Animal models of chronic pain: Advances and challenges for clinical translation
SIGNIFICANCE Chronic pain is pervasive and among the most difficult challenges in medicine. An assortment of animal models has been developed to study the underlying causes of chronic pain. These animal models are instrumental in the discovery of candidate therapeutic targets, but the translational success from animals to humans critically depends on a thorough appreciation of the inherent benefits and limitations of each model. This review highlights common animal models used to study pain and the major challenges of translating preclinical discoveries into realized pain therapies.
Abstract
Chronic pain is a global problem that has reached epidemic proportions. An estimated 20% of adults suffer from pain, and another 10% are diagnosed with chronic pain each year (Goldberg and McGee, 2011). Despite the high prevalence of chronic pain (an estimated 1.5 billion people are afflicted worldwide), much remains to be understood about the underlying causes of this condition, and there is an urgent requirement for better pain therapies. The discovery of novel targets and the development of better analgesics rely on an assortment of preclinical animal models; however, there are major challenges to translating discoveries made in animal models to realized pain therapies in humans. This review discusses common animal models used to recapitulate clinical chronic pain conditions (such as neuropathic, inflammatory, and visceral pain) and the methods for assessing the sensory and affective components of pain in animals. We also discuss the advantages and limitations of modeling chronic pain in animals as well as highlighting strategies for improving the predictive validity of preclinical pain studies. © 2016 Wiley Periodicals, Inc.
SCOPE OF THE CHRONIC PAIN PROBLEM
Pain is often considered a curse that society is better off without. On the surface, the notion of a pain-free existence seems ideal, but the ability to sense pain is vital to our survival and is a key component of the body's natural defense. Acute nociceptive pain is a normal function of the nervous system that provides important sensory information about the environment, and it is an early warning mechanism that protects against noxious heat, extreme cold, chemical irritants, and mechanical tissue damage. These noxious stimuli activate peripheral nociceptors, triggering action potentials that propagate into the dorsal horn of the spinal cord, where incoming nociceptive signals are actively processed and filtered before being relayed to the brain. Activation of specific centers within the brain produces a rich tapestry of sensory, emotional, autonomic, and motor responses that collectively shapes our experience and perception of pain.
The importance of perceiving pain is most striking in patients with congenital insensitivity to pain (CIP), a rare genetic disorder linked to altered expression of several genes (Nagasako et al., 2003; Oertel and Lötsch, 2008). In particular, a null mutation in the SCN9A gene encoding for the Nav1.7 sodium channel is causally linked to the inability of CIP patients to feel thermal and mechanical pain (Cox et al., 2006). Without acute nociceptive pain, individuals with CIP may continue to engage in harmful behavior that puts them at risk of severe injuries and even death (Protheroe, 1991). In stark contrast to CIP patients, who feel no pain, chronic pain patients have a heightened sensitivity to pain. Chronic or pathological pain results from abnormal functioning of the nervous system, with pain persisting far beyond the resolution of the injury or insult. The cardinal feature of chronic pain is pain hypersensitivity, which manifests as spontaneous pain (pain in the absence of an overt stimulus), allodynia (pain resulting from an innocuous stimulus), and/or hyperalgesia (an exaggerated pain response to a noxious stimulus). These opposing pain disorders, CIP and chronic pain, illustrate the paradox of pain; although acute pain is necessary and protective, chronic unremitting pain confers no known physiological advantage and can be so severe that individuals sometimes prefer death. Great strides have been made toward understanding the mechanisms of acute pain, yet little is known about the core processes that transform acute pain to chronic pain. A peculiar aspect of pain is the great variability among individuals in the likelihood of transitioning from acute to chronic pain. The location, type, or extent of injury is not necessarily predictive of the severity or chronicity of the ensuing pain. For example, the extent of cartilage degradation is not predictive of pain reported by osteoarthritis patients. It has, however, become apparent that chronic pain is not simply long-lasting acute pain and that the underlying mechanisms of these two types of pain are fundamentally distinct: acute pain is a physiological function of the normal nervous system, whereas chronic pain is the manifestation of a pathologically altered nervous system (Woolf and Salter, 2000).
Chronic pain affects more than 1.5 billion people worldwide and is a major burden on health care systems, costing over 600 billion dollars annually in the United States alone (Global Industry Analysts Inc., 2011; The National Academies Press; 2011). Chronic pain can be broadly classified into chronic neuropathic pain (arising from nerve injury or disease) and chronic inflammatory pain (pain as a result of persistent or unresolved inflammation). However, this broad classification is simplistic because many chronic pain conditions have both a neuropathic and inflammatory component and others, such as cancer pain, visceral pain, and idiopathic pain, do not fall into either category. Multitudes of people with diseases such as arthritis, diabetes, AIDS, multiple sclerosis, postherpetic neuralgia, fibromyalgia, and nerve trauma are plagued with chronic pain (Kehl et al., 2000; Dinser, 2008; Jaggi et al., 2011). Chemotherapy, antiviral therapies, and surgical procedures are also common instigators of chronic pain (Jaggi et al., 2011). Therefore, it is important to recognize that there is not one overarching, singular condition called chronic pain but rather, there are multiple etiologies of pain, each resulting from different pathologies and differing in the clinical presentation of signs and symptoms.
Chronic pain has often been considered simply a consequence of disease, a symptom rather than a disease in and of itself. This view is beginning to shift, and increasing importance is being placed on considering chronic pain as a distinct entity. An urgency to study the fundamental causes of chronic pain has led to a growing body of discoveries that suggests commonalities in the cellular and molecular mechanisms that underlie chronic pain conditions (Stucky et al., 2001; Reichling and Levine, 2009; Phillips and Clauw, 2011; Descalzi et al., 2015). Despite these commonalities, there are also divergent mechanisms (some subtle, others profoundly different) that may dictate an individual's likelihood for developing chronic pain and his or her responsiveness to existing pain therapies (Wiesenfeld-Hallin, 2005; Apkarian et al., 2009; Sorge et al., 2012). To address differences in the presentation of pain symptoms across chronic pain conditions, an array of preclinical animal models has been developed to recapitulate the underlying pathology, duration, and comorbidities of pain phenotypes (Mogil, 2009). This variety of preclinical pain models is essential for understanding the molecular and cellular mechanisms that underlie distinct pain conditions.
There is extensive and clear evidence that chronic pain, regardless of the cause, is triggered by a functional reorganization of nociceptive circuitry, leading to persistent changes along the entire nociceptive pathway of the nervous system (Honore et al., 2000; Dubin and Patapoutian, 2010; Baliki et al., 2014). Persistent changes in the cellular architecture of the nervous system is a form of long-lasting plasticity involving a constellation of ion channels and signaling cascades that conspires to reduce nociceptive threshold, increase nociceptive output, and amplify pain signaling (Woolf, 1983, 2011; Woolf and Salter, 2000; Pfau et al., 2011; von Hehn et al., 2012). This altered neuronal output manifests as peripheral or central sensitization (Cervero, 1995; Graven-Nielsen and Arendt-Nielsen, 2002; Carlton et al., 2009), which culminates in aberrant pain signaling described by patients as “burning” pain with intermittent “shooting” or “electric shock” sensations that occur spontaneously or in response to stimuli such as touch, temperature change, and movement (Bouhassira et al., 2008; Bourinet et al., 2014). The severity of these symptoms varies greatly among individuals; while some patients experience neurological deficits suggestive of significant nerve injury but remain free from pain, others report severe pain despite retaining normal nerve function (Kehlet et al., 2006). Much of this variability is influenced by etiological, genetic, and environmental factors that converge to produce a diversity of pathological changes within the nervous system (Lacroix-Fralish and Mogil, 2009; Costigan et al., 2010; Nissenbaum et al., 2010; von Hehn et al., 2012; Sorge et al., 2012; Trang and Salter, 2012).
The spinal cord has emerged as a key locus for pathological adaptations that drive aberrant pain signaling (Honore et al., 2000). Specifically, increased output of spinal lamina I neurons is causally implicated in the sequelae of chronic pain (Bester et al., 2000; Coull et al., 2003). Lamina I neurons normally respond only to noxious stimuli, but after peripheral nerve injury or ongoing inflammation, the output of these neurons is transformed from nociceptive-specific to wide-dynamic-range responses (Keller et al., 2007). This functional shift in lamina I neurons may provide a neuronal correlate for allodynia, hyperalgesia, and spontaneous pain, which are the three cardinal symptoms that torment individuals with chronic pain and are notoriously resistant even to the powerful pain-relieving effects of opioid analgesics (Wiffen et al., 2013; Percie du Sert and Rice, 2014; Trang et al., 2015).
Although we are beginning to put together mechanistic pieces of the pain puzzle, a desperate lack of effective treatment options for chronic pain patients remains. The development of novel and more efficacious therapies requires a thorough understanding of the mechanistic underpinnings of chronic pain and the design and testing of new drugs; arguably, these milestones will be best achieved through the use of animal models. Indeed, preclinical animal models confer several advantages in that they: 1) allow the detailed study of chronic pain at the cellular and molecular level; 2) facilitate identification, design, and testing of candidate pain-relieving compounds; and 3) inform us on the safety of these novel compounds before testing and use in the human population. This review outlines the predominant methods used to measure acute nociception and common animal models employed to study persistent pain. Along the way, we provide a commentary on the use of animal models for the study of chronic pain, touching on how these models have advanced our knowledge of acute and chronic pain conditions, and discuss some of the major challenges associated with translating research with animals to effective therapies in the clinic.
ANIMAL MODELS OF PAIN
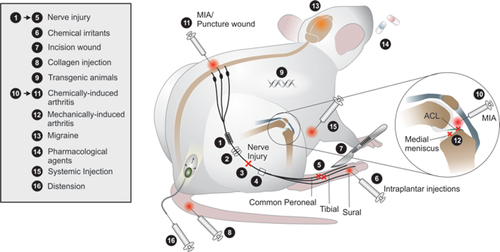
Since the early 1900s, there has been a steady increase in the use of, and the reliance on, animal models to study fundamental biological processes and causes of disease (Mogil, 2009). Among the 105 Nobel Prizes in Physiology or Medicine, 89 of these prizes acknowledged medical breakthroughs gained through the use of animal research (Buckmaster, 2014). Thus, a compelling case has been made for the use of animal models for understanding the pathophysiology of diseases and for the design of novel drugs and therapies. In particular, the study of pain relies extensively on preclinical animal models to assess the sensory and psychological complexities of this condition. The diverse neurobiological processes engaged by different types of chronic pain conditions have required the development of a battery of preclinical pain models (Fig. 1).
Rodents are employed in an overwhelming majority of preclinical pain studies (Mogil, 2009). However, the use of alternate vertebrates and invertebrates, such as zebrafish, fruit flies (Drosophila sp.), and nematodes (Caenorhabditis elegans), can also be advantageous for studying the genetic and molecular mechanisms of acute and chronic pain (Way and Chalfie, 1989; Gonzalez-Nunez and Rodríguez, 2009; Milinkeviciute et al., 2012). Each organism confers a distinct advantage for studying pain; the behavioral complexity of the rat allows for analysis of the affective components of pain (Johansen et al., 2001; Panksepp and Lahvis, 2011), whereas simpler organisms, such as Drosophila, can facilitate the discovery of novel molecular players involved in the detection of noxious stimuli (Caldwell and Tracey, 2010; Mogil et al., 2010). There is not one “best organism” for the study of pain; rather, the use of different organisms has been instrumental for constructing and integrating a comprehensive breadth of genetic, molecular, cellular, and behavioral knowledge on normal and aberrant pain processing in the central and peripheral nervous systems.
Models of Neuropathic Pain
In rodents, the most common experimental approach for inducing peripheral neuropathy is traumatic nerve injury (full or partial) via ligation, transection, or compression of the sciatic nerve (Wall et al., 1979; Bennett and Xie, 1988; Seltzer et al., 1990), distal branches of the sciatic nerve (Decosterd and Woolf, 2000; Lee et al., 2000; Vadakkan et al., 2005), infraorbital nerve (Imamura et al., 1997), or trigeminal nerve roots (Ahn et al., 2009; Yang et al., 2009). These nerve injury models are surrogates of the neuropathic pain that can arise following physical nerve trauma in humans. In contrast, preclinical models of central neuropathic pain are scarce, with most resorting to mechanically or chemically induced injuries to the spinal column (Hains and Vera-Portocarrero, 2011). Metabolic and chemically induced neuropathies in rodents have also been used to mimic peripheral neuropathic pain associated with chemotherapy, anti-HIV therapy, and diabetes (Wang and Wang, 2003; Authier et al., 2009). Animal models of HIV-associated pain (Wallace et al., 2007) and HIV therapy-induced pain (Joseph et al., 2004) along with preclinical models of postherpetic neuralgia, multiple sclerosis pain, and diabetic neuropathy are also used to study pain associated with disease-specific pathology (Fleetwood-Walker et al., 1999; Fox et al., 1999; Takasaki et al., 2000; Aicher et al., 2004; Lynch et al., 2008; Obrosova, 2009). Thus, many preclinical neuropathic pain models are available to study analogous human neuropathic pain conditions, each with strengths and limitations for addressing a wide range of experimental questions (for review see Berge, 2011; Challa, 2015; Toia et al., 2015). The design of prospective therapeutic strategies relies on the veracity of these models in recapitulating the heterogeneous pathoetiology of neuropathic pain in humans.
Models of Inflammatory Pain and Arthritis
In addition to direct nerve damage or degeneration, chronic pain can arise from persistent inflammation. A myriad of inflammatory mediators are released after injury that can lead to peripheral and central sensitization (Ji et al., 2014). The ensuing inflammatory events typically subside after tissue healing, but in some circumstances, the initial acute inflammation can persist and develop into chronic inflammatory pain. Localized inflammatory responses are commonly modeled in animals by injecting a chemical irritant, such as formalin, complete Freund's adjuvant (CFA; Stein et al., 1988), or carrageenan (Martin et al., 1984), into the paw (Murray et al., 1988). Each chemical irritant produces a unique time course of pain responses lasting from minutes (e.g., formalin) to weeks (e.g., CFA), allowing for short- or long-term studies of local inflammation (Zhang and Ren, 2011).
Arthritis is one of the most debilitating types of chronic inflammatory disease is arthritis, which afflicts approximately 52 million Americans (Barbour et al., 2012; Centers for Disease Control and Prevention, 2013) and nearly 50% of the global population by age 85 (Murphy et al., 2008). To better understand the underlying mechanisms of arthritis pain and to develop novel effective treatments, animal models have been developed to recapitulate specific clinical etiologies of arthritis. Rheumatoid arthritis (RA) is the most common type of autoimmune arthritis (Alamanos et al., 2006), in which persistent joint inflammation leads to articular breakdown and chronic pain. Animal models have been developed to study the clinical etiology of RA with inducible and spontaneous models, e.g., collagen-induced arthritis (Myers et al., 1997) and K/BxN mouse (Monach et al., 2007). Studies using these models have led to an improved understanding of RA and the development of effective treatments, such as cytokine scavengers (against tumor necrosis factor-α, or interleukin-1β) (Williams et al., 1992; van den Berg et al., 1994), and direct depletion of B-cells (using rituximab), which suppresses adaptive immune pathways (Edwards et al., 2004). In contrast to RA, the clinical presentation of osteoarthritis (OA) includes minimal joint inflammation (Bhatia et al., 2013), and animal models of OA reflect this. OA occurs following breakdown of articular cartilage over time, often as a result of trauma, infection, or disease (Alshami, 2014). Surgically induced models closely simulate the clinical syndrome of post-traumatic OA, in which a transection or tear of a ligament, such as the anterior cruciate ligament (ACL) or medial meniscus, causes destabilization of the knee joint, altered load bearing, and the development of OA-like pain (Fang and Beier, 2014). OA can also be modeled chemically by intra-articular injection of toxins (commonly, monosodium-iodoacetate (MIA), papain, or CFA) that cause widespread chondrocyte death, resulting in inflammation and pain (Guzman et al., 2003). Surgically induced models (e.g., ACL transection) of OA take 4–8 weeks to develop a pain phenotype (Ruan et al., 2013) and are thought to mimic the progression of post-traumatic OA in humans more closely. In contrast, chemically induced models (e.g., intra-articular MIA injection) cause OA-like pain in less than 1 week (Marker and Pomonis, 2012) but do not follow the temporal progression of the clinical disorder. The shorter onset of pathology is conducive for high-throughput screening and testing of potential targets, which is a primary reason for the widespread popularity of chemically induced models of OA, despite reservations that these models may less accurately mirror the human OA condition.
Models of Dysfunctional Pain and Other Pain Disorders
The dearth of preclinical models has been a limitation for studying certain types of chronic pain disorders, including migraine, chronic back pain, musculoskeletal pain, and visceral pain. Migraine pain, for example, has been difficult to model in the preclinical setting because of its diverse underlying causes and triggers; in humans, migraine attacks can be spontaneous or evoked, and are highly variable in terms of onset, presentation, and progression (Eikermann-Haerter and Moskowitz, 2008). Animal models of migraine typically employ vasodilation by arteriovenous shunting or pharmacological agents, or by direct electrical stimulation to areas innervated by the trigeminal nerves (e.g., pial, dural, and large cerebral blood vessels; De Vries et al., 1999; Eikermann-Haerter and Moskowitz, 2008). In addition, several genetic mutations identified in human migraine patients have been developed into knock-in mouse models for studying migraine pain (Eikermann-Haerter and Moskowitz, 2008). Animal models of migraine are highly specialized, whereas models of chronic back pain have adopted elements from pre-existing pain models and applied them to local regions of the back. Kim et al. (2011) used MIA to induce back pain as a result of lumbar facet joint osteoarthritis. This group recently developed another model that involved puncture-induced injury to the lumbar facet joint, which reproduced a spectrum of signs and symptoms that are displayed in human chronic back pain patients (Kim et al., 2015). Although these models of back pain are relatively new, animal models of visceral pain have been in use for nearly three decades. For instance, colorectal distension was first used in 1988 to study visceral hypersensitivity (Ness and Gebhart, 1988). The general concepts of this distension model have been applied to study an assortment of visceral pain syndromes in animals, including the bowel, biliary system, urinary tract and bladder, and reproductive organs (Ness, 1999). Distention models have also been employed experimentally in healthy human individuals and people with functional gastrointestinal disorders, such as inflammatory bowel disease. Consequently, the use of distention models to study visceral pain syndromes in animals and humans facilitates the predictive success of findings from these models. Finally, incision pain models (typically involving incision to the plantar surface, tail, or gastrocnemius) are used to study acute postsurgical pain (Brennan, 1999; Kamerman et al., 2007; Kim et al., 2010; Spofford et al., 2011; Masaki et al., 2016; Zhu et al., 2016). These models closely recapitulate surgical pain in humans (Brennan, 1999) and have proved instrumental in understanding the pathogenesis and management of postsurgical pain. Thus, the growing repertoire of preclinical models is advantageous for teasing apart mechanistic commonalities as well as differences that underlie diverse pain disorders.
PAIN ASSESSMENT IN ANIMAL MODELS
Evoked Measurements of Pain
Animals cannot self-report, which makes assessing the extent and severity of pain a major challenge inherent to all animal pain models. The underlying assumption in each model is that an animal's behavior in response to noxious stimuli can be reliably and objectively evaluated. A battery of behavioral tests has therefore been developed to evaluate the severity and progression of pain. Most of these behavioral tests measure the latency to withdraw or escape from a thermal or mechanical stimulus; a longer latency equates to a higher nociceptive threshold. Commonly employed tests such as the tail-flick or tail-immersion test are believed to reflect spinal-mediated nociceptive responses (Le Bars et al., 2001), whereas the hot–cold plate test engages both spinal and supraspinal nociceptive mechanisms (Gårdmark et al., 1998; Le Bars et al., 2001). These acute nociceptive tests are important tools for assessing the efficacy and potency of pre-existing and novel analgesic compounds, but they are also commonly employed to measure altered nociceptive thresholds in a variety of chronic pain models. Regardless of the application, these behavioral measures in rodents are proxies for the considerably more subjective and complex perception of pain in humans.
Several techniques have been developed to assess mechanical allodynia (e.g., von Frey filaments; Frey, 1896), mechanical hyperalgesia (e.g., Randall-Selitto paw pressure apparatus; Randall and Selitto, 1957), and thermal hypersensitivity (radiant heat [e.g., Hargreaves apparatus; Hargreaves et al., 1988], acetone test [Yoon et al., 1994], hot–cold plate [Woolfe and Macdonald, 1944], and water bath [D'Amour and Smith, 1941]). Measurements of mechanical allodynia are particularly reliable for detecting altered nociceptive thresholds in nerve-injured or chronically inflamed animals (Muley et al., 2016; Reitz et al., 2016). Traditionally, measures of thermal hyperalgesia involve the application of a constant, high-threshold thermal stimulus (Bölcskei et al., 2010), and, although this technique has proved valuable for assessing the antinociceptive properties of analgesics, it can be unreliable for detecting lower nociceptive thresholds in animals with persistent pain (Lavich et al., 2005). An alternative approach advocates the use of nonstatic thermal stimuli (in which the intensity is gradually increased), which provides a more reproducible and sensitive measurement of hyperalgesia in animals with persistent pain (Bölcskei et al., 2010).
Spontaneous Measurements of Pain
Some of the tests used to evaluate nociceptive thresholds in laboratory animals hold great clinical validity for assessing and diagnosing aberrant pain sensitivity in human patients. For example, the von Frey filament test, which assesses mechanical threshold in laboratory animals, is akin to tests used to assess mechanical allodynia in humans (Tena et al., 2012). Likewise, response to hot or cold stimuli is used in both preclinical and clinical settings to evaluate thermal hyperalgesia (Jensen and Finnerup, 2014). Although there are direct translational applications for some of the behavioral nociceptive tests employed in the laboratory, a major criticism of many of these tests is that they measure evoked pain, which is reported in only 15–50% of chronic pain patients (Backonja and Stacey, 2004). By contrast, it is estimated that 100% of chronic pain patients are afflicted by spontaneous pain (Freynhagen et al., 2011). Although spontaneous pain is a debilitating feature of chronic neuropathic pain, its study has been stifled by a critical lack of assessment tools. Recently, the grimace scale has emerged as a highly reliable and reproducible assay for spontaneous pain (Langford et al., 2010). The grimace scale, developed for use in mice, rats, rabbits, cats, and horses, grades orofacial features that correlate with pain and vary depending on the pain severity (such as tightening or closing of the eyes and presence of nose and cheek bulge), which is similar to pain assessment scales used in human infants and nonverbal adults (Keating et al., 2012; Leach et al., 2012; Matsumiya et al., 2012; Costa et al., 2014; Holden et al., 2014). Although the grimace scale is valuable for assessing acute responses to pain, its utility for monitoring the progression and chronicity of pain is less reliable because animals, just like humans (Craig et al., 1991), learn to mask behaviors demonstrating pain or weakness (Matsumiya et al., 2012).
Other non-reflexive measurements of pain include weight bearing (in which the distribution between paws/gait is analyzed; Schött et al., 1994; Bove et al., 2003), home cage monitoring for abnormal behaviors (such as altered locomotor activity or grooming; Houghton et al., 1997; Goulding et al., 2008; Richardson, 2015), and open-field tests (Bailey and Crawley, 2009; Parent et al., 2012). Animals can also be subjected to free-choice tests (place conditioning, place-escape tests; Sufka, 1994; Davoody et al., 2011; Fuchs and McNabb, 2012), which correlate ongoing pain with reward processes. In combination with pharmacological and genetic interventions, place conditioning is used to assess an animal's motivation to seek pain relief and indirectly assess the effectiveness of the treatment based on the animal's preference or aversion to the environment that has been paired with the treatment. Although measures of spontaneous pain are less direct and less conducive to high-throughput testing, there are numerous advantages to measuring non-reflexive or spontaneous pain in preclinical studies. One major advantage is that many assessments of spontaneous pain do not require the presence of the experimenter in the testing room and can even be conducted in the animal's home cage. This ostensibly decreases stress experienced by the animal through handling or experimenter presence and can lead to a more “natural” pain phenotype produced by the animal, as stress is a component of testing known to alter nociceptive threshold and mask pain behaviors (Lester and Fanselow, 1985; Amit and Galina, 1986; Keogh and Cochrane, 2002; Butler and Finn, 2009).
Affective and Emotional Assessments of Pain
In addition to measurements of spontaneous or evoked pain, there has been a push toward evaluating the affective and emotional consequences of pain. The emotional toll of chronic pain has often been overlooked in preclinical studies, not necessarily because of ignorance but likely because there has been a lack of reliable experimental models. Indeed, it is estimated that 20–40% of chronic pain patients experience anxiety and depression (Meyer-Rosberg et al., 2001; Twillman, 2007). Studies investigating the interrelationship between affect and chronic pain have used the forced-swim, open-field, or elevated plus-maze tests, which evaluate anxiety-like behaviors in animals with persistent pain (Bailey and Crawley, 2009; Yalcin et al., 2014). Home-cage monitoring can reveal more subtle changes in behavior, such as locomotor activity (Buvanendran et al., 2008), burrowing, and voluntary wheel running (Cobos et al., 2012), that can provide additional insights into the wellbeing of an animal (Richardson, 2015). Despite the availability of behavioral assays to monitor quality of life or levels of stress and anxiety, animal models of chronic pain are limited by their ability to recapitulate fully the comorbid diseases known to associate with the human chronic pain condition. From a clinical perspective, it is important to recognize that the patient's concerns extend beyond the management of evoked pain; improved therapies must therefore address the full spectrum of symptoms associated with each chronic pain phenotype (Harden and Cohen, 2003).
LIMITATIONS OF PAIN RESEARCH AND FUTURE DIRECTIONS
There are, admittedly, limitations to the use of animal models for the study of pain; however, the discoveries made from these studies have been instrumental in advancing our understanding of the mechanistic underpinnings of pain states and in the development and testing of novel analgesic compounds. Although there are examples of targets that show tremendous promise at the preclinical stage that fail to translate into realized therapies, there are also numerous examples of translational success. One such success story is ziconotide, a derivative of ω-conotoxin (specifically ω-MVIIA) that blocks N-type voltage-gated calcium channels (McGivern, 2007). In preclinical rodent studies, ziconotide produced antiallodynic and antihyperalgesic effects in models of chronic inflammatory (Sluka, 1998; Smith et al., 2002) and neuropathic (Chaplan et al., 1994; Yamamoto and Sakashita, 1998; Berman et al., 2000; Scott et al., 2002) pain while also alleviating hyperexcitability of dorsal horn neurons following intraplantar formalin administration (Diaz and Dickenson, 1997). After successful phase III clinical trials, ziconotide was approved as a nonopioid analgesic for the management of chronic inflammatory and neuropathic pain (Wallace et al., 2006; Williams et al., 2008). Ziconotide is now widely used and highly regarded by clinicians as being more efficacious, long lasting, and tolerable than classical analgesics, such as opioids (McGivern, 2007). Successful drug development (e.g., ziconotide) with animal studies makes a strong case for the importance of preclinical pain research. However, the predictive validity of targets identified in animal models does not always translate to effective pain therapies in humans. It is no secret that there are substantially more failures than successes in novel therapies developed from preclinical animal studies. Some drugs that show promise at the preclinical phase (i.e., attenuate pain states and exhibit high efficacy and low toxicity) fail during clinical trials. Most notably, MK-869, a neurokinin-1 antagonist, and ralfinamide, an NMDA receptor antagonist, showed great promise in animal models, but in clinical trials these compounds did not demonstrate sufficient efficacy and produced a spectrum of negative side effects in humans (Hill, 2000; Priest and Kaczorowski, 2007). Despite the rigorous testing of these and other novel compounds in preclinical models, there remains a high rate of translational failure, which has led some to question the utility and veracity of animal models in the discovery of new therapies. Some argue that novel therapies are best developed within the clinical setting, whereas the opposing argument advocates the initial testing of novel compounds in animals as an important step for determining efficacy and for ensuring safety before testing in humans.
There is significant homology in the genes and molecules expressed by higher organisms, yet the differences that do exist in neurochemistry between humans and animals have provided challenges in predicting the pharmacological characteristics of novel compounds in the clinic. One such example is the development of orally available cannabinoid receptor type 1 (CB1) agonists for pain relief in humans. CB1 receptors were identified as viable analgesic targets in rodents, in which activation of CB1 receptors (via endogenous endocannabinoid ligands or synthetic compounds) dampened the development and expression of pain in numerous animal models of chronic pain (Martin et al., 1999a, 1999b; Panikashvili et al., 2006; Petrosino et al., 2007; Palmer et al., 2008; Karbarz et al., 2009; Kinsey et al., 2009), including models of neuropathic pain (Blake et al., 2006; Ashton and Milligan, 2008). There is also evidence that smoked cannabis may be effective for alleviating pain in humans, suggesting that CB1 receptors could be a viable drug target for treating pain in humans. However, orally consumable CB1 receptor agonists produced psychotropic and debilitating side effects in humans (Carlini, 2004; Ware et al., 2010), including tremendous neurological damage and one instance of death during phase I trials of a novel fatty acid amide hydrolase inhibitor, without producing similar effects in rodents. This highlights that, although preclinical animal studies have proved valuable in identifying new analgesic agents (e.g., CB1 receptor agonists), there may be unanticipated side effects in humans that result in failure at the clinical trials stage (Zogopoulos et al., 2013).
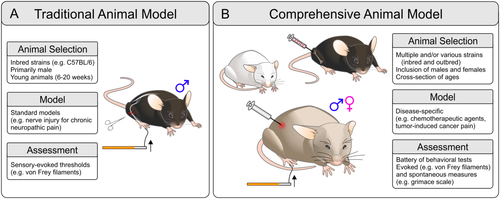
Although the predictive validity of translational therapies from animals to humans could be improved, it is important to note that most clinically approved drugs have been shown to be “reverse translational” to animals; i.e., compounds effective in humans are often effective analgesics in animal models (Kontinen and Meert, 2002; Mogil, 2009). This suggests that the lack of success in drug development from animal models to human clinical application is not necessarily due to the failure of animal models to simulate mechanisms of pain in humans, but rather to other aspects of translational research (such as behavioral assay methodologies, animal model selection, differences in drug pharmacology in humans vs. rodents, and consideration of the effect of social interactions and comorbidities) that may be contributing factors in the failure of drug translation to the clinic. Another challenge for translating preclinical discoveries is the profound heterogeneity of chronic pain in humans. There are a diversity of causes (injury, infection, disease) that can instigate the development of chronic pain and a multitude of genetic and environmental influences that can dictate an individual's susceptibility for developing chronic pain (Kvaal et al., 1999; Fillingim et al., 2008; Young Casey et al., 2008; Nissenbaum et al., 2010; Sorge et al., 2012; Vehof et al., 2014). This is in stark contrast to the homogeneity often associated with the design of animal studies and the controlled environment afforded by the laboratory setting, which may not ideally translate into a heterogeneous human population. With these limitations in mind, the selection of an appropriate animal model is imperative, and specific consideration must be given to the choice of organism (invertebrate vs. vertebrate), age, strain, and sex. Moreover, the method used to produce the disease phenotype (e.g., nerve injury, tumor-induced pain) should closely mimic the disease phenotype in humans, and the techniques used to monitor progression of the disease symptoms (e.g., von Frey, grimace scale) must reflect the chief complaint reported by the human population (Fig. 2).
The transition from acute to chronic pain is a major clinical problem and a tipping point in the development of pathological pain. This transition represents an important therapeutic window of opportunity, but the challenge has been in the development of appropriate animal models and in the identification of therapeutic targets. Hyperalgesic priming, produced by inflammatory or neuropathic insults, is a common preclinical model for studying the transition toward chronic pain in animals (Ferrari et al., 2015; Tillu et al., 2015; Araldi et al., 2016a; Kim et al., 2016; Yang et al., 2016). A distinct advantage of this model is the delay (approximately 72 hr) from the acute painful event that induces priming to the onset of a long-lasting primed state (Ferrari et al., 2015). This discrete window is exploited to investigate the transition toward pain chronicity, and it is understood that the hyperalgesic priming can be triggered by a myriad of inflammatory mediators, such as prostaglandin E2, adenosine, serotonin, cytokines and trophic factors (Ferrari et al., 2014; Kandasamy and Price, 2015; Araldi et al., 2016a, 2016b; Sun and Chen, 2016), and analgesic agents such as opioids (Joseph et al., 2010; Araldi et al., 2015; Kandasamy and Price, 2015). The concept of hyperalgesic priming as a mechanistic switch for acute to chronic pain applies to both inflammatory and neuropathic pain states because inflammation is often an underlying component in both pathologies (Dimitroulas et al., 2014; Zhang et al., 2015). The convergent and divergent mechanisms that underlie inflammatory vs. neuropathic pain indicate that there may not always be a clear mechanistic delineation between these conditions, especially as it applies to the progression from acute to chronic pain.
Pain afflicts individuals across all stages of life, but the prevalence of certain types of chronic pain conditions varies greatly across different age demographics (Chakour et al., 1996; Jones et al., 2001; Yezierski, 2012). For instance, chronic diseases associated with pain, such as gout, diabetic neuropathy, and osteoarthritis, are more common in the geriatric population (Schmader, 2002; Arden and Nevitt, 2006; Roddy et al., 2007), yet preclinical studies rarely use older animals (Mogil, 2009). In fact, many pain studies use young rodents (6–20 weeks of age) to model pain phenotypes that are primarily expressed in the aging human population. The underlying assumption is that pain mechanisms are similar for animals of all ages; this assumption is now on shaky ground because there is growing evidence for an age-dependent divergence in cellular and behavioral pain responses (Bodnar et al., 1988; Yezierski, 2012; Weyer et al., 2016). For example, Weyer and colleagues (2016) recently demonstrated that young mice display greater inflammatory mechanical sensitization than their aged counterparts and that this difference is due to age-dependent alterations in nociceptor sensitization (Weyer et al., 2016). Thus, fundamental differences exist with respect to how the nervous system responds to injury in young vs. old subjects. Another potential confound of age is an altered responsiveness of the nervous system to drug treatment (Mangoni and Jackson, 2004). This is a particularly salient consideration among the aged population because of changes in drug metabolism and excretion, increased incidences of drug–drug interactions, and higher prevalence of comorbidities that impact responses to pharmacological interventions (Pickering et al., 2001; McLachlan et al., 2011).
It is well established that genetics also has a profound influence on pain processing and response to analgesics. A recent study conducted in mice by Sorge et al. (2012) reported considerable interstrain variability in the development of mechanical allodynia following peripheral nerve injury. This variability was associated with a nucleotide polymorphism in the P2rx7 gene that encoded a single amino acid change that differentially affects only one of the two modes of P2X7 receptor (P2X7R) action, pore formation, leaving cationic channel function completely intact. In mice, the ability of P2X7R to pore form dictated sensitivity to both neuropathic and inflammatory pain. In humans, susceptibility haplotypes for the P2rx7 gene were discovered in two distinct types of persistent pain, chronic postmastectomy pain and osteoarthritis; individuals with low P2X7R pore formation presented less pain in both populations (Sorge et al., 2012). The findings by Sorge et al. (2012) and a growing number of other studies showing interstrain differences (Mills et al., 2001; Shir et al., 2001; Wijnvoord et al., 2010; Zhou et al., 2014) provide a cautionary tale against making overinterpretations based solely on findings from one animal strain. A poignant example of this pertains to the overuse of C57BL/6 mice in pain research. This mouse strain displays atypical responses to noxious stimuli, is prone to autotomy following nerve injury, expresses hypersensitive baseline responses to noxious thermal stimuli (such as in the tail immersion task), and is comparatively resistant to developing thermal and mechanical sensitivity following nerve injury (Mogil et al., 1999; Mekada et al., 2009; Sorge et al., 2012). Therefore, pain phenotypes observed in C57BL/6 mice or in transgenic mice back-crossed with the C57BL/6 genotype may not be representative of a global pain phenotype or, for that matter, even generalizable to the mouse population at large.
There is also an emerging realization that sexual dimorphism has an enormous impact on the development and presentation of chronic pain. However, the vast majority of preclinical studies (79%) have used exclusively male rodents (Mogil, 2012). The overrepresentation of males is contrary to epidemiological evidence showing a substantially higher prevalence of chronic pain among females (Torrance et al., 2006; Mogil, 2012). Across a range of chronic pain conditions, there is a 5.5% excess of female patients compared with male patients (Mogil, 2012). Exclusion of female subjects from preclinical experiments has led to the development of therapies that are rooted in targets and processes identified in male subjects. This sexual dimorphism in drug response is most strikingly illustrated by dextromethrophan (DXM), a compound originally developed as a cough suppressant and nonaddictive substitute for codeine and co-opted as an adjuvant treatment to enhance the analgesic actions of opioid drugs (Caruso et al., 2000). Promising preclinical studies were conducted exclusively in males. However, in human trials involving both male and female subjects, it became apparent that DXM was not nearly as effective in female patients (Heiskanen et al., 2002). Follow-up studies in rodents revealed that coadministration of DXM with morphine failed to produce any further enhancement of analgesia in female subjects, whereas it was efficacious in male subjects (Nemmani et al., 2004). Thus, the lesson learned from the trials and tribulations of DXM is that a complete mechanistic picture can be achieved only by inclusion of both sexes.
Recent studies have identified several divergent mechanisms that may account for sexual dimorphisms in pain. Sorge et al. (2015) reported that release of brain-derived neurotrophic factor (BDNF) from microglia is necessary for the development and expression of mechanical allodynia in nerve-injured animals (Sorge et al., 2015). However, BDNF mediates this critical role exclusively in male rodents, as blocking this pathway in female mice did not attenuate pain thresholds (Sorge et al., 2015). Another study by Sorge et al. (2011) found that mechanical allodynia in males, but not in females, is dictated by activity of toll-like receptor 4 expressed on spinal microglia (Lehnardt et al., 2003; Sorge et al., 2011). Thus, microglia appear to be a critical cellular point of sexual divergence in the development of neuropathic pain (Mapplebeck et al., 2016). In contrast, female mice have been shown to use an alternate cellular pathway involving the recruitment of adaptive immune cells into the central nervous system to express comparable pain phenotypes (Sorge et al., 2015). Exclusion of females from preclinical pain studies may provide an explanation for the lack of effective pain therapies in women, but there is little explanation for why female animals are underutilized in preclinical studies. A hesitation to incorporate female animals in pain studies has come from the perception that testing female subjects at different phases of the estrous cycle produces a confounding variable in pain studies (Frye et al., 1993; Martínez-Gómez et al., 1994; Bradshaw et al., 2000); however, substantive data to back this claim are few. Rather, there are converging and compelling lines of evidence indicating that sex differences in nociception are robust and are not influenced by changing hormonal levels throughout the phases of the estrous cycle in multiple strains of mice (Mogil et al., 2000). The inclusion of male and female animals is particularly important when considering the interaction between sex and genotype. Sex differences in pain studies have been reported to be dependent on strain, and similar interactions between sex/genotype have been observed in response to exogenous opioid analgesics and endogenous opioid mediated mechanisms (Bodnar et al., 1988; Mogil et al., 2000). Thus, there is a complex interplay between sex and genotype, and the interaction between these two variables can greatly impact the pain phenotype and subsequent interpretations drawn from preclinical studies.
SUMMARY
Chronic pain is an immense clinical and preclinical problem. On the clinical front, there is an urgent requirement for better diagnostic tools and therapies for treating pain in patients. This clinical requirement has fuelled an explosion in the number of preclinical animal models that are used to study a wide array of acute and chronic pain conditions. In using these animal models, the challenge on the preclinical front is to capture the sensory and emotional complexities of the human pain experience accurately and reliably. This review has discussed ways in which animal models have been instrumental in constructing an understanding of how key genes, cells, and circuits contribute to the behavioral alterations that are considered surrogates of chronic pain. However, there are limitations and caveats to the use of these models that must be acknowledged when translating discoveries from the bench to the bedside. Open dialogue must, therefore, be maintained with respect to discoveries made in preclinical animal models and the clinical realities and challenges that chronic pain presents in the human patient. This open dialogue will engender the translation of discoveries from bench to bedside and back to bench again that will ultimately give rise to better pain therapies and management strategies.
ACKNOWLEDGMENTS
The authors thank Dr. Nikita Burke and Michael Mousseau for their helpful comments on the review.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflicts of interest.
ROLE OF AUTHORS
NEB, HL-P, and TT wrote and revised the review. HL-P and CYF created the figures.