltage: 5,000 V, source temperature 350 C, declustering potential 50 V, collision energy variable (see value in parentheses), ion source gas 20 liters/min, curtain gas: 25 liters/min. The following transitions were monitored (acquisition time 150 msec/transition): N,N-dimethylsphingosine (Q1/Q3, m/z, CE in parenthesis): 328.2/310.2 (26 V); 328.2/280.2 (32 V); 328.2/110.2 (42 V). PSY (Q1/Q3, m/z, CE in parenthesis): 462.5/444 (25 V); 462.5/282 (30 V);462.5/264 (27 V); 462.5/252 (39 V). Transitions 328.2/310.2 (N,N-dimethylsphingosine) and 462.5/282 (PSY) were used for quantitation purposes.
Annexin V/Propidium Iodide Cell Death Assay
For annexin V/propidium iodide (PI) cell death assay, MO3.13 cells were plated on standard 24-well plates and, after treatments, processed as described by Voccoli et al. (2014). Briefly, cells were harvested and centrifuged for 5 min at 330g, and the pellets obtained were washed with PBS, centrifuged again; resuspended in binding buffer (10 mmol/liter HEPES, 135 mmol/liter NaCl, 5 mmol/liter CaCl2) containing annexin V-FITC conjugate 1 μM (Invitrogen-Life Technologies, Carlsbad, CA) and PI 100 nM (Sigma-Aldrich); and incubated in ice for 30 min. Flow cytometry was performed by using an S3 flow cytometer (Bio-Rad) equipped with 488- and 561-nm diode-pumped solid-state lasers. Annexin V-FITC was excited with the 488-nm laser, and the emitted fluorescence was collected through a 586/25-nm bandpass filter; PI was excited with the 561-nm laser, and its fluorescence was collected through a 615/25-nm bandpass filter. Data were analyzed in ProSort (Bio-Rad) by dividing the scatterplot of annexin V vs. PI into four quadrants representing four clearly distinguishable populations of cells, viable cells (R4 quadrant), apoptotic cells (R5), necrotic cells (R3), and secondary necrotic cells (R2). The four quadrants were set on control conditions, and the cellular debris was excluded from the quantification by gating cells on a forward-scatter/side-scatter (FSC/SSC) graph; at least 25,000 gated events were acquired for each sample.
ROS Detection by Flow Cytometry and Microplate Fluorimetry
For ROS detection, cells were plated in standard 24-well plates and exposed to the treatments previously described, washed with 500 μl/well of prewarmed PBS with calcium and magnesium, and then stained with carboxyl-H2-DCFDA (H2-DCFDA; Life Technologies) for 30 min at 37 °C. DCFA was diluted in DMSO to obtain a 10 μM working solution, and it was used at 2.5 μM final concentration (diluted in prewarmed PBS with calcium and magnesium). Negative (PBS with no staining solution) and positive (treated with 100 mM H2O2 diluted in 0.2% FBS medium for 1 hr) control wells were also prepared. For flow cytometry, the staining cells were treated with 200 μl trypsin for 5 min at 37 °C, harvested in 500 μl complete medium, centrifuged, resuspended in 500 μl prewarmed PBS with calcium and magnesium, and filtered in polypropylene Greiner round-bottomed tubes (Sigma Aldrich). To detect ROS production, the S3 flow cytometer previously described was used. The oxidation product of carboxyl-H2DCFDA was excited with the 488-nm laser, and the emitted fluorescence was collected through a 525/30-nm bandpass filter. More specifically, the dedicated ProSort software was used to create a protocol in which plot types, a region gate to exclude debris, and regions to estimate the percentage of cells with different characteristics were defined. Histograms were plotted as cell counts vs. emitted fluorescence intensity area. Background fluorescence was eliminated by setting a threshold gate on the histogram of the negative control (dashed line in Fig. 6a, top left). Only cells with fluorescence greater than the value of the gate were considered positive for enhanced ROS production. For each sample at least 20,000 gated cells were acquired.
To avoid artifacts from possible oxidative stress originating from cell detachment, we also evaluated ROS production by reading DCF fluorescence from adhered cells by using a microplate fluorescence reader (GloMax Multimode Readers; Promega, Madison, WI). For this measure, immediately after the 30 min staining, the working solution was discarded and the treatment medium (previously collected from each well and put aside in a new 24-well plate) was placed back to allow a sort of recovery for the cells (as suggested by Eruslanov and Kusmartsev, 2010). The oxidation product of carboxyl-H2DCFDA was excited at 475 nm and the emission intensity was measured at 500–550 nm. After the measurement, cells were treated with 200 μl of trypsin for 5 min at 37 °C, harvested in 500 μl complete medium, centrifuged, resuspended in 1 ml complete medium, stained with trypan blue, and counted by means of a Countess automated cell counter (Invitrogen). The mean fluorescence intensity coming from each well was finally normalized to the corresponding cell number and averaged for each repetition (n = 3 independent experiments, each performed in triplicate) and finally normalized to the control condition. The signal coming from the control condition was consistent with that of the unstained sample (data not shown).
Statistical Analysis
Data are reported as mean ± SEM obtained from at least three independent experiments (in figure legends, “n” indicates the number of experiments performed). Data were statistically analyzed in Prism 6.00 (GraphPad Software, San Diego, CA; RRID:SCR_002798). For parametric data, Student's t-test (unpaired, two-tailed) or one-way ANOVA (Tukey's or Dunnett's multiple-comparisons test) was used; the mean values obtained in each repeated experiment were assumed to be normally distributed about the true mean. Statistical significance refers to results for which P < 0.05 was obtained.
RESULTS
Psychosine Induces Autophagy
To monitor the effects of PSY on the autophagic pathway, human oligodendrocyte MO3.13 cells were treated with PSY 10 μM for 24 hr, and LC3-I/-II and p62 protein expression levels and LC3 localization were investigated. First, we monitored LC3 cell localization by LC3-YFP transfection and confocal fluorescence microscopy. This method allowed us to evaluate autophagosome formation in living cells by visualizing and quantifying the presence of LC3-YFP-positive puncta. At the beginning of autophagosome formation, in fact, LC3 is converted into a cleaved form (LC3-II) and inserted into autophagosome membranes, becoming less diffuse and becoming concentrated in so-called puncta (Proikas-Cezanne, 2007; Travassos et al., 2010). LC3-YFP-based assessment of autophagosome has been reported to be both more sensitive and specific compared with other techniques, including electron microscopy (Heiseke et al., 2009). As shown in Figure 1a, control cells are characterized by a diffused fluorescent signal and a low number of autophagosomes (2.4 ± 0.3 autophagosomes/cell). Conversely, PSY-treated cells revealed increased LC3 aggregation (5.3 ± 0.3 autophagosomes/cell), thus indicating autophagy activation (P < 0.01 PSY vs. control, Student's t-test).
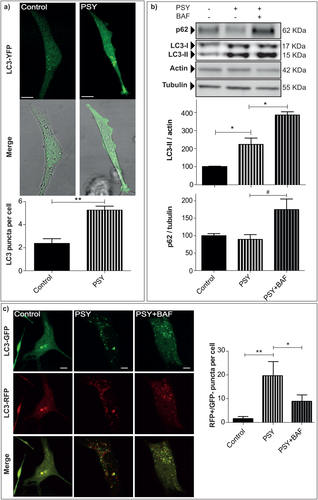
Psychosine induces autophagy. a: MO3.13 cells were transiently transfected with LC3-YFP, treated with 0.2% FBS medium alone (control) or PSY 10 μM (PSY) and imaged after 24 hr by live-cell confocal microscopy. The number of autophagosomes was quantified as the amount of LC3 puncta per cell in each condition; **P < 0.01 PSY vs. control, Student's t-test; At least 15 cells for each condition were quantified per experiment. b: To monitor the induction of autophagy activity, the LC3-I/-II and p62 protein expression levels were quantified in MO3.13 cells differently treated as follow: Control and PSY (10 µM), in the presence or not of Bafilomycin 200 nM (PSY + BAF). Results were normalized to the actin or tubulin content and and averaged the control condition within each experiment; for LC3-II: *P < 0.05, PSY vs. control and vs. PSY + BAF, Dunnett's test (n = 3); for p62: #P < 0,05, PSY + BAF vs. PSY, Student's t-test. c: To detect the autolysosomes formation MO3.13 cells were transfected with LC3-RFP-GFP, treated with 0.2% FBS medium alone (control), PSY, PSY + BAF and imaged after 24 hr by live-cell confocal microscopy. The number of autolysosomes was quantified as the amount of the LC3-RFP-positive/LC3-GFP-negative puncta per cell; **P < 0.01 PSY vs. control and *P < 0.05 PSY + BAF vs. PSY Tukey's test (n = 3); At least 12 cells for each condition were quantified per experiment. Scale bars = 10 μm.
Then, the expression of the two isoforms of LC3, LC3-I an LC3-II, was assayed by Western blotting. The conversion (cleavage) of LC3-I (17 kDa, a cytoplasmic form) into LC3-II (15 kDa, a cleavage form) is correlated with autophagic activity. Representative blots and their quantification are given in Figure 1b. We found a significant increase of LC3-II levels in PSY-treated cells with respect to the control condition and a further increase in cells treated with PSY together with BAF (P < 0.05 PSY vs. control and vs. PSY + BAF, Dunnett's test). BAF is a drug that blocks the fusion between lysosomes and autophagosomes, and it is typically used to prevent LC3-II degradation. This control condition is needed to preclude that the measured LC3-II increase induced by PSY can stem from impaired autophagosome-lysosome fusion instead of augmented autophagy (Mizushima et al., 2007; Rubinsztein et al., 2009).
Together with LC3, p62 is an autophagic activity marker that was examined by Western blotting. p62 Is a ubiquitin-binding protein that can link ubiquitinated proteins to the autophagic machinery, making their degradation possible within the lysosome. As shown in Figure 1b, the levels of p62 were significantly increased in cells treated with PSY and BAF compared with those measured for cells treated only with PSY (P < 0.05 PSY + BAF vs. PSY, Student's t-test).
To verify directly that PSY does not impair autolysosome formation, we measured the lysosomally driven GFP quenching after transfection with the LC3-RFP-GFP construct. GFP is a stably folded protein, relatively resistant to lysosomal protease. However, the low pH inside the lysosomes quenches the fluorescent signal of GFP, making it difficult to trace the delivery of LC3-GFP into lysosomes (Kabeya et al., 2000; Bampton et al., 2005). In contrast, RFP exhibits a more stable fluorescence in acidic compartments, allowing LC3-RFP-GFP constructs to label autophagosomes as GFP- and RFP-positive (GFP+/RFP+) puncta, and autolysosomes as GFP-negative and RFP-positive (GFP–/RFP+) puncta (Mizushima et al., 2010). As shown by the representative confocal fluorescence images in Figure 1c, a quite evident increase in both autophagosomes and autolysosomes was detected in PSY-treated cells with respect to the control condition, whereas we found an increase of autophagosomes only in PSY + BAF-treated cells caused by the blockage of autophagosome maturation. The number of autolysosomes under the different conditions was finally quantified as the number of GFP–/RFP+ puncta, confirming the significant increase of autolysosome formation following PSY administration (Fig. 1c; P < 0.01 PSY vs. control and P < 0.05 PSY + BAF vs. PSY, Tukey's test). Taken together, these data indicate an activation of the autophagic machinery following PSY administration in MO3.13 cells.
Li Increases Autophagy in Psychosine-Treated Cells
To investigate which Li concentration could be tolerated by MO3.13 cells, a dose–response vitality curve was obtained in the presence of increasing concentrations of Li (Fig. 2a). To this end, cells were stained with annexin V/PI and analyzed by flow cytometry (for details see Materials and Methods). A decreasing trend in cell survival was found by increasing concentration from 10 to 100 mM (P < 0.05 Li 100 mM vs. control, Dunnett's test). Given these results and that Li is therapeutically used at serum concentrations between 0.2 and 2 mM in humans (Motoi et al., 2014; Malhi et al., 2013), 1 mM and 2 mM were chosen for all our experiments. These values are are typically used also for in vitro studies (Dwivedi and Zhang, 2015).
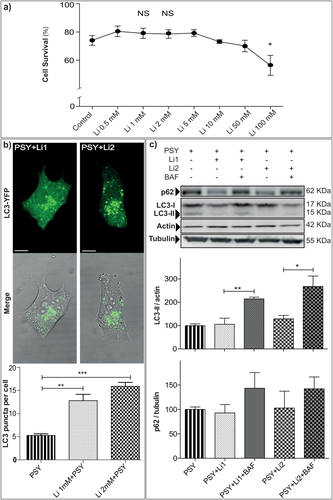
Li increases autophagy in psychosine-treated cells. a: Li dose–response viability curve obtained by annexin V/PI staining and flow cytometry; *P < 0.05, Li 100 mM vs. control, Dunnett's test (n ≥ 4). b: MO3.13 cells were transiently transfected with LC3-YFP, treated with PSY 10 μM (PSY), PSY and Li 1 mM (Li1 + PSY), or Li 2 mM (Li2 + PSY) and imaged after 24 hr by live-cell confocal microscopy. The number of autophagosomes was quantified as the amount of LC3 puncta per cell in each condition; **P < 0.01 Li1 + PSY vs. PSY and ***P < 0.001 Li2 + PSY vs. PSY, Dunnett's test (n = 3). At least 15 cells for each condition were quantified per experiment. c: To investigate the autophagy activation in PSY-treated MO3.13 cells after Li treatments further, LC3-II and p62 levels were quantified in cells treated with Li1 + PSY, Li2 + PSY, in the presence or not of Bafilomycin 200 nM (Li1 + PSY + BAF, and Li2 + PSY + BAF). Results were normalized to the actin or tubulin content and and averaged for each repetition toward the PSY condition; for LC3-II: **P < 0.01 Li1 + PSY + BAF vs. Li1 + PSY and *P < 0.05 Li2 + PSY + BAF vs. Li2 + PSY, Tukey's test; for p62: *P < 0.05 Li1 + PSY + BAF vs. Li1 + PSY, Dunnett's test (n = 3). Insets: Representative blot panels. Scale bars = 10 μm.
MO3.13 cells were thus pretreated with Li 1 mM or 2 mM and then given PSY 10 μM for 24 hr (Li1 + PSY, Li2 + PSY). To investigate the effect of Li on autophagy in PSY-treated cells, LC3-I/-II and p62 protein expression levels and LC3 localization were evaluated by confocal imaging and Western blotting, as for PSY alone (Sec 3.1). We found that LC3-positive puncta in Li1 + PSY (13 ± 2 autophagosomes/cell)- and Li2 + PSY (15.9 ± 0.8 autophagosomes/cell)-treated cells were significantly more abundant than in cells treated with PSY alone (P < 0.01 PSY vs. Li1 + PSY and P < 0.001 PSY vs. Li2 + PSY, Dunnett's test), indicating an upregulation of the autophagosome formation machinery (Fig. 2b). We next investigated the levels of LC3-I/-II by Western blotting (Fig. 2c). Li1 + PSY and Li2 + PSY treatments did not lead to statistically significant LC3-II variations with respect to the control condition (PSY treatment alone). Nonetheless, addition of BAF to Li1 + PSY- and Li2 + PSY-treated cultures could increase the LC3-II expression markedly (P < 0.01 Li1 + PSY vs. Li1 + PSY + BAF and P < 0.05, Li2 + PSY vs. Li2 + PSY + BAF, Tukey's test), demonstrating that the autophagy flux is not blocked by Li. A comparable trend was found for p62 (Fig. 2c). In this case also, Li1 + PSY and Li2 + PSY treatments compared with PSY alone led to similar p62 levels, and BAF administration together with Li led to enhanced p62 expression, although this did not reach statistical significance. Overall, these data demonstrate that cells given Li and PSY tend to produce more LC3-posivite autophagosomes without impairment of the autophagy flux with respect to cells treated with PSY alone, in agreement with the hypothesis that Li can stimulate autophagy in the presence of an excess of exogenous PSY in MO3.13 human oligodendrocytes.
Psychosine Content in PSY- and Li-Treated Cells
To investigate whether stimulation of autophagy by Li could led to a clearance of the PSY content in oligodendrocytes, PSY levels were quantified in MO3.13 treated cells (PSY, Li1 + PSY and Li2 + PSY) by HPLC/MS analysis; untreated MO3.13 cells were used as negative control. This measure turned out to be rather difficult because of parasitic signals originating from residual noncellular PSY present in the lipid extract (for a detailed description of the sample preparation for HPLC/MS analysis see Materials and Methods). For this reason, to gain a better comparison of different experiments, we normalized data within each experiment to the positive control (PSY-treated cells). As the representative mass spectrometry time/count plot of Figure 3a shows, our analytical method allowed us clearly to detect and quantify the PSY signals in our positive control (cell treated with PSY only); as expected, no PSY peak was present in our negative control (data not shown).

Evaluation of PSY content in PSY-treated MO3.13 cells after Li administration. a: Representative time/count plot of the monitored transition for HPLC-MS quantification of PSY in PSY-treated MO3.13 cell lysates. The monitored transitions are shown at top right; the selected transitions for quantitative purpose are: transitions 328.2/310.2 for N,N-dimethylsphingosine and transition 462.5/282 for PSY. b: PSY content in PSY-treated MO3.13 cells after Li administration (Li1 + PSY, Li2 + PSY); values are normalized to the PSY condition within each experiment (n = 7).
The quantification of seven independent experiments is summarized by the graph in Figure 3b, in which cells pretreated with Li revealed a reduction of PSY average levels compared with cells treated with PSY alone. In particular, PSY mean values decreased by about 25% and 20% in cells pretreated with Li 1 mM and Li 2 mM, respectively. Although the above-mentioned noise did not allow us to reach statistical significance, the measured decreasing tendency of the PSY content in cells given Li suggests that the autophagic activity induced by Li can succeed in removing some of the exogenously introduced PSY.
Li Improves Viability of Psychosine-Treated Cells
MO3.13 cells were treated with PSY 10 μM for 24 hr, and viability measured by annexin V/PI staining and flow cytometry (as described in Materials and Methods). An increased mortality rate (Fig. 4b,c; P < 0,001 PSY vs. control, Student's t-test) was measured, as we previously reported (Voccoli et al., 2014). Next, we investigated the effect of Li on PSY-treated cell viability. Representative FACS scatterplots are shown in Figure 4a for the PSY, Li1 + PSY, and Li2 + PSY conditions and for the untreated control. Already from these plots it is possible to notice qualitatively that Li can reduce the number of dots present in R3 quadrants with respect to that measured for cells treated only with PSY, indicating a reduction of secondary necrosis with the following recovery of viability. The quantification of this experiment is given in Figure 4b,c; the administration of Li significantly increased the percentage of viable cells (P < 0.05 Li1 + PSY vs. PSY and P < 0.01 Li2 + PSY vs. PSY, Dunnett's test; Fig. 4b) and decreased secondary necrosis (P < 0.01 Li1 + PSY vs. PSY and P < 0.001 Li2 + PSY vs. PSY, Dunnett's test; Fig. 4c) compared with cells treated with PSY alone.
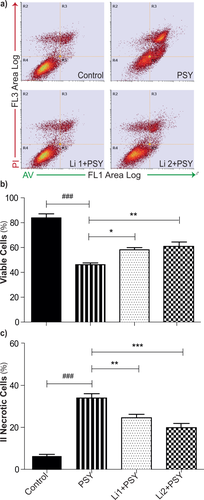
Li treatment improves cell viability in PSY-treated MO3.13 cells. a: Representative dot-plots of MO3.13 cells stained with annexin V/PI and analyzed by flow cytometry: R4, R5, R3 and R2 quadrants define, respectively, the healthy, apoptotic, secondary necrotic, and necrotic populations. b: The number of viable cells is reported in percentage of the total cell number. ###P < 0.001 PSY vs. control, Student's t-test; *P < 0.05 Li1 + PSY vs. PSY and **P < 0.01 Li2 + PSY vs. PSY, Dunnett's test (n = 4). c: The number of II necrotic cells is reported in percentage of the total cell number; ###P < 0.001 PSY vs. control, Student's t-test; **P < 0.01 Li1 + PSY vs. PSY and ***P < 0.001 Li2 + PSY vs. PSY (n = 4).
To investigate further whether the improved viability could be caused by Li-induced activation of autophagy, MO3.13 cells were treated with PEI. PEI is an inhibitor of the autophagy pathway that, by elevating intracellular levels of IP3 (Williams et al., 1999), has an effect opposite that of Li (Sarkar et al., 2005). First, to test which PEI concentrations could be tolerated by MO3.13 (Fig. 5a), a dose–response curve was obtained with increasing PEI concentrations. Only concentrations greater than 100 μM were toxic (P < 0.05 PEI 100 μM vs. control and P < 0.001 PEI 250 μM vs. control, Dunnett's test). Given these results and according to the literature (Sarkar et al., 2005), a concentration of 25 μM was chosen for the experiments. As shown in Figure 5b,c, cells given both PSY and PEI showed significantly less viability than cells exposed only to PSY (P < 0.05 PEI + PSY vs. PSY, Dunnett's test). We note that this cannot be ascribed to an additive cytotoxic effect of PEI because the PEI concentration was chosen to be fully tolerated by the cells. Therefore, downregulation of the autophagic pathway activated by Li makes MO3.13 cells less tolerant to PSY, in agreement with the viability rescue that we obtained with Li. These experiments demonstrate that Li pretreatment can reduce the cytotoxicity of PSY in MO3.13 cells. However, the viability rescue obtained in the presence of PSY can be ascribed to Li-driven autophagy activation.
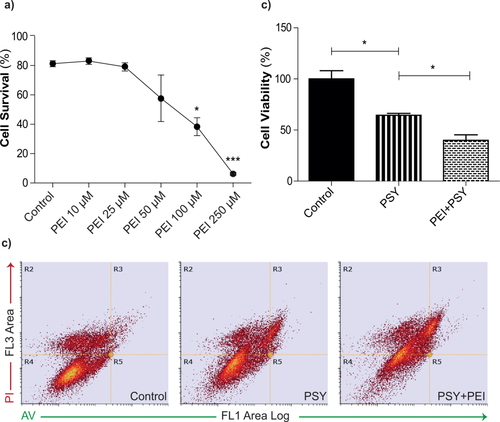
PEI decreases cell viability of PSY-treated MO3.13 cells. a: Representative dot plots of MO3.13 cells treated with 0.2% FBS medium alone (control), PSY 10 μM (PSY), or PSY followed by PEI 25 μM (PEI + PSY); cells were stained with annexin V/PI and analyzed by flow cytometry: the R4 quadrant defines the viable cells and it was set on control condition. b: PEI dose–response viability curve obtained by annexin V/PI cell death assay; *P < 0.05 PEI 100 μM vs. control, ***P < 0.001 PEI 250 μM vs. control, Dunnett's test (n ≥ 3). c: PEI-induced reduction of viable cells in PSY-treated MO3.13 cells; *P < 0.05 PSY + PEI vs. PSY and *P < 0.05 control vs. PSY, Dunnett's test (n = 3).
Improved Viability Does Not Correlate With Reduced Cytoplasmic Oxidative Stress
Finally, it is known that PSY-induced cell death is associated with increased ROS production (Voccoli et al., 2014, Hawkins-Salsbury et al., 2012), so ROS levels were investigated under our experimental conditions by H2-DCFDA staining and flow cytometry. As expected, MO3.13 cells treated with PSY revealed an augmented cytoplasmic ROS with respect to control conditions (Fig. 6). Interestingly, the additional administration of Li, both 1 and 2 mM, did not ameliorate the oxidative stress (P > 0.01 Li1 + PSY and Li2 + PSY vs. PSY, Dunnett's test), though leading to better cell viability (see above). To avoid possible stress originating from cell detachment before flow cytometry, we performed another set of experiments in which the dye fluorescence was read from adhered cells by microplate fluorimetry (for details see Materials and Methods). The result of these measurements is summarized in Figure 6c, confirming what we obtained by flow cytometry. These final experiments show that Li protection from PSY cannot be ascribed to a nonspecific reduction of cytosolic oxidative stress.
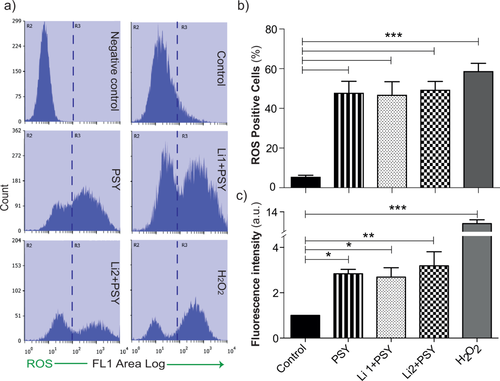
ROS detection in PSY- and Li-treated MO3.13 cells. a: Representative histogram plots of MO3.13 cells treated with 0.2% FBS medium alone (control), PSY 10 μM (PSY), PSY and Li 1 mM (Li1 + PSY), or Li 2 mM (Li2 + PSY); H2O2 100 mM was used as positive control. Cells were stained with carboxyl-H2DCFDA and analyzed by flow cytometry. The R3 region was set by the histogram plot obtained for the unstained samples (negative control). b: Cytoplasmic ROS levels increase after PSY administration and remain constant after additional Li treatments; ***P < 0.001 PSY, Li1 + PSY, Li2 + PSY, and H2O2 vs. control, Dunnett's test (n = 4). c: DCF fluorescence from adhered cells measured by microplate fluorimetry. Cells treated with PSY revealed an increased amount of cytoplasmic ROS with respect to the control condition; *P < 0.05 PSY and PSY + Li1 vs. control, ***P < 0.001 PSY + Li2 vs. control, Dunnett's test (n = 3). For each condition, the mean fluorescence intensity was normalized to the correspondent cell number and to the control condition within each experiment.
DISCUSSION
This study demonstrates that Li pretreatment can reduce exogenous PSY cytotoxicity in the human oligodendrocyte cell line MO3.13 and that this correlates with the activation of the autophagic pathway. GLD is a lysosomal storage disorder characterized by the intracellular accumulation of PSY, a cytotoxic sphingolipid, whose cytotoxicity has been clearly demonstrated in many studies, especially for oligodendrocytes. This lies at the basis of the so-called PSY hypothesis (Suzuki, 1998), according to which the GLD phenotype would originate almost exclusively from the presence of a high concentration of PSY in the central nervous system (CNS), causing demyelination and progressive, rapid neurodegeneration. Although peripheral neuropathy is also present in GLD, and evidence is recently emerging that GALC loss of function also has a negative impact at cellular and tissue levels before PSY accumulation can be detected (Smith et al., 2011; Giacomini et al., 2015), PSY clearance from the CNS remains the primary therapeutic action to be pursued. To this end, enzyme replacement therapies (ERTs) that aim to restore or replace the defective enzymatic activity (Lee et al., 2005, 2007) were developed and tested in the GLD murine model with alternating results, mainly because of the well-known difficulty in delivering large molecules to the CNS. Gene therapies seem at the moment the most promising approaches, especially if combined with other methods such as bone marrow transplantation and neural stem cell therapy (Li and Sands, 2014; Ricca et al., 2015). These methods often were successful in restoring GALC activity in the CNS and in ameliorating GLD models in vitro and in vivo, but they could still not satisfactorily rescue the pathological phenotype. For this reason, a belief that additional treatments should be introduced in combination, aiming specifically to correct molecular dysregulations, is emerging. The mechanisms by which PSY imparts toxicity are still to be fully understood, and research on molecular pathogenesis in this context is necessary to discover new pharmacological targets. Our study addresses for the first time autophagy in GLD with the aim of understanding whether this could be a possible therapeutic target with combined therapies. Remarkably, for medical purposes, drugs for inducing or inhibiting autophagy are already available for clinical practice (Rubinsztein et al., 2012).
For our experiments we decided to use an accepted model of GLD in vitro for testing against PSY excess, which consists is a human model of oligodendrocytes, the MO3.13 cell line, cultured in an excess of exogenous PSY. This model, although somewhat simplified, has already successfully allowed unravelling novel molecular mechanisms affected by the presence of PSY (Haq et al., 2003; Giri et al., 2006, 2008). By using a 10 μM PSY concentration in previous work, we quantitatively demonstrated apoptotic cell death in the same model by annexinn V/PI staining and flow cytometry (Voccoli et al., 2014). These data gave us the basic information for trying to rescue viability by acting on autophagy. Given the results obtained by Ribbens et al. (2013), which suggested an upregulation of autophagosomes formation induced by PSY, we hypothesized that by helping this degradation pathway we would be able to help cells in PSY clearance and improve their tolerance to PSY, with a consequent increase in cell viability.
First, we characterized the autophagic pathway in the presence of PSY. To this end, LC3-I/-II and p62 expression were measured by Western blotting, and autophagosome/autolysosome formation was imaged by LC3-GFP/LC3-RFP-GFP transfection and confocal microscopy. Consistently with the results cited above, exogenous PSY administration could stimulate autophagy also in MO3.13 cells. LC3-positive vesicle formation was indeed significantly augmented with respect to the case of nontreated cells (Fig. 1a), indicating an enhanced production of autophagosomes. At the molecular level, although we did not found a clear trend for p62, LC3-II expression was upregulated. The amount of LC3-II is indeed closely correlated with the number of autophagosomes, serving as a good indicator of autophagosome formation. However, because LC3-II itself is degraded by autophagy, LC3 immunoblotting is sometimes interpreted inappropriately: an LC3-II increase upon treatment might in fact also indicate a blockage of the autophagic degradation. As suggested by Mizushima et al. (2007), we repeated the experiment in the presence of BAF, an inhibitor of autophagosome–lysosome fusion, to block LC3-II degradation. LC3-II expression was in this case further increased, indicating that autophagy was not blocked at the level of autophagosome/lysosome fusion. p62 Can bind LC3, thus serving as a selective substrate of autophagy, and for this reason it would be expected to follow LC3 levels. Actually, the expression level of p62 can also change independently of autophagy (Ju et al., 2010; Puissant et al., 2012; Xie et al., 2013), making data interpretation difficult. In our case, although we did not find a variation induced by PSY, the treatment showed an increasing trend consistent with what we measured for LC3-II. To show that the autophagic machinery is active to its final stage, we imaged the delivery of LC3-RFP-GFP to the lysosomes by confocal imaging in living cells. An important increase in the number of autolysosomes was detected in cells treated with PSY that, as expected, did not occur in the presence of BAF (Fig. 1c). Therefore, we can conclude that exogenous PSY did not block autophagy in our experimental model, which instead showed quite an evident autophagy activation.
To stimulate autophagy further, we chose to use Li, which is clinically accepted for the treatment of bipolar disorders and whose neurotrophic and neuroprotective properties are known (Malhi et al., 2013). Pretreatment with Li was effective in regulating autophagy in various neuropsychiatric diseases such as Huntington's disease, Alzheimer's disease, Parkinson's disease, prion disease, and amyotrophic lateral sclerosis (Motoi et al., 2014). Interestingly, autophagy was not always upregulated. Conversely, it was reported that in a mouse model of cerebral ischemia and Alzheimer's disease Li can instead lead to downregulation. We thus repeated the analysis described above in cells pretreated with Li and exposed to PSY. As shown in Figure 2b, autophagosome formation was clearly stimulated by Li. The molecular analysis of LC3-I/-II and p62 was instead less straightforward. Nonetheless, the increasing trend of both these proteins upon blocking of autophagic degradation by BAF is consistent with the measured augmented number of autophagosomes and with autophagic flux upregulation.
Therefore, the cytotoxic effect of PSY was essayed upon Li pretreatment and was diminished. The cell culture was indeed less sensitive to PSY, and cell death was reduced with a corresponding improvement in cell viability (see Fig. 4). To test our hypothesis in which this therapeutic effect originates from a more efficient PSY degradation, PSY accumulation in cells was quantified. Although a decreasing trend was found in PSY intracellular content, this variation was not large enough to reach statistical significance with our experimental pool. For this reason, we must suppose that other mechanisms not related to PSY clearance might contribute to the observed cell viability rescue. For example, cytotoxic molecules other than PSY, yet accumulating in Krabbe's disease (KD), might be better digested by Li-stimulated autophagy. Smith et al. (2015) indeed identified the presence of misfolded protein aggregates in both human and murine KD brains, consisting primarily of aggregated α-synuclein, and demonstrated that administration of PSY facilitates α-synuclein aggregation in vitro. In light of this, and given that autophagy stimulation has been successfully targeted to ameliorate some α-synucleinopathies (Decressac et al., 2013; Hu et al., 2016; Tian et al., 2016), we can speculate about a connection between the clearance of some aggregate-prone protein and improved cell viability. Interestingly, cytosolic ROS production was not affected by Li (Fig. 6). Oxidative stress has been one of the first targets tested for counteracting PSY cytotoxicity. Administration of the antioxidant N-acetylcysteine (NAC) in cell cultures exposed to exogenous PSY gave good results for ameliorating cell viability, but, unfortunately, almost no effect was seen with its administration in TWI mice as dietary supplement (Hawkins-Salsbury et al., 2012). As in our case, Li did not exert its protective effect by reducing ROS production; other mechanisms related to PSY or other cytotoxic protein reduction could come into play. Altered autophagy and accumulation of lipids, especially cholesterol and glycosphingolipids, have been reported for a wide range of LSDs, including those without primary defects in glycosphingolipid or cholesterol degradation (Settembre et al., 2008a, 2008b). These lipids are the principal constituents of lipid rafts, specialized membrane microdomains rich in signaling molecules and that influence membrane fluidity (Tommasino et al., 2015). Remarkably, lipid rafts were reported to be disrupted in GLD, and this was demonstrated to be caused by PSY accumulation in lipid rafts. Lipid rafts are present not only in the plasma membrane but also in other parts of the cell such as the endoplasmic reticulum, mitochondria-associated membranes, Golgi, mitochondria, and lysosomes (Lingwood et al., 2010; Sorice et al., 2012) and are involved in many signaling cascades, including autophagy. It is possible that the abnormal accumulation of PSY affects the physiology of lysosomal membranes, making the autophagy pathway a good target for counteracting some of the PSY effects. Intriguingly, also amyloidogenic proteins, that, as previously mentioned, rapidly fibrillate in the presence of PSY, have been demonstrate to interacts with raft glycosphingolipids (Fantini et al., 2011). Finally, also inflammation, a neglected part of GLD, could come into play. It is known that the inflammasome, a molecular platform activated by stress that regulates the activity of caspases and cytokines, colocalizes with the autophagosome and that a blocking of autophagy potentiates inflammasome activity, whereas stimulating autophagy limits it (Shi et al., 2012). Recently it has been demonstrated that PSY-treated oligodendrocytes show activation of TLR2 signaling, with consequent innate immune system activation (Snook et al., 2014); indeed, it is possible that autophagy activation cold help the cell in attenuating the inflammation response. Although additional studies are required to elucidate the molecular mechanism correlated with the Li improvement in cell viability in PSY-treated oligodendrocytes, these data give a strong rationale to investigate further the modulation of the autophagic pathway in GLD.
CONCLUSIONS
This study, investigates autophagy as a therapeutic target to counteract PSY cytotoxicity in the human oligodendrocyte cell model MO3.13. We reported that PSY causes upregulation of the autophagic flux at the level of autophagosomes formation and LC3-II and p62 expression. We showed that pretreatment with Li, a drug clinically used to treat bipolar disorders, can further stimulate autophagy, improving cell tolerance to PSY. We measured improved cell viability, with a corresponding reduction of cell apoptotic death. Finally, our data show that PSY-induced cytosolic oxidative stress is not reduced by Li, although it might help in PSY degradation. Altogether these data provide novel information on the intracellular pathways activated during PSY-induced toxicity and suggest the autophagy pathway as a potential novel therapeutic target for ameliorating the GLD phenotype.
ACKNOWLEDGMENTS
We acknowledge Dr. Mario Costa for having kindly provided the LC3-YFP construct.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest.
ROLE OF AUTHORS
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: ADG, MC. Acquisition of data: ADG, LA, SA, GS, IT. Analysis and interpretation of data: All authors. Drafting of the manuscript: All authors. Statistical analysis: ADG, IT, MC. Obtained funding: MC. Study supervision: MC.