Activation of the prelimbic medial prefrontal cortex induces anxiety-like behaviors via N-Methyl-D-aspartate receptor-mediated glutamatergic neurotransmission in mice
Abstract
We investigated the possible roles of the prelimbic medial prefrontal cortex (PL) in the regulation of anxiety-like behaviors by pharmacologically activating the terminals of neuronal inputs or postsynaptic efferent neurons with a sodium channel activator veratrine. The extracellular glutamate levels were measured by in vivo microdialysis, and the behaviors were assessed with the open field (OF) test in mice simultaneously. The samples were collected every 10 min for 60 min, as basal levels of glutamate. The medium containing drugs were perfused for 30 min. The OF test was performed in the last 10 min of drug perfusion. After the drug treatments, the perfusion medium containing drugs was switched back to perfusion medium without drugs, and then samples were collected for another 90 min. The extracellular glutamate levels were significantly elevated after local perfusion of veratrine in the PL. At the same time, perfusion of veratrine in the PL produced anxiety-like behaviors in mice. Local coperfusion of a sodium channel blocker, lamotrigine, completely diminished the veratrine-induced elevated extracellular glutamate levels and the behavioral changes. Local coperfusion of an NMDA receptor antagonist, MK-801, but not a non-NMDA (AMPA/kainate) receptor antagonist, CNQX, completely diminished the behavioral changes without any effects on the veratrine-induced elevated extracellular glutamate levels. This study demonstrates that the activation of the PL with veratrine induces anxiety-like behaviors via NMDA receptor-mediated glutamatergic neurotransmission in mice. © 2014 Wiley Periodicals, Inc.
The neural mechanisms underlying anxiety have not been clearly elucidated. Lesion studies have demonstrated that the rodent medial prefrontal cortex (mPFC) is critical for the expression of anxiety-like behaviors (Lacroix et al., 2000; Sullivan and Gratton, 2002; Shah and Treit, 2003). In addition, there have been several studies that used pharmacological inactivation of the mPFC to investigate the neural mechanism of anxiety. One article reported that the inactivation of the mPFC by the GABAA agonist muscimol induced anxiolytic-like effects in the rat elevated plus maze (EPM) test (Shah et al., 2004). Another study showed that inactivation of the mPFC by the calcium channel blocker CoCl2 also induced anxiolytic-like effects in the rat EPM test and the light–dark test (Lisboa et al., 2010). Together, these findings suggest that the mPFC is critical for the expression of anxiety-like behaviors.
The mPFC is a heterogeneous cortical structure composed of several nuclei, including the prelimbic cortex (PL) and the infralimbic cortex (Van Eden and Uylings, 1985; Uylings et al., 2003; Hoover and Vertes, 2007). Although many studies have addressed the roles of the mPFC without distinguishing between these subnuclei of mPFC, other studies have shown specific involvement of the PL of the mPFC in the regulation of anxiety-like behaviors. The PL in rat mPFC receives glutamatergic inputs from the thalamus (Pirot et al., 1994), hippocampus (Jay et al., 1992; Gigg et al., 1994), and amygdala (Mcdonald, 1996). Maaswinkel et al. (1996) reported that the selective electrolytic lesion of the PL induced the anxiety-like behaviors in rat EPM test, but not in the rat open field (OF) test. On the other hand, the selective electrolytic lesion of the PL induced anxiety-like behaviors in the rat EPM test and the OF test (Jinks and McGregor, 1997). The pharmacological inactivation of the PL induced by the NMDA receptor antagonist AP5 decreased the anxiety-like behaviors in rat EPM tests (Stern et al., 2010). Similar effects were observed with pharmacological inactivation of the PL induced by CoCl2, the adrenaline b1 receptor antagonist atenolol, and the muscarine receptor antagonist scopolamine in rat EPM tests (Stern et al., 2010). Also, pharmacological inactivation of the PL by the GABAA agonist muscimol attenuated freezing after exposure to a threatening cat odor (Chan et al., 2011). In contrast, de Visser et al. (2011) reported that PL inactivation induced by concomitant treatment of a GABAA agonist, muscimol, and a GABAB agonist, baclofen, increased the anxiety-like behaviors in rat EPM tests. Inactivation of the PL by a sodium channel blocker, lidocaine, also increased the anxiety-like behaviors induced by trimethylthiazoline, a component of fox feces (Fitzpatrick et al., 2011). Therefore, consistent results for the possible roles of the PL of the mPFC in the regulation of anxiety-like behaviors were not obtained from previous electrolytic lesion studies or pharmacological inactivation studies.
We have carried out pharmacological activation studies to investigate the possible role of the PL in regulation of anxiety-like behaviors. The local perfusion of a sodium channel activator, veratrine, activates the action potential invasion of the synaptic terminal or postsynaptic efferent neurons through the voltage-gated sodium channels and thereby increases glutamatergic neurotransmission in the rat mPFC (Hashimoto et al., 1995; Golembiowska and Dziubina, 2002). Therefore, the possible involvements of NMDA and non-NMDA (AMPA/kainate) glutamate receptors were also examined.
MATERIALS AND METHODS
Animals
Seven-week-old male C57BL6 mice (Japan SLC, Shizuoka, Japan) were used. Mice had free access to food and water in an animal room maintained at 23°C ± 1°C on a 12-hr light–dark cycle (lights were automatically switched on at 8:00 AM). The study protocol was approved by the Institutional Animal Care and Use Committee of the National Center of Neurology and Psychiatry (approval No. 2010001).
Drugs
The drugs used in the present study were veratrine hydrochloride, lamotrigine, MK-801 maleate (Sigma, St. Louis, MO), and CNQX disodium salt (Ascent Scientific, Bristol, United Kingdom). The dose of veratrine (30 and 100 μM) was determined according to a previous article (Hashimoto et al., 1995). The doses of lamotrigine (30, 100 μM), MK-801 (1, 10 μM), and CNQX (1, 10 μM) were determined according to our preliminary studies. For lamotrigine, MK-801, and CNQX, doses without affecting the extracellular glutamate levels and the anxiety-like behaviors were used in this study (Supp. Info. Fig. 1, Supp. Info. Table I). All drugs were dissolved in perfusion medium (NaCl 117 mM, KCl 4.02 mM, CaCl2/2H2O 2.3 mM) and locally administered by perfusion medium through a microdialysis probe.
Microdialysis Study
Mice were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and placed in a stereotactic apparatus (Kopf 900; David Kopf Instruments, Tujunga, CA). A microdialysis guide cannula was implanted into the PL (AP +1.9 mm, L +0.3 mm, V −1.8 mm from bregma). The guide cannula was secured to the skull using a bone anchor screw and dental acrylic cement. Postoperatively, animals were housed in single cages and were allowed to recover from surgery for at least 24 hr before microdialysis experiments. Microdialysis probes (A-I-3-01; EICOM, Kyoto, Japan) with 1.0-mm-long membranes were continuously perfused with perfusion medium at a flux rate of 2.0 μl/min using a gas-tight syringe pump (ESP-64; EICOM).
Immediately after probe insertion, each mouse was attached to a swivel unit (TSU-20; EICOM) to allow free movement. The microdialysis sampling started 60 min after the onset of probe perfusion. Samples of dialysate were collected every 10 min for 60 min as basal levels of glutamate. After determination of basal levels of glutamate, the perfusion medium was switched to medium containing drugs by using a liquid switch (SI-60; EICOM). The medium containing drugs was perfused for 30 min. The OF test was performed in the last 10 min of drug perfusion. After drug treatments, the perfusion medium containing drugs was switched back to perfusion medium without drugs, and then samples of dialysate were collected for 90 min. The location of the dialysis probes was verified at the end of each experiment in 40-mm-thick brain slices.
High-Performance Liquid Chromatography Assay
The glutamate content of the samples was analyzed by high-performance liquid chromatography with an electrochemical detector (HTEC-700; EICOM). Briefly, a reverse-phase column (Eicompack GU-GEL, 4.6 × 150 mm; EICOM) for separation and a glutamate oxidase immobilized reactor column (E-ENZYMPAK, 3 × 4 mm; EICOM) for conversion of glutamate to hydrogen peroxide were used at 33°C. The potential of the platinum electrode (WE-PT; EICOM) was set at +0.45 V (vs. Ag/AgCl). Composition of the mobile phase for the measurement of glutamate was 60 mM ammonium chloride buffer (pH 7.6) with 0.7 mM hexadecyl-trimethyl-ammonium bromide (Nacalai Tesque, Kyoto, Japan).
OF Test
Each mouse was placed in a habituation cage (width 200 × depth 200 × height 330 mm) for at least 2 hr, for adaptation to the new environment before the test. The OF apparatus consisted of a square area (500 × 500 mm) with opaque walls 50 cm high and was placed in indirect light (50 Lux). The mice attached to the swivel unit were gently placed in a corner of the open field facing the opaque walls and allowed to freely explore the apparatus for 10 min. The total distance travelled and the percentage of distance travelled in the center area (30 × 30 cm) or time spent in the center area were automatically recorded using a video camera system (Smart Jr; Panlab, Barcelona, Spain). In addition, grooming duration and rearing counts were recorded. Grooming duration and rearing counts could be used to assess anxiety-like and/or exploratory behaviors in rodents (Perrot-Sinal et al., 2004; Takeda et al., 2007; Enginar et al., 2008). After removal of each animal, the apparatus was cleaned.
Statistical Analysis
Data are expressed as mean ± SEM. Statistical significance of the differences in microdialysis data was assessed by two-way repeated-measures ANOVA with interaction (time × drug treatment). Statistical significance of the differences in the OF test data was assessed by one-way factorial ANOVA. Post hoc individual group comparisons were made using the Bonferroni test for multiple comparisons. These analyses were performed in Graphpad Prism (Graphpad Software, La Jolla, CA). P < 0.05 was considered statistically significant.
RESULTS
Changes in the Extracellular Glutamate Levels and the Behaviors in the OF Test After Local Perfusion of Veratrine in the PL
The changes in the extracellular glutamate levels of the PL and the behaviors were examined simultaneously after local perfusion of veratrine. Veratrine (30, 100 μM) perfusion significantly and dose dependently increased the extracellular glutamate levels 10, 20, 30, and 40 min after the treatment (two-way repeated-measures ANOVA with interaction F24,396 = 16.38, P < 0.0001; Bonferroni test 30 μM, t = 6.599 and t = 5.374, P < 0.01, respectively; Bonferroni test 100 μM, t = 9.542, t = 12.96, t = 12.59, and t = 5.682, P < 0.01, respectively; Fig. 1A). Basal levels of glutamate were 0.41 ± 0.06 μM.
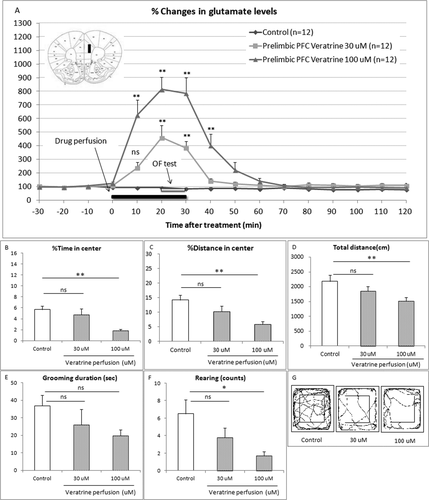
On the other hand, veratrine (30, 100 μM) perfusion significantly and dose dependently decreased the percentage of time spent (one-way ANOVA F2,33 = 7.529, P = 0.002; Bonferroni test t = 0.8803, P > 0.05, t = 3.713, P < 0.01, respectively; Fig. 1B), the percentage of distance in the center area (one-way ANOVA F2,33 = 7.166, P = 0.0026; Bonferroni test t = 1.823, P > 0.05, t = 3.785, P < 0.01, respectively; Fig. 1C), the total distance (one-way ANOVA F2,33 = 7.529, P = 0.0314; Bonferroni test t = 1.388, P > 0.05, t = 2.776, P < 0.01, respectively; Fig. 1D), and the rearing counts (one-way ANOVA F2,33 = 4.187, P = 0.0240; Bonferroni test t = 1.641, P > 0.05, t = 2.885, P < 0.05, respectively; Fig. 1F) compared with the control group. Veratrine (30, 100 μM) perfusion dose dependently, but not significantly, decreased the grooming duration (one-way ANOVA F2,33 = 1.753, P = 0.1891; Bonferroni test t = 1.179, P > 0.05, t = 1.849, P > 0.05, respectively; Fig. 1E). Figure 2D shows the comprehensive images of movement in the OF.
Effects of Lamotrigine on Veratrine-Induced Elevated Extracellular Glutamate Levels and Behavioral Changes
To confirm the involvement of the voltage-gated sodium channels, we examined the effect of coadministration of a selective sodium channel blocker, lamotrigine, on the elevated extracellular glutamate levels and the behavioral changes induced by local perfusion of veratrine in the PL. As expected, veratrine (100 μM) perfusion increased the extracellular glutamate levels 10, 20, and 30 min after the treatment compared with the control group (two-way repeated measured ANOVA with interaction F36,432 = 13.83, P < 0.0001; Bonferroni test t = 9.152, t = 14.17, and t = 10.68, P < 0.01, respectively; Fig. 2A). Lamotrigine (30, 100 μM) coperfusion with veratrine dose dependently attenuated the veratrine-induced elevated extracellular glutamate levels 10, 20, and 30 min after the treatment (Bonferroni test 30 μM, t = 2.288, P > 0.05, t = 4.091, P < 0.01 and t = 2.409, P > 0.05, respectively; Bonferroni test 100 μM, t = 0.07518, t = 0.1230, and t = 0.02689, P > 0.05, respectively, compared with the control group). Basal levels of glutamate were 0.43 ± 0.06 μM.
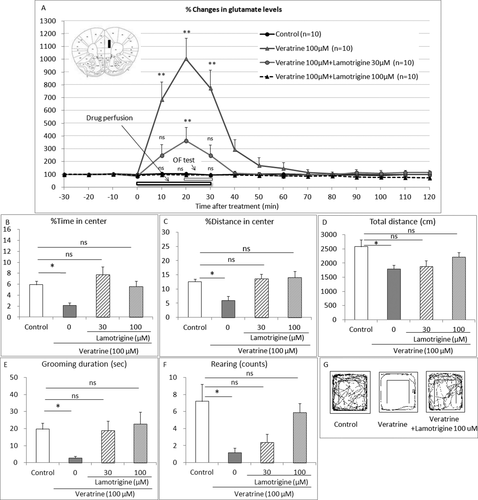
On the other hand, as expected, veratrine (100 μM) perfusion decreased the percentage of time in the center (one-way ANOVA F3,35 = 6.102, P = 0.0019; Bonferroni test t = 2.820, P < 0.05; Fig. 2B), the percentage of distance in the center (one-way ANOVA F3,35 = 6.023, P = 0.002; Bonferroni test t = 3.054, P < 0.05; Fig. 2C), the total distance (one-way ANOVA F3,35 = 3.519, P = 0.0249, Bonferroni test t = 2.934, P < 0.05; Fig. 2D), the grooming duration (one-way ANOVA F3,35 = 3.698, P = 0.0206, Bonferroni test t = 2.593, P < 0.05; Fig. 2E), and the rearing counts (one-way ANOVA F3,35 = 5.06, P = 0051, Bonferroni test t = 3.389, P < 0.05; Fig. 2F) compared with the control group. Lamotrigine (30, 100 μM) coperfusion diminished the veratrine-induced anxiety-like behaviors and did not affect the percentage of time in the center (Bonferroni test t = 1.365, t = 0.2235, P > 0.05, respectively; Fig. 2B), the percentage of distance in the center (Bonferroni test t = 0.5144 and t = 0.6516, P > 0.05, respectively; Fig. 2C), the total distance (Bonferroni test t = 2.006 and t = 0.01482, P > 0.05, respectively; Fig. 2D), the grooming duration (Bonferroni test t = 0.1226 and t = 0.436, P > 0.05, respectively; Fig. 2E), or the rearing counts (Bonferroni test t = 2.711 P < 0.05 and t = 0.7208, P > 0.05, respectively; Fig. 2F) compared with the control group. Figure 2G shows the comprehensive images of movement on the OF.
Effects of the NMDA Receptor Antagonist MK-801 on Veratrine-Induced Elevated Extracellular Glutamate Levels and the Behavioral Changes
To investigate the possible involvement of NMDA receptor-mediated glutamatergic neurotransmission, we examined the effect of the NMDA receptor antagonist MK-801 on the elevated extracellular glutamate levels and the anxiety-like behaviors induced by local perfusion of veratrine in the PL. As expected, veratrine (100 μM) perfusion increased the extracellular glutamate levels 10, 20, and 30 min after the treatment compared with the control group (two-way repeated measure ANOVA with interaction F36,432 = 3.38, P < 0.0001; Bonferroni test t = 2.652, P > 0.05, t = 6.651 and t = 6.343, P < 0.01, respectively; Fig. 3A). MK-801 (1, 10 μM) coperfusion with veratrine did not affect the veratrine-induced elevated extracellular glutamate levels 10, 20, and 30 min after the treatment (Bonferroni test 1 μM, t = 2.412, P > 0.05, t = 6.638 and t = 4.343, P < 0.01, respectively; Bonferroni test 10 μM, t = 4.054, t = 6.638 and t = 5.200, P < 0.01, respectively, compared with the control group). Basal levels of glutamate were 0.47 ± 0.05 μM.
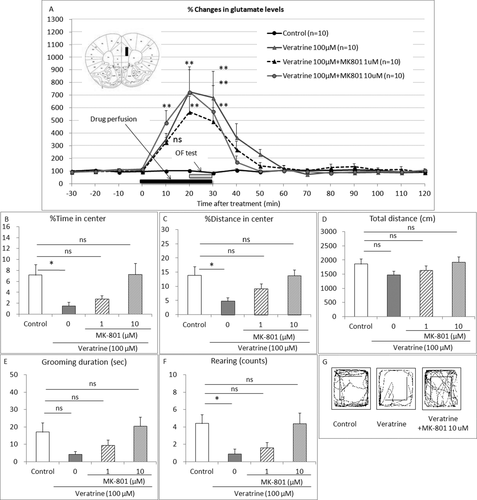
On the other hand, as expected, veratrine (100 μM) perfusion decreased the percentage of time in the center (one-way ANOVA F3,36 = 4.321, P = 0.0106; Bonferroni test t = 2.787, P < 0.05; Fig. 3B), the percentage of distance in the center (one-way ANOVA F3,36 = 4.113, P = 0.0131; Bonferroni test t = 3.013, P < 0.05; Fig. 3C), the total distance (one-way ANOVA F3,36 = 1.417, P = 0.2536, Bonferroni test t = 1.552, P > 0.05; Fig. 3D), the grooming duration (one-way ANOVA F3,36 = 3.291, P = 0.0314, Bonferroni test t = 2.238, P > 0.05; Fig. 3E), and the rearing counts (one-way ANOVA F3,36 = 4.295, P = 0.0109, Bonferroni test t = 2.786, P < 0.05; Fig. 2F) compared with the control group. MK-801 (1, 10 μM) coperfusion diminished the veratrine-induced anxiety-like behaviors, the percentage of time in the center (Bonferroni test t = 2.148 and t = 0.0744, P > 0.05, respectively, compared with the control group; Fig. 3B), the percentage of distance in the center (Bonferroni test t = 1.584 and t = 0.05966, P > 0.05, respectively, compared with the control group; Fig. 3C), the total distance (Bonferroni test t = 0.927 and t = 0.2729, P > 0.05, respectively, compared with the control group; Fig. 3D), the grooming duration (Bonferroni test t = 1.317 and t = 0.6067, P > 0.05, respectively, compared with the control group; Fig. 3E), and the rearing counts (Bonferroni test t = 2.229 and t = 0.0001, P > 0.05, respectively; Fig. 3F). Fig. 3G shows the comprehensive images of movement on the OF.
Effects of Non-NMDA Receptor Antagonist CNQX on Veratrine-Induced Elevated Extracellular Glutamate Levels and Behavioral Changes
To investigate the possible involvement of non-NMDA receptor-mediated glutamatergic neurotransmission, we examined the effect of the non-NMDA receptor antagonist CNQX on the elevated extracellular glutamate levels and the anxiety-like behaviors induced by local perfusion of veratrine in the PL. As expected, veratrine (100 μM) perfusion increased the extracellular glutamate levels 10, 20, and 30 min after the treatment compared with the control group (two-way repeated measure ANOVA with interaction F36,468 = 4.83, P < 0.0001; Bonferroni test t = 4.438, t = 8.164 and t = 7.954, P < 0.01, respectively; Fig. 4A). CNQX (1, 10 μM) coperfusion with veratrine did not affect the veratrine-induced elevated extracellular glutamate levels 10, 20, or 30 min after the treatment (Bonferroni test 1 μM, t = 3.725, t = 8.819 and t = 7.800, P < 0.01, respectively; Bonferroni test 10 μM, t = 3.614, P < 0.05, t = 8.142 and t = 6.111, P < 0.01, respectively, compared with the control group). Basal levels of glutamate were 0.43 ± 0.07 μM.
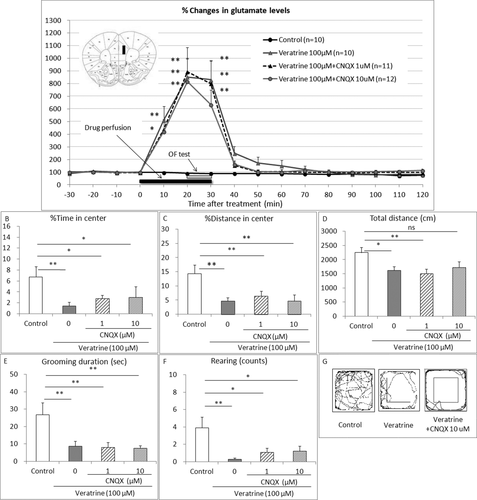
On the other hand, as expected, veratrine (100 μM) perfusion decreased the percentage of time in the center (one-way ANOVA F3,39 = 5.572, P = 0.0028; Bonferroni test t = 3.885, P < 0.01; Fig. 4B), the percentage of distance in the center (one-way ANOVA F3,39 = 16.39, P < 0.001; Bonferroni test t = 5.905, P < 0.05; Fig. 4C), the total distance and rearing counts (one-way ANOVA F3,39 = 3.98, P = 0.0145; Bonferroni test t = 2.662, P < 0.05; Fig. 4D), the grooming duration (one-way ANOVA F3,39 = 6.042, P = 0.0018, Bonferroni test t = 3.299, P < 0.01; Fig. 4E), and the rearing counts (one-way ANOVA F3,39 = 4.766, P = 0.0063, Bonferroni test t = 3.536, P < 0.01; Fig. 4F) compared with the control group. CNQX (1, 10 μM) coperfusion with veratrine did not affect the veratrine-induced anxiety-like behaviors, the percentage of time in the center (Bonferroni test t = 2.941 and t = 2.871, P < 0.05, respectively, compared with the control group; Fig. 4B), the percentage of distance in the center (Bonferroni test t = 4.949 and t = 6.198, P < 0.01, respectively, compared with the control group; Fig. 4C), the total distance (Bonferroni test t = 3.229 P < 0.05, and t = 2.353, P > 0.05, respectively; Fig. 4D), the grooming duration (Bonferroni test t = 3.528 and t = 3.684, P < 0.01, respectively, compared with the control group; Fig. 4E), or the rearing counts (Bonferroni test t = 2.824 and t = 2.718, P < 0.05, respectively, compared with the control group; Fig. 4F). Figure 4G shows the comprehensive images of movement on the OF.
DISCUSSION
In the present study, the extracellular glutamate levels of the PL in mice were significantly increased after local perfusion of veratrine. Perfusion of veratrine in this region of mPFC induced anxiety-like behaviors simultaneously. Indeed, veratrine perfusion in the PL in mice decreased the percentage of time spent in the center, the percentage of distance in the center area, the grooming duration, and the rearing counts compared with the control group. In this study, the total distance was also decreased with the veratrine perfusion. The decreased activity also would be considered a form of anxiety in which a behavioral inhibition system is activated (Muigg et al., 2009). This is the first report demonstrating that the activation of the PL induces anxiety-like effects in mice.
In this study, local coperfusion of an NMDA receptor antagonist, MK-801, in the PL completely diminished the veratrine-induced behavioral changes, without affecting the veratrine-induced elevated extracellular glutamate levels. Stern et al. (2010) previously reported that the local administration of another NMDA receptor antagonist, AP-5, in rat PL produced anxiolytic-like effects in the EPM test. It was suggested that the activation of glutamatergic neurotransmission mediated by NMDA receptor in the PL is responsible for the anxiety-like behaviors induced by veratrine. In addition, our results demonstrated that veratrine activated the presynaptic terminals but did not directly activate the postsynaptic voltage-dependent sodium channels in the PL. On the other hand, a non-NMDA receptor antagonist, CNQX, produced no effects on the veratrine-induced behavioral changes. Our results are consistent with a previous study (Bi et al., 2013), which demonstrated that the local administration of CNQX in rat PL produced no effects on the rat OF test.
Veratrine acts on sodium channel subtypes, including NaV1.1, NaV1.2, and NaV1.3, which are widely distributed in the central nervous system (Maurice et al., 2001; Catterall et al., 2005). This study confirms the involvement of the voltage-gated sodium channels in the elevated extracellular glutamate levels and the behavioral changes induced by local perfusion of veratrine in the PL. The local coperfusions of a selective sodium channel blocker, lamotrigine, which had been reported to inhibit the presynaptic glutamate release in rats (Hashimoto et al., 1995; Waldmeier et al., 1996), completely diminished the veratrine-induced elevated extracellular glutamate levels in the PL and the behavioral changes.
Previous articles have reported that intraperitoneal administration of lamotrigine produced anxiolytic-like effects in rats (Mirza et al., 2005; Munro et al., 2007; Sugiyama et al., 2012). The present study used only doses of lamotrigine without any effects on the extracellular glutamate level in the PL or the anxiety-like behaviors by themselves. It is possible that the higher doses of lamotrigine could affect the basal glutamate release in the PL and the basal behaviors. On the other hand, it was reported that pharmacological inactivation of the PL by another sodium channel blocker, tetrodotoxin and lidocaine, did not affect anxiety-like behavior in the rat OF test (Corcoran and Quirk, 2007; Fitzpatrick et al., 2011). There is another possibility, that lamotrigine could effectively attenuate the elevated glutamate levels only in the hyperactivated regions of the brain.
Finally, it was strongly suggested that a compound with inhibitory action on glutamatergic function in the PL would be a possible candidate for the novel agent for the treatment of anxiety disorders. Indeed, lamotrigine was found to be effective in treating patients with posttraumatic stress disorder in a small placebo-controlled trial (Hertzberg et al., 1999). Based on the patterns of afferent projections, the PL in rodents appears to represent a region homologous to the lateral/dorsolateral PFC of primates (Hoover and Vertes, 2007). Further study is needed to elucidate the possible involvement of the lateral/dorsolateral PFC in the regulation of human anxiety.
The present study has a limitation. The OF test is very useful but is not a specific behavioral test for anxiety-like behavior. Therefore, we tried to assess the anxiogenic-like effect of veratrine using the EPM test in our preliminary experiment. However, the sham-operated control mice with probe and tubing for in vivo microdialysis expressed substantial fear of the height and did not enter the open arm on the EPM. Therefore, we could not examine the anxiogenic-like effect of veratrine with the EPM test simultaneously with in vivo microdialysis. A similar observation had been previously reported by Thoeringer et al. (2007). Further investigation would be needed to strengthen our findings.
CONCLUSIONS
This study demonstrates that activation of the PL with the sodium channel activator veratrine induces anxiety-like behaviors via NMDA receptor-mediated glutamatergic neurotransmission in mice.




