Influence of N-methyl-D-aspartate receptors on ouabain activation of nuclear factor-κB in the rat hippocampus
Abstract
It has been shown that ouabain (OUA) can activate the Na,K-ATPase complex and mediate intracellular signaling in the central nervous system (CNS). Inflammatory stimulus increases glutamatergic transmission, especially at N-methyl-D-aspartate (NMDA) receptors, which are usually coupled to the activation of nitric oxide synthase (NOS). Nuclear factor-κB (NF-κB) activation modulates the expression of genes involved in development, plasticity, and inflammation. The present work investigated the effects of OUA on NF-κB binding activity in rat hippocampus and the influence of this OUA-Na,K-ATPase signaling cascade in NMDA-mediated NF-κB activation. The findings presented here are the first report indicating that intrahippocampal administration of OUA, in a concentration that did not alter Na,K-ATPase or NOS activity, induced an activation of NF-κB, leading to increases in brain-derived neurotrophic factor (Bdnf), inducible NOS (iNos), tumor necrosis factor-α (Tnf-α), and B-cell leukemia/lymphoma 2 (Bcl2) mRNA levels. This response was not linked to any significant signs of neurodegeneration as showed via Fluoro-Jade B and Nissl stain. Intrahippocampal administration of NMDA induced NF-κB activation and increased NOS and α2/3-Na,K-ATPase activities. NMDA treatment further increased OUA-induced NF-κB activation, which was partially blocked by MK-801, an antagonist of NMDA receptor. These results suggest that OUA-induced NF-κB activation is at least in part dependent on Na,K-ATPase modulatory action of NMDA receptor in hippocampus. The interaction of these signaling pathways could be associated with biological mechanisms that may underlie the basal homeostatic state linked to the inflammatory signaling cascade in the brain. © 2011 Wiley Periodicals, Inc.
The enzyme Na,K-ATPase is responsible for maintaining the electrochemical gradient of sodium and potassium ions, thus regulating ionic homeostasis in tissues and cells (Gloor,1997). This enzyme can also act as a signal transducer and a nuclear factor-κB (NF-κB) activator by interacting with neighboring membrane proteins and organized cytosolic cascades of signaling proteins (Haas et al.,2000; J. Liu et al.,2000; Aizman et al.,2001; Xie and Askari,2002; L. Lui et al.,2007; Desfrere et al.,2009). In fact, there is now evidence that ouabain (OUA), an endogenously synthesized steroid hormone (Scheiner-Bobis and Schoner,2001), is a ligand of the Na,K-ATPase enzyme (Bagrov et al.,2009). This compound dose dependently inhibits the enzyme activity by binding to the extracellular domains of α1, α2-, or α3 -Na,K-ATPase. However, binding of OUA to Na,K-ATPase can also activate various intracellular pathways, such as mitogen-activated protein (MAP) kinase phosphorylation (Haas et al.,2000; Dmitrieva and Doris,2003) and intracellular calcium signaling cascades (Aizman et al.,2001; Yuan et al.,2005). In addition, mutations of Na,K-ATPase are known to cause rapid-onset dystonia-Parkinson diseases (De Carvalho Aguiar et al.,2004).
NF-κB, the activity of which is attributed to the Rel/NF-κB family proteins forming homo- and heterodimers through the combination of the subunits p65 (or RelA), p50, p52, c-Rel, and RelB, can be activated by lipopolysaccharide (LPS), cytokines such as tumor necrosis factor (TNF-α) and interleukin-1β (IL-1β), and reactive oxygen species (Hoffmann and Baltimore,2006; Mattson and Meffert,2006). NF-κB, which is constitutively expressed in the cytoplasm, is inhibited by a family of molecules termed inhibitor-κB (IκBs). IκB binds NF-κB and masks its nuclear localization signal, thus retaining it in the cytoplasm (Ghosh et al.,1998). Inducers of NF-κB act by intracellular signaling pathways that activate the IκB kinases (IKKs), which phosphorylate two specific N-terminal serines of IκBα, resulting in IκBα polyubiquitination and degradation in the 26S proteasome (Ghosh and Karin,2002). When IκB is degraded, NF-κB migrates to the nucleus, modulating the transcription of several genes associated with neurodegenerative or neuroprotector actions (Kaltschmidt et al.,1994; Camandola and Mattson,2007).
Glutamate, the major excitatory neurotransmitter in the central nervous system (CNS), is at basal concentrations crucial for brain functions such as learning and memory (McEntee and Crook,1993). However, at high concentrations, the excessive stimulation of N-methyl-D-aspartate (NMDA), which is an ionotropic excitatory glutamate receptor, has been associated with neurodegenerative processes (Choi,1988). It has been shown that the NMDA receptor is involved in a variety of neurological disorders such as epilepsy and Huntington's and Alzheimer's diseases as well as in brain ischemia and stroke (Lipton,2004). NMDA can activate NF-κB (Lipsky et al.,2001), and its activation may constitute an adaptive response to protect neurons against adverse effects, such as accumulation of amyloid-β peptide (Barger et al.,1995; Mattson et al.,1997; Kawamoto et al.,2008; Mattson,2008).
NF-κB can be activated by glutamate-NMDA and OUA-Na,K-ATPase complex signaling cascades. One of the hypothesis to explain OUA-induced NF-κB activation proposes that this hormone inhibits the Na,K-ATPase activity, leading to increased intracellular Na+ concentration that transactivates Na+/Ca2+ exchangers. Cytosolic Ca2+ waves, in turn, will be sufficient to activate a downstream signaling cascade that induces activation of NF-κB or evokes the release of glutamate (Blaustein and Lederer,1999; L. Liu et al.,2007). In addition, evidence has also suggested that OUA inhibition of Na,K-ATPase can affect signaling events in mammalian cells through the AMPK (5′-adenosine monophosphate-activated protein kinase)-mediated response to reduction of cellular ATP (Soltoff and Heeden,2008).
OUA can regulate NMDA binding and receptor activity (Reinés et al.,2001, 2004; Bersier and Rodríguez de Lores Arnaiz,2009) as well as the glutamate transporters (Rose et al.,2009), so we have hypothesized that OUA-Na,K-ATPase could modulate the activation of NF-κB by NMDA receptors, since this action could be of relevance for normal as well as pathological conditions such as Alzheimer's and Parkinson's disease. In this report, we suggest that OUA-induced NF-κB activation is at least in part dependent on Na,K-ATPase activation of NMDA receptor in rat hippocampus.
MATERIALS AND METHODS
Chemicals and Kits
Routine reagents, MK-801 (dizocilpine), Dowex AG 50 Wx-8, and OUA were purchased from Sigma (St. Louis, MO); NMDA was obtained from Research Biochemicals International (Natick, MA); γ-32P-ATP (3,000 Ci/mmol, 10 Ci/ml) was purchased from DuPont-New England Nuclear (Boston, MA); gel shift assay system kit for NF-κB was purchased from Promega (Madison, WI); and Bio-Rad protein assay kit was purchased from Bio-Rad (Hercules, CA). Antibodies against subunits p50 (200 μg/ml), p65 (200 μg/ml), p52 (200 μg/ml), and c-Rel (200 μg/ml) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Animal Treatment and Surgical Procedures
Adult male Wistar rats (300–350 g; Biomedical Sciences Institute, University of São Paulo) were kept under a 12/12-hr light-dark cycle and allowed free access to food and water. All animal treatments were performed between 9:00 and 11:00 AM.
After anesthesia with a mixture of ketamine and xylazine (75 and 10 mg/kg i.p., respectively), the rats were positioned in a stereotaxic instrument. Guide cannulae (26-gauge, 10 mm) were lowered to the dorsal hippocampus (coordinates: anterior-posterior –3.5 mm from bregma, medial-lateral 2.0 mm, and dorsal-ventral 2.7 mm from skull, according to an atlas of the rat brain; Paxinos and Watson,2004). Dental acrylic was used to fix the cannula to the skull, and dummy cannulae (33-gauge, 10.5 mm) were inserted into the guide cannulae. When appropriate, the 30-gauge infusion cannulae were fitted into the guide cannulae. The tip of the infusion cannula protruted 1.0 mm beyond the tip of the guide cannula and was aimed at the pyramidal cell layer of CA1, as indicated in Figure 1. Each animal received bilateral infusions of 1 μl OUA (10 nM or 10 μM; Bersier and Rodríguez de Lores Arnaiz,2009; X.L. Liu et al.,2007) or 1 μl vehicle (0.9% saline) in different sides of the hippocampus over 5 min. In some experiments, 1 μl NMDA (1 μM) or vehicle (0.9% saline) was injected in each side 1 hr before OUA (10 nM) or saline opposite hippocampus injections. For assessment of the effects of MK-801 (3 mg/kg; Glezer et al.,2003), rats were treated with i.p. injection of the drug or vehicle (0.9% saline) for 20 min prior to OUA (10 nM) or saline hippocampus injection.
After the last injection, the infusion cannula was left in place for at least 2 min and then slowly withdrawn. One hour after the last injection animals were killed by decapitation following procedures approved by the Ethical Committee for Animal Research (CEEA) of the Biomedical Sciences Institute of the University of São Paulo. Brain sections of pilocarpine (360 mg/kg, i.p. after a scopolamine methylnitrate injection 1 mg/kg, 30 min before pilocarpine)-treated animals were used as positive controls (Fujikawa,1996). Brains were removed and immersed in cold phosphate-buffered saline (PBS). Dorsal hippocampus was rapidly dissected, quickly immersed in liquid nitrogen, and stored at –80°C for later use.
Detection of Neuronal Cell Death and Histological Analysis
The presence of neuronal cell death was investigated with the Fluoro-Jade B (FJB) method (Schmued and Hopkins,2000). Briefly, every twelfth section of the whole rostrocaudal extent of each brain was mounted onto poly-L-lysine-coated slides, dried under a vacuum for 2 hr, dehydrated through graded concentrations of alcohol (50%, 70%, and 100%; 1 min), rehydrated through graded concentrations of alcohol (100%, 70%, and 50%; 1 min), and rinsed for 1 min in distilled water. The sections were then dipped and shaken in potassium permanganate solution (0.06%) for 10 min and rinsed for 1 min in distilled water. Slides were next dipped and shaken into a solution containing a mixture of 0.0004% FJB (Histochem, Jefferson, AR) plus 0.1% acetic acid (Sigma-Aldrich) plus 0.0002% 4,6-diamidino-2-phenylindole (DAPI; Molecular Probes, Eugene, OR) for 20 min. The slides were thereafter rinsed three times in distilled water (1 min each), dried, dipped in xylene three times (2 min each), and coverslipped with DPX prior to observation by fluorescence microscopy. Nissl stain was used as a general index of cellular morphology that may be altered in response to the different treatments.
Electrophoretic Mobility Shift Assay NF-κB Consensus Oligonucleotide
Nuclear extract of each hippocampus was prepared as previously described (Rong and Baudry,1996). Briefly, hippocampal structures were homogenized using a Dounce homogenizer in cold PBS supplemented with 0.5 mM DTT, 0.5 mM PMSF, 2 μg/ml leupeptin, 2 μg/ml antipain, and 3 mM sodium ortovanadate and centrifuged at 4°C for 30 sec at 12,000g. The supernatants (S1) were reserved for immunoblot and enzymatic assays, and the pellets were resuspended in lysis buffer (10 mM HEPES, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.1 mM EDTA, 0.5 mM DTT, 0.5 mM PMSF, 2 μg/ml leupeptin, 2 μg/ml antipain, and 3 mM sodium ortovanadate) and incubated on ice for 10 min. After addition of NP-40 (10%), samples were vigorously mixed and centrifuged for 30 sec at 12,000g. Supernatant was discarded, and the pellet was resuspended in extraction buffer (20 mM HEPES, pH 7.9, 25% glycerol, 1.5 mM MgCl2, 300 mM NaCl, 0.25 mM EDTA, 0.5 mM DTT, 0.5 mM PMSF, 2 μg/ml leupeptin, 2 μg/ml antipain, and 3 mM sodium ortovanadate), incubated 20 min on ice, and centrifuged for 20 min at 12,000g at 4°C. The resulting supernatants containing nuclear proteins were stored at –80°C. Protein concentration was determined using the Bio-Rad protein reagent. Electrophoretic mobility shift assay (EMSA) for NF-κB was performed by using the gel shift assay kit from Promega, as described previously (Rong and Baudry,1996). 32P-NF-κB double-stranded consensus oligonucleotide probe (5′-AGTTGAGGGGACTTTCCCAGGC-3′; 20,000 cpm) and nuclear extracts (15 μg) were used. DNA–protein complexes were separated by electrophoresis through a 6% nondenaturing acrylamide:bis-acrylamide (37.5:1) gel in 0.5× Tris-borate/EDTA (TBE) for 2 hr at 150 V. Gels were vaccuum dried and analyzed by autoradiography. For competition experiments, NF-κB and transcription initiation factor II (TFIID; 5′-GCAGAGCATATAAGGTGAGG TAGGA-3′) unlabeled double-stranded consensus oligonucleotide was included in 20-fold molar excess over the amount of 32P-NF-κB probe to detect specific and nonspecific DNA–protein interactions, respectively. Unlabeled oligonucleotides were added to the reaction mixture 20 min before the radioactive probe. Supershift assays, using antibodies against different NF-κB subunits (p50, p52, cRel and p65, 1:20 dilution), were also conducted according to the protocol of the manufacturer (Santa Cruz Biotechnology) before the incubation of nuclear extracts with the labeled oligonucleotide. Autoradiographs were visualized with a photodocumentation system DP-001-FDC (VilberLourmat) and quantified in NIH ImageJ software (http://rsb.info.nih.gov/ij). Several exposure times were analyzed to ensure the linearity of the band intensities.
Immunobloting of Nuclear and Cytosolic p65 NF-κB Subunit
Electrophoresis was performed using 10% polyacrylamide gel and the Bio-Rad mini-Protean III apparatus. In brief, the proteins present in the hippocampus cytosolic (20 μg) and nuclear fractions (10 μg) were size separated in 10% SDS-PAGE (90 V). The proteins were blotted onto a nitrocellulose membrane (Bio-Rad) and incubated with the specific antibody (p65 sc-372; Santa Cruz Biotechnology). The Ponceau method of immunoblotting was used to ensure equal protein loading (Salinovich and Montelaro,1986). Proteins recognized by antibodies were revealed by the ECL technique, following the instructions of the manufacturer (Amersham Biosciences). To standardize and quantify the immunoblots, we used the photodocumentation system DP-001-FDC (VilberLourmat) and NIH ImageJ, respectively. Several exposure times were analyzed to ensure the linearity of the band intensities. β-Actin antibody (sc-1616; Santa Cruz Biotechnology) was used as an internal control for the experiments. Results were expressed in relation to the intensity of β-actin.
RT-PCR Determination of iNos, Tnf-α, Bdnf, Bcl2, and Bax mRNA Levels
The effect of OUA on NF-κB-modulated gene expression in the hippocampus of rats was measured. Total RNA was isolated with Trizol reagent (Invitrogen, Carlsbad, CA) according to the instructions of the manufacturer. Semiquantitative reverse transcription-PCR (RT-PCR) amplification was performed using the ThermoScript RT kit (Invitrogen) according to the instructions of the manufacturer. The primer sequences were iNos (GenBank access No. 012611.3, 651 bp), 5′- GTGCTAATGCGGAAGGTCAT A-3′ (sense) and 5′-CCAAATGTGCTTGTCACCACA-3′ (antisense); TNF (GenBank access No. 012675.3, 82 bp), 5′-AGGCGCTCCCCAAAAAGATG-3′ (sense) and 5′-GAT GGCGGAGAGGAGGCTGA-3′ (antisense); Bax (GenBank access No. 017059.1, 260 bp), 5′-TGAACTGGACAAC AACATGGAGC-3′ (sense) and 5′-GGTCTTGGATCCAGA CAAACAGC-3′ (antisense); Bcl2 (GenBank access No. 016993.1, 271 pb), 5′-GGAGGATTGTGGCCTTCTTTG AG-3′ (sense) and 5′-TATGCACCCAGAGTGATGCA GGC-3′ (antisense); Bdnf (GenBank access No. 012513.3, 304 bp), 5′-ATGCTCAGCAGTCAAGTGCC-3′ (sense) and 5′-AGCCTTCCTTCGTGTAACCC-3′ (antisense); and Iκbα (GenBank access No. 020529.2, 329 pb), 5′-CATGAAGAG AAGACACTGACCATGGAA-3′ (sense) and 5′-TGGATAG AGGCTAAGTGTAGACACG-3′ (antisense). The PCR conditions consisted of 5 min at 94°C; different numbers of cycles of 94°C for 45 sec, 63°C (iNos, Bax, Bcl2), 60°C (Tnf-α), 59°C (Bdnf) for 45 sec; and 72°C for 90 sec depending on the gene studied (30 cycles: Bcl2; 33 cycles: iNos, Tnf-α; 35 cycles: Bdnf; 40 cycles: Bax) and a final extension at 72°C for 10 min. Glyceraldehyde-3-phosphate dehydrogenase (Gapdh; GenBank access No. 017008.3, 264 bp) was also amplified as an internal PCR control using the following primers: 5′-CGGGAAGCTTGTGATCAATGG-3′ (sense) and 5′-GGCAGTGATGCCATGGACTG-3′ (antisense). In this case, the temperature cycling conditions were as follows: 5 min at 94°C, 20 cycles of 94°C for 45 sec, 63°C for 45 sec, and 72°C for 1 min and 30 sec and a final extension at 72°C for 10 min. Gel electrophoresis of the PCR product was performed using an ethidium bromide-containing agarose gel (2%), and resulting bands were visualized under UV light. The photographs were captured by photodocumentation system DP-001-FDC (VilberLourmat), and the optical density of the bands was determined in NIH ImageJ. Results were expressed in relation to the intensity of Gapdh mRNA levels.
Measurement of Na,K-ATPase Activity
Na,K-ATPase activity was determined by assaying Pi released from ATP hydrolysis. This inorganic compound forms a complex with molybdate, which can be read spectrophotometrically at 700 nm (Esmann,1988). For this colorimetric ATP assay, hippocampus supernatant (S1) was centrifuged (12,000g for 15 min at 4°C). The supernatant was reserved for nitric oxide synthase (NOS) activity assay and the particulate fraction was resuspended in a buffer containing 0.32 M sucrose, 20 mM HEPES, 1 mM EDTA, 1 mM DTT, and 1 mM PMSF, pH 7.4. Protein content was determined in particulate samples by Bio-Rad colorimetric assay (Bradford,1976). Na,K-ATPase activity was tested by adding 10 μg of the particulate fraction (in 40 μl buffer) to 360 μl buffer containing 3 mM ATP, 120 mM NaCl, 2 mM KCl, 3 mM MgCl2, and 30 mM histidine (pH 7.2), with or without OUA (3 μM or 3 mM). After 20 min of incubation at 37°C, Na,K-ATPase activity was measured. The reaction was terminated by the addition of a quenching solution (0.6 ml) containing 1 N H2SO4 and 0.5% ammonium molybdate. Formation of a phosphomolybdate complex was determined spectrophotometrically at 700 nm (Esmann,1988). The total ATPase, Mg-ATPase, α1- and α2/3-Na,K-ATPase activities were linearly related up to 20 min. In rodents, the α1-Na,K-ATPase isoform is 1,000 times less sensitive to the cardiac glycoside than the α2/3-Na,K-ATPase measured as the difference between ouabain-untreated and ouabain-treated samples. The high-affinity α2/3-isoform fraction was calculated by subtracting the activity obtained with 3 μM ouabain from total ATPase activity. To determine the low-affinity fraction (α1-subunit-associated Na,K-ATPase activity), the values obtained in the presence of 3 μM OUA were subtracted from those obtained in the presence of 3 mM OUA. The Na,K-ATPase activity was expressed as nmol/mg protein · min.
NOS Activity Assay
The supernatant (described above) was passed through a Dowex AG 50 Wx-8 (Na+ form) column to remove the endogenous arginine. The arginine-free eluent was used to assay the NOS activity. Protein content of these samples was determined using the Bio-Rad protein assay kit. NOS activity in each sample was determined according to a method already described (Mckee et al.,1994). Briefly, the NOS assay reaction medium of 200 μl contained 100 mM HEPES, pH 7.4, 1 mM NADPH, 0.45 mM CaCl2, 1 μM L-[2,3-3H]arginine (0.5 μCi), and 100 μl hippocampus cytosolic protein (0.2 μg/μl). The reaction mixture was incubated for 30 min at 37°C and stopped by the addition of stop buffer containing 20 mM Hepes at pH 5.5. The entire reaction mixture was passed through a column packed with the Na+ form of Dowex AG 50 Wx-8 resin. The flow-through fraction containing [3H]citrulline was counted for radioactivity using a Beckman 6000 liquid scintillation counter. The NOS activity was expressed as nmol citrulline/mg protein · min.
Statistical Analysis
Results are expressed as mean ± SEM of the indicated number of experiments. Statistical comparisons for NF-κB activation and NOS and Na,K-ATPase activities were performed by one-way ANOVA, followed by the Newman-Keuls test. RT-PCR and immunoblot assays were analyzed via Student's t-test. All analyses were performed in the Prism 4 software package (GraphPad Software, San Diego, CA).
RESULTS
OUA Treatment and Integrity of the Neuronal Elements
Histological analysis showed that cannulae were implanted just above the dorsal hippocampus in both sides of the brain. The main site of injections was located in the CA1 region, and a photomicrograph of a coronal brain section of a typical rat showing site of microinjection is illustrated in Figure 1. FJB is a polyanionic fluorescein derivative that has been shown to be a sensitive and reliable marker for histochemical localization of neuronal degeneration (Schmued and Hopkins,2000). Figure 2 shows examples of images from brain sections from OUA-infused rats and from rats that had been injected with pilocarpine in a parallel series of experiments. The latter revealed marked cell death in the hippocampus after pilocarpine injections. In the present experiments, on the other hand, no obvious changes were observed 1 hr after treatments, and sides ipsilateral to the injection were similar to the contralateral areas after OUA infusion (Fig. 2).
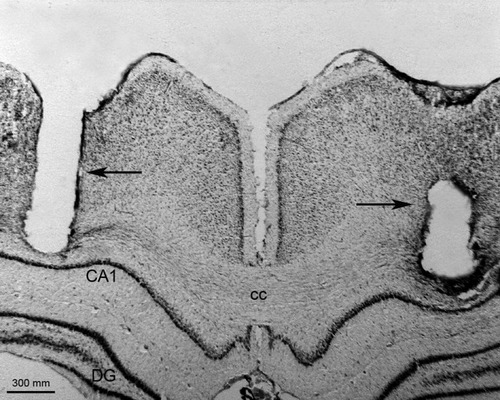
Digital image of a coronal brain section from one representative rat, showing bilateral guide cannula tracks just above the microinjection sites in the dorsal hippocampus (arrows). CA1, field CA1 of hippocampus; cc, corpus callosum; DG: dentate gyrus.
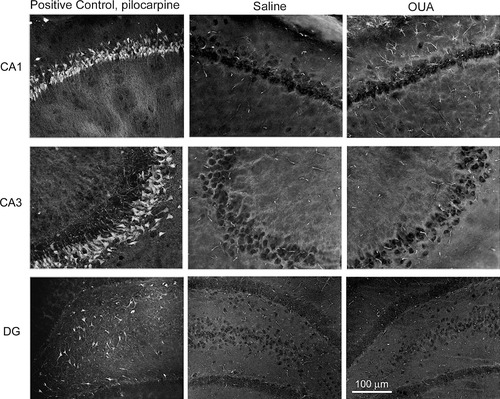
FJB fluorescence signals of control and pilocarpine- and OUA-treated rats. Brain sections of pilocarpine (360 mg/kg, i.p.), OUA (10 nM), or saline (injected in the opposite hippocampus) were investigated by FJB. Pilocarpine treated-animals were used as positive controls, because subpopulations of cells within CA1, CA3, and the dentate gyrus (DG) are known to undergo neuronal cell death 24 hr after this treatment, generating strong and selective fluorescent FJB signals. Such positive cells were never observed in the brains of OUA-treated rats 1 hr after the infusion or in the brains of control rats infused with saline solution.
OUA-Induced Modulation of α2/3-Na,K-ATPase and NF-κB Activities and Gene Expression in Rat Hippocampus
EMSAs were performed to study the effects of OUA treatment on NF-κB binding activity in rat hippocampus. OUA induced a time- and dose-dependent activation of NF-κB (Fig. 3A,B). Nuclear extracts from the control and OUA-treated tissues presented a similar pattern of three DNA–protein complexes (Fig. 3A,B). Corresponding densitometric analyses showed that the p65p50 value to saline group was 74.0 ± 5.0, and to OUA 10 nm and 10 μM values were 104.7 ± 1.4* and 135.0 ± 5.5**, respectively. The p50p50 value to the saline group was 92.6 ± 4.0 and to OUA 10 nm and 10 μM 115.7 ± 1.2* and 134.0 ± 6.4**, respectively (*P < 0.001 vs. control-treated opposite hippocampus; **P < 0.05 vs. OUA [10 nM]-treated animals [one-way ANOVA, followed by Newman-Keuls test; Fig. 3C]).
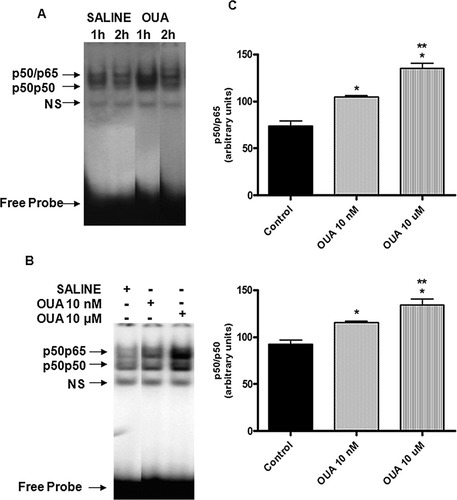
Dose-response curve and the time-course curve of the effect of OUA (10 nM and 10 μM) on NF-κB activation in rat hippocampus. A: Nuclear proteins (10 μg) were extracted from rat hippocampus obtained 1 and 2 hr after intrahippocampal injection with OUA (10 nM) or saline (injected in the opposite hippocampus). The positions of both protein–DNA complexes observed are indicated (p50p65 and p50p50). B: Nuclear proteins (10 μg) were extracted from rat hippocampus obtained 1 hr after intrahippocampal injected with OUA (10 nM and 10 μM) or saline (injected in the opposite hippocampus). The positions of both protein––DNA complexes observed are indicated (p50p65 and p50p50). NS, nonspecific binding. The localization of free probe is also indicated. Results are representative of three experiments. C: Densitometric analysis (arbitrary units, A.U.) of the bands p50p65 and p50p50 presented in B. Results are expressed as mean + SEM from four individual experiments. ∗︁P < 0.001 vs. control-treated opposite hippocampus; ∗︁∗︁P < 0.05 vs. OUA (10 nM)-treated animals (one-way ANOVA followed by Newman-Keuls test).
The complexes p50p65 and p50p50 were displaced by an excess of unlabeled NF-κB but not by TFIID double-stranded oligonucleotide consensus sequence, demonstrating the specificity of NF-κB/DNA binding interaction (Fig. 4). The NS complex was less efficiently displaced by unlabeled 32P-NF-κB probe (Fig. 4). Supershift analysis indicated that the antibody against the p65 subunit was able to shift the DNA–protein interaction observed for p50p65. The antibody against the p50 subunit shifted complex 2 and induced a partial decrease of the p50p65 complex. The presence of antibodies against the p52 and c-Rel subunits did not affect the DNA–protein complexes (Fig. 4). Taken together, these results indicated that p50p65 heterodimers and p50p50 homodimers were included in NF-κB DNA. NS complex was not displaced by the antibodies, so it was not considered to be a member of the NF-κB family (Fig. 4). In fact, the same effect was observed in the immunoblot assay; OUA (10 nM) caused an increase of p65 NF-κB subunit translocation to the nucleus (Fig. 5A) and a decrease of p65 expression in the cytosol (Fig. 5A) 1 hr after hormone intrahippocampal injection. Densitometric analyses showed that control value of p65 nuclear/β-actin was 66.3 ± 2.3 and to OUA (10 nM) was 93.5 ± 1.3*; control values of p65 cytosol/β-actin was 190.4 ± 6.2 and to OUA (10 nM) was 152.2 ± 11.5* (*P < 0.05 vs. control-treated opposite hippocampus, Student's t-test). In addition, OUA (10 nM) induced both an increase in pIκBα and a decrease in IκB in the cytosol (Fig. 5C). Corresponding densitometric analyses of these blots showed that the mean of the value of pIκBα/IκB ratio was 0.8 ± 0.1 and to OUA was 10.3 ± 1.6*, and the IκB/β-tubulin ratio was 0.26 ± 0.03 and to OUA was 0.07 ± 0.01 (*P < 0.05 vs. saline, Student's t-test; Fig. 5D).
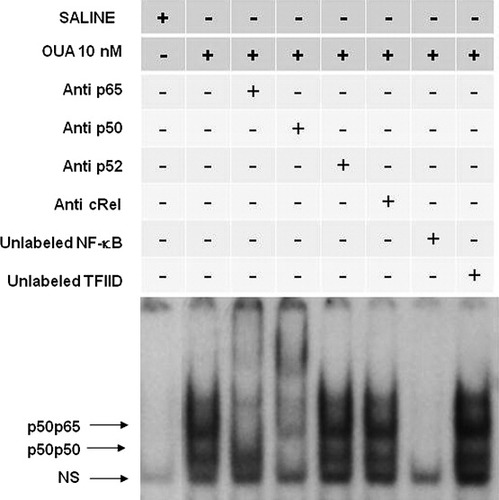
Competition and supershift assay of NF-κB activation by OUA (10 nM) in rat hippocampus. Competition studies were performed using nuclear extract (10 μg) from hippocampus of rats 1 hr after received intrahippocampal injection of OUA (10 nM) in the absence or presence of unlabeled specific (NF-κB consensus sequence, 20-fold molar excess) or nonspecific (TFIID consensus sequence, 20-fold molar excess) oligonucleotide, as indicated. Supershift assays were performed with the same nuclear extract (10 μg) incubated in the absence and presence of antibodies against subunits p50 (1:20 dilution), p65 (1:20 dilution), and p52 and cRel (1:20 dilution), as indicated. Antibodies were added 20 min prior to addition of the radiolabeled NF-κB consensus oligonucleotide. The positions of specific NF-κB/DNA binding complexes p50p50 and p50p65 are indicated. NS, nonspecific binding. The localization of the probe is also indicated. Results are representative of three experiments.
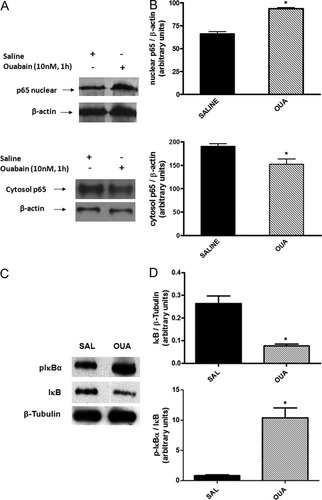
Effect of OUA (10 nM) on p65 subunit NF-κB translocation and pIκBα/IκB levels in rat hippocampus. Protein extracts were extracted from rat hippocampus obtained 1 hr after intrahippocampal injection with OUA (10 nM) or saline (injected in the opposite hippocampus). A: Representative Western blot autoradiographies of p65 nuclear and cytosolic and β-actin. B: Densitometric analysis (arbitrary units, A.U.) of p65 nuclear/β-actin and cytolosic/β-actin ratios of groups presented in A. Results are expressed as mean + SEM. from four individual experiments. ∗︁P < 0.05 vs. control-treated opposite hippocampus (Student's t-test). C: Representative Western blot autoradiographies of pIκBα, cytosolic IκB, and β-tubulin. D: Densitometric analysis (arbitrary units, A.U.) of cytosolic pIκBα/IκB and IκB/β-tubulin ratios of groups presented in C. Results are expressed as mean + SEM from four individual experiments. ∗︁P < 0.001 vs. saline (Student's t-test).
The effects of OUA (10 nM) on the expression of some NF-κB target genes were then evaluated by RT-PCR assay. Densitometric analysis showed that OUA induced an increase of Bdnf, iNos, Tnf-α, and Bcl2 mRNA levels. No change in mRNA Bax or Iκbα bands was observed (Bcl2 [saline = 1.9 ± 0.1 and OUA = 2.8 ± 0.2*, n = 7]; Bax [saline = 1.9 ± 0.1 and OUA = 2.0 ± 0.2, n = 7]; Bndf [saline = 4.8 ± 0.6 and OUA = 7.2 ± 0.2*, n = 6]; iNos [saline = 0.9 ± 1.6 and OUA = 2.2 ± 0.2*, n = 7]; Tnf [saline = 0.8 ± 0.1 and OUA = 1.3 ± 0.2*, n = 7]; Iκbα [saline = 2.8 ± 0.1 and OUA = 2.8 ± 0.2, n = 7]; *P < 0.05 vs. saline, Student's t-test; Fig. 6A,B).
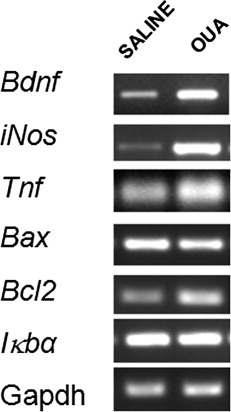
Effects of OUA (10 nM) on levels of mRNA for Bcl2, Bax, Bdnf, Tnf, Iκbα, and iNos in rat hippocampus by RT-PCR. Representative PCR photographs and densitometric analysis (arbitrary units, A.U.) of the specific bands of OUA-treated group. mRNA levels is presented as ratios of target gene to GAPDH expression. Data are presented as mean ± SEM from at least six individual experiments. ∗︁P< 0.05 vs. control-treated opposite hippocampus (Student's t-test).
The dose of 10 μM OUA caused a decrease of the total Na,K-ATPase activity (Fig. 7A), more specifically for the α2/3-Na,K-ATPase in the hippocampus of rats, and no changes for the α1-Na,K-ATPase activity (Fig. 7B). A lower OUA dose (10 nM) did not alter the enzyme activity. The control values were 310.2 ± 6.8 nmol/mg protein · min to total ATPase; 208 ± 7.6 nmol/mg protein · min to Mg-ATPase; 65.1 ± 4.8 nmol/mg protein · min to α1-Na,K-ATPase; and 36.7 ± 4.1 nmol/mg protein · min to α2/3-Na,K-ATPase. The OUA values were 308.8 ± 8.7 nmol/mg protein · min to total ATPase; 208.7 ± 7.4 nmol/mg protein · min to Mg-ATPase; 64.5 ± 4.2 nmol/mg protein · min to α1-Na,K-ATPase; 42.8 ± 3.8 nmol/mg protein · min to α2/3-Na,K-ATPase to OUA 10 nM; and 295.6 ± 4.5 nmol/mg protein · min to total ATPase*; 209.5 ± 5.8 nmol/mg protein · min to Mg-ATPase; 62.0 ± 6.9 nmol/mg protein · min to α1-Na,K-ATPase; and 24.1 ± 3.9 nmol/mg protein · min to α2/3-Na,K-ATPase* to OUA 10 μM, respectively (*P < 0.05 vs. control treated opposite hippocampus, one-way ANOVA followed by Newman-Keuls test).
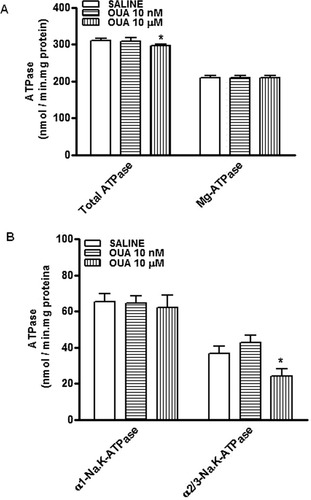
Effects of different doses of OUA (10 nM and 10 μM) on total ATPase, Mg-ATPase (A), α1-Na,K-ATPase, and α2,3-Na,K-ATPase (B) activities in rat hippocampus. Na,K-ATPase activity was tested by adding 10 μg of the particulate fraction from rat hippocampus obtained 1 hr after intrahippocampal injection with OUA (10 nM and 10 μM) or saline (injected in the opposite hippocampus). α2/3-Na,K-ATPase activity was measured by subtracting the activity obtained with 3 μM ouabain from total Na,K-ATPase activity. To determine the α1-subunit-associated Na,K-ATPase activity, the Na,K-ATPase activity measured with 3 μM OUA was subtracted from the activity obtained with 3 mM OUA (Mg-ATPase). Results are expressed as nmol of Pi released/mg protein · min (mean + SEM) from three individual experiments. ∗︁P < 0.05 vs. control treated opposite hippocampus (one-way ANOVA followed by Newman-Keuls test).
NMDA Increase OUA Activation of NF-κB Activity in Rat Hippocampus
Both OUA (10 nM) and NMDA (1 μM) caused NF-κB activation (Fig. 8). When NMDA (1 μM) was injected 1 hr before OUA (10 nM) intrahippocampal injection, a synergistic activation of NF-κB was observed (Fig. 8A). The densitometric analyses showed that the mean value to saline group was 72.5 ± 2.7; OUA (10 nm) was 117.4 ± 3.7*; NMDA (1 μM) was 102.4 ± 5.9*; and NMDA (1 μM) + OUA (10 nm) was 132.5 ± 2.4** (*P < 0.05 vs. control treated opposite hippocampus; **P < 0.05 vs. OUA or NMDA treated hippocampus, one-way ANOVA followed by Newman-Keuls test; Fig. 8B).
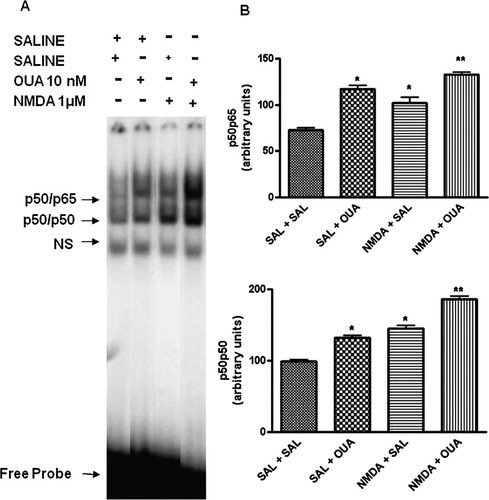
Effect of OUA (10 nM) on NF-κB activation induced by NMDA (1 μM) in rat hippocampus by EMSA. A: Nuclear proteins (10 μg) were extracted from hippocampus of rats pretreated with NMDA (1 μM, intrahippocampal) or saline 1 hr before of the OUA (10 nM) or saline intrahippocampal injection. B: Densitometric analysis (arbitrary units, A.U.) of the p50p65 and p50p50 bands presented in A. Results are expressed as mean ± SEM from four individual experiments. ∗︁P < 0.05 vs. control treated opposite hippocampus; ∗︁∗︁P < 0.05 vs. OUA or NMDA treated opposite hippocampus (one-way ANOVA followed by Newman-Keuls test).
The upper complexes induced by NMDA injection were displaced by an excess of unlabeled NF-κB, but not by TFIID doubled-stranded oligonucleotide consensus sequence, demonstrating a similar pattern of the specificity of NF-κB/DNA binding interaction obtained after OUA (Fig. 9). Supershift analysis also confirmed that the antibodies against the p65 and p50 subunits were able to shift DNA–protein interaction present in the upper complexes. The presence of antibodies against the subunits p52 and c-Rel did not affect DNA–protein complexes (Fig. 9). The lower complex was not displayed by the antibodies and was not considered to be related to NF-κB family (Fig. 9).
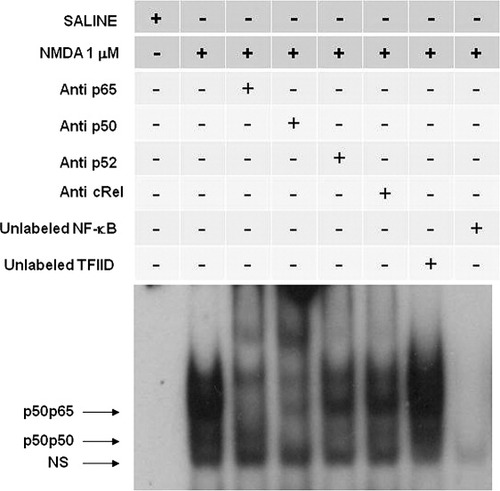
Competition and supershift assay of NF-κB activation by NMDA (1 μM) in rat hippocampus. Competition studies were performed using nuclear extract (10 μg) from hippocampus of rats 2 hr after received intrahippocampal injection of NMDA (1 μM) in the absence or presence of unlabeled specific (NF-κB consensus sequence, 20-fold molar excess) or nonspecific (TFIID consensus sequence, 20-fold molar excess) oligonucleotide, as indicated. Supershift assays were performed with the same nuclear extract (10 μg) incubated in the absence and presence of antibodies against subunits p50 (1:20 dilution), p65 (1:20 dilution), and p52 and cRel (1:20 dilution), as indicated. Antibodies were added 20 min prior to addition of the radiolabeled NF-κB consensus oligonucleotide. The position of specific NF-κB/DNA binding complexes p50p50 and p50p65 are indicated. NS, nonspecific binding. The localization of the probe is also indicated. Results are representative of three experiments.
OUA (10 nM) did not affect NOS activity. However, NMDA (1 μM) either itself or in the presence of OUA (10 nM), caused an increase of this enzyme activity (Fig. 10) and also increased total Na,K-ATPase activity (Fig. 11A), which was linked specifically to α2/3-Na,K-ATPase activity, because no changes were observed for α1-Na,K-ATPase (Fig. 11B). The mean of control value was 47.7 ± 2.2; OUA (10 nM) was 54.2 ± 3.4; NMDA (1 μM) was 80.9 ± 2.0*; OUA (10 nM) + NMDA (1 μM) was 85.8 ± 2.2* to NOS activity (*P < 0.05 vs. control [Sal + Sal]-treated opposite hippocampus, one-way ANOVA followed by Newman-Keuls test). By the same token, NMDA (1 μM) alone or in the presence of OUA (10 nM) also increased total Na,K-ATPase activity (Fig. 11A), which was linked specifically to α2/3-Na,K-ATPase activity, because no changes were observed for α1-Na,K-ATPase (Fig. 11B). The controls values were 310.5 ± 8.4 nmol/mg protein · min to total ATPase; 197.8 ± 9.4 nmol/mg protein · min to Mg-ATPase; 68.7 ± 5.2 nmol/mg protein · min to α1-Na,K-ATPase; 44.0 ± 5.7 nmol/mg protein · min to α2/3-Na,K-ATPase, respectively. The OUA values were 312.7 ± 7.4 nmol/mg protein · min to total ATPase; 204.7 ± 8.7 nmol/mg protein · min to Mg-ATPase; 69.5 ± 4.2 nmol/mg protein · min to α1-Na,K-ATPase; 38.5 ± 6.8 nmol/mg protein · min to α2/3-Na,K-ATPase, respectively. The NMDA values were 345.8 ± 6.8 nmol/mg protein · min to total ATPase*; 203.2 ± 6.9 nmol/mg protein · min to Mg-ATPase; 74.0 ± 6.8 nmol/mg protein · min to α1-Na,K-ATPase; 68.6 ± 6.4 nmol/mg protein · min to α2/3-Na,K-ATPase*. The NMDA + OUA values were 343.6 ± 8.7 nmol/mg protein · min to total ATPase*; 210.7 ± 5.4 nmol/mg protein · min to Mg-ATPase; 72.4 ± 6.8 nmol/mg protein · min to α1-Na,K-ATPase; 69.5 ± 4.7 nmol/mg protein · min to α2/3-Na,K-ATPase* (*P < 0.05 vs. control [Sal + Sal], one-way ANOVA followed by Newman-Keuls test).
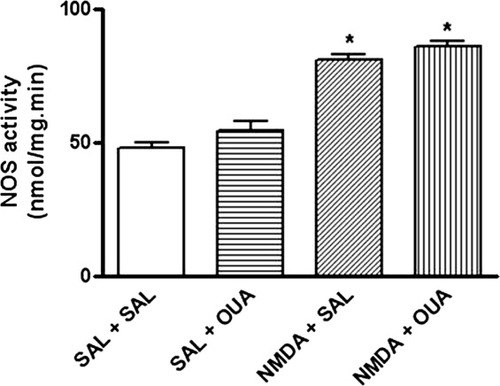
Effects of NMDA (1 μM), OUA (10 nM), and OUA (10 nM) + NMDA (1 μM) on cytosolic NOS activity in rat hippocampus. The enzyme activity was determine in supernatant fraction from rat hippocampus obtained of rats pretreated with OUA (10 nM) or saline 1 hr prior treatment with NMDA (1 μM, intrahippocampal) or saline. Relative NOS activity (L-[3H]citrulline production) is expressed as nmol/mg protein · min (mean ± SEM) from three individual experiments. ∗︁P < 0.05 vs. control-treated opposite hippocampus (one-way ANOVA followed by Newman-Keuls test).
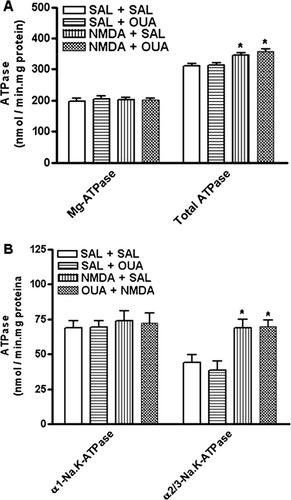
Effects of NMDA (1 μM), OUA (10 nM), and OUA (10 nM) + NMDA (1 μM) on total ATPase, Mg-ATPase (A), α1-Na,K-ATPase and α2,3-Na,K-ATPase (B) activities in rat hippocampus. Na,K-ATPase activity was tested by adding 10 μg of the particulate fraction from rat hippocampus obtained of rats pretreated with OUA (10 nM) or saline 1 hr prior treatment with NMDA (1 μM, intrahippocampal) or saline. α2/3-Na,K-ATPase activity was measured by subtracting the activity obtained with 3 μM OUA from total Na,K-ATPase activity. To determine the α1-subunit-associated Na,K-ATPase activity, the Na,K-ATPase activity measured with 3 μM OUA was subtracted from the activity obtained with 3 mM OUA (Mg-ATPase). Results are expressed as nmol of Pi released/mg protein · min (mean ± SEM) from three individual experiments. ∗︁P < 0.05 vs. control-treated opposite hippocampus (one-way ANOVA followed by Newman-Keuls test).
Finally, administration of MK-801 (3 mg/kg, i.p.), an antagonist of NMDA receptors, partially reduced the OUA-induced activation of NF-κB binding activity without changing the pattern of 32P-NF-κB/protein complexes observed (Fig. 12A). The p65p50 values of densiometric analysis were saline group = 82.9 ± 11.4; OUA 10 nm = 175.8 ± 9.9*; MK-801 = 95.8 ± 7.6; MK-801 + OUA = 121.5 ± 8.9**. In addition, the p50p50 value of densiometric analysis were: saline group = 92.8 ± 5.8; OUA 10 nm = 176.9 ± 9.9*; MK = 801 = 95.8 ± 7.6; MK-801 + OUA = 117.8 ± 5.7** (*P < 0.05 vs. saline; **P < 0.05 vs. OUA treated hippocampus, one-way ANOVA followed by Newman-Keuls test; Fig. 12B).
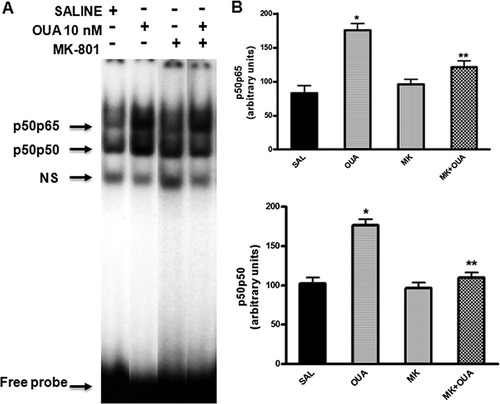
Effects of MK-801 on NF-κB activation induced by OUA (10 nM) in rat hippocampus by EMSA. A: Nuclear proteins (10 μg) were extracted from hippocampus of rats pretreated with MK-801 (3 mg/kg, i.p.) or saline 20 min prior to OUA (10 nM) or saline intrahippocampal injection. B: Densitometric analysis (arbitrary units, A.U.) of the NF-κB bands presented in A. Results are expressed as mean ± SEM from four individual experiments. ∗︁P < 0.05 vs.control-treated opposite hippocampus; ∗︁∗︁P < 0.05 vs. OUA-treated hippocampus (one-way ANOVA followed by Newman-Keuls test).
DISCUSSION
OUA is an endogenous steroid hormone thought to be synthesized in the adrenal glands (Schneider et al.,1998); the adrenal secretion of endogenous OUA has been identified both in vivo and in vitro (Doris and Stocco,1989; Boulanger and Vanhoutte,1991; Ludens et al.,1992; Beck et al.,1996; Hinson et al.,1998). In fact, adrenocorticotrophic hormone (ACTH) and angiotensin II have been shown to stimulate the adrenal secretion of endogenous OUA (Laredo et al.,1994, 1997). OUA is also present in mammalian tissues, such as the hypothalamus (Kawamura et al.,1999), anteroventricular third ventricular region (AV3V; Pamnani et al.,1981; Songu-Mize et al.,1982; Bealer et al.,1983), and human plasma (Hamlyn et al.,1982). Evidence indicates that circulating levels of OUA increase rapidly in blood of humans and dogs during salt and volume expansion (De Wardener et al.,1961), during physical exercise (Bauer et al.,2005), in pregnancy, in newborn infants (Schoner and Scheiner-Bobis,2007; Bagrov and Shapiro,2008), in a volume-dependent hypertension model (Haddy and Overbeck,1976), and in patients with congestive heart failure (Schoner,2001; Bagrov et al.,2009).
For the nervous system, evidence supports a dual role of NF-κB in neurodegenerative diseases; activation of NF-κB in neurons promotes their survival, whereas activation in glial and immune cells mediates pathological inflammatory processes (Camandola and Mattson,2007). The findings presented here are the first report indicating by EMSA that intrahippocampal administration of OUA induces an activation of NF-κB in that rat brain structure. It is interesting to note that most of the transcriptional activity altered by OUA intrahippocampal administration is mediated through p50p65 heterodimers and p50p50 homodimers. The former is a potent transcriptional activator of genes that can be activated in response to synaptic activity and thereby provides a mechanism of signaling that likely plays an important role in neuroinflammatory response; the later is a stimulus-specific repressor of gene activation that is also relevant to modulate the activation of inflammatory response gene (Pereira and Oakley,2008).
It is well known that OUA can inhibit the Na,K-ATPase activity, leading to increased intracellular Na+ concentration that transactivates Na+/Ca2+ exchangers, which thereby produce cytosolic Ca2+ waves that are sufficient to elicit downstream signaling events such as increase of NF-κB activity (Blaustein and Lederer,1999; L. Liu et al.,2007). It has been shown in peripheral systems that low doses of OUA (10–100 nM) protected kidney cell cultures from serum deprivation, an effect apparently occurring in parallel with NF-κB activation (Li et al.,2006).
Multiple Na,K-ATPase isoforms can be functionally differentiated by OUA in the brain (Sweadner,1989; Richards et al.,2007). In rodents, the α1-Na,K-ATPase isoform is 1,000 times less sensitive to the cardiac glycoside than the α2/3-isoform. In fact, our data show that a concentration of 10 μM of OUA caused a decrease in α2/3-Na,K-ATPase activity in the hippocampus of rats and no changes in the α1-Na,K-ATPase activity, whereas the lower concentration (10 nM), which also activated NF-κB, did not alter either isoform's activity. Therefore, the inhibition of Na,K-ATPase pumping activity by OUA is not the only mechanism responsible for triggering the activation of NF-κB in hippocampus. Although 10 μM of OUA caused greater activation of NF-κB, the dose of OUA used during the other experiments was 10 nM, because the aim of our study was to evaluate possible mechanisms involved in the signaling pathway of the digitalic without the influence of sodium pump activity.
Data indicate that the activation of NF-κB by OUA (10 nM) could be at least partially linked to the increase of Bdnf, iNos, Tnf-α, and Bcl2 mRNA levels. In fact, we cannot rule out that these genes can also be modulated by other transcription factors that might be activated by OUA. These results suggest that OUA might play an important role in modulating genes associated with inflammatory response as well as in cell survival signaling (Kawamoto et al.,2008). In fact, OUA has both toxic (Valente et al.,2003; Orlov et al.,2004) and protective actions in cells (Li et al.,2006; Pasdois et al.,2007), including neurons (Golden and Martin,2006).
OUA has been shown to evoke release of glutamate by reversing glutamate transporter function (Rossi et al.,2000). Glutamate-induced NMDA activation has been shown to induce NF-κB activation in several brain structures (Guerrini et al.,1995; Kaltschmidt et al.,1995; Munhoz et al.,2006), which is linked to the NO pathway (Glezer et al.,2003). Consistently with these studies, NMDA (1 μM) caused NF-κB activation and increased the activity of NOS in hippocampus, whereas OUA (10 nM) activated NF-κB but not NOS activity. In addition, when NMDA was injected 1 hr before OUA intrahippocampal injection, we observed a potentiation of NF-κB activation but not of NOS activity. NMDA exhibited a similar pattern of specificity of NF-κB/DNA binding interaction obtained after OUA injection. The present results confirm that OUA can regulate NMDA binding and receptor activity (Reinés et al.,2001, 2004; Bersier and Rodríguez de Lores Arnaiz,2009) as well as the glutamate transporters (Rose et al.,2009). In the present work, administration of MK-801 (3 mg/kg), an antagonist of NMDA receptors, partially reduced the OUA-induced activation of NF-κB binding activity, suggesting that the OUA activation of this transcription factor is at least in part dependent on Na,K-ATPase activation of NMDA receptor in hippocampus. We also observed that NMDA in the presence or absence of OUA induced a similar increase of total Na,K-ATPase activity, which was specifically linked to α2/3-Na,K-ATPase activity. The present results confirm our previous evidence that glutamate increased α2/3-Na,K-ATPase activity through NO/carbon monoxide cascade in the CNS (Nathanson et al.,1995; Munhoz et al.,2005) and suggest that OUA activates NF-κB by modulating NMDA receptors in a non-NO-dependent pathway.
Na,K-ATPase has been shown to be associated with the inositol 1,4,5-triphosphate receptor to generate intracellular Ca2+ oscillations (Miyakawa-Naito et al.,2003). In addition, OUA (100 μM) induced calcium oscillation and a calcium-dependent p65–NF-κB translocation to the nucleus in hippocampal astrocyte cultures (X.L. Liu et al.,2007). The authors of this study suggest, based on coimmunoprecipitation studies, that Ca2+ oscillations involve a multiprotein complex consisting of ankyrin-B, inositol 1,4,5-triphosphate receptor, and Na,K-ATPase. Therefore, the potentiation of NF-κB activation when NMDA was injected 1 hr before OUA intrahippocampal injection probably occurred through a calcium-dependent pathway in glial cells. However, we cannot rule out that part of the OUA-induced NF-κB activation could be linked to calcium activation pathway in neurons.
Despite OUA modulating genes involved with inflammatory response, no signs of neurodegeneration were observed via FJB and Nissl stain techniques, suggesting that OUA at nanomolar concentration did not induce any toxic effect. It is interesting to note that OUA did not change the mRNA levels of Bax. The Bcl2 gene family includes both proapoptotic and antiapoptotic members that enhance or block cell death (Hengartner,2000). Thus, the Bax gene generates a protein (BAX), which is known to promote apoptosis in mammalian cells (Oltvai et al.,1993; Fig. 13), whereas the Bcl2 genes generate the BCL-2 proteins, which in turn neutralize the actions of BAX. Therefore, the lack of changes in Bax mRNA levels after OUA injection suggests that this hormone does not activate proapoptotic pathways at nanomolar concentration.
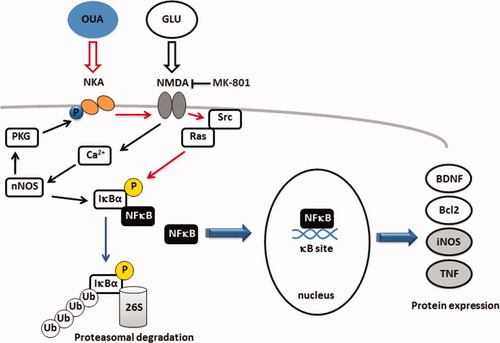
Simplified model of the modulation of NF-κB activation by OUA-Na,K-ATPase and NMDA signaling cascade in rat hippocampus and its implications for gene regulation. Activation of NMDA receptor increases nNOS and α2,3-Na,K-ATPAse activity and activates NF-κB in CNS. OUA (10 nM) can activate the Na,K-ATPase complex that results in the activation of NF-κB in the CNS, leading to an increase of Bdnf, iNos, Tnf, and Bcl2 mRNA levels. The OUA effect was not linked to changes in nNOS and Na,K-ATPase activity. Na,K-ATPase has been shown to stimulate calcium and Src-Ras-MAP kinase cascade, which in turn can activate NF-κB (red arrows). NMDA treatment further increases OUA-induced NF-κB activation, but MK-801, an antagonist of the NMDA receptor, partially blocked OUA activation of this transcription factor. Na,K-ATPase, in addition to its established role as an ion pump, can also function as a signal transducer receptor activating NMDA receptor as well as other, neighboring plasma membrane proteins.
A recent report also showed that this concentration of OUA stimulates neurite outgrowth in human neuroblastoma cells (Desfrere et al.,2009). The authors also showed that OUA stimulates dendritogenesis in primary culture of cortical neurons via activation of a transcriptional program that involves cyclic AMP response element (CRE)-binding protein (CREB)- and CRE-mediated gene expression, primarily through a CaM (calmodulin) kinase-dependent signaling pathway. OUA induced this effect at concentrations (≤1 μM) that do not change the resting membrane potential, suggesting that Na,K-ATPase signal transduction cascade mediates the process (Desfrere et al.,2009). However, OUA can also assemble with epidermal growth factor receptor and therefore activates Src-Ras-MAP kinase, which in turn can activate NF-κB in cultured cardiac myocytes (Xie and Askari,2002).
The involvement of NMDA receptors, Src-Ras, and MAPK is consistent with NF-κB mediating adaptive responses of the cells to the cellular signaling induced by sublethal concentrations of Aβ in cultured cerebellar cells (Kawamoto et al.,2008). Taken together, our findings suggest that Na,K-ATPase, in addition to its established role as an ion pump, can also function as a signal transducer receptor. When OUA is bound to Na,K-ATPase, it may activate the NMDA receptor as well as other, neighboring plasma membrane proteins. OUA-induced NF-κB activation seems to be at least in part dependent on Na,K-ATPase's modulatory action of NMDA receptor in hippocampus, and this transcription factor can modulate genes that may play important roles in adaptive neuroplasticity and neuroprotection processes (Albensi and Mattson,2000; Srinivasan et al.,2004; Mattson, 2008b).
Several studies have provided evidence that NF-κB mediates at least in part the neuroprotective effects of NMDA receptor activation in cerebellar granule neurons and that BDNF is a key NF-κB target involved in NMDA neuroprotection (Lipsky et al.,2001; Jiang et al.,2005; Kawamoto et al.,2008). In addition, it hasbeen shown that NF-κB mediating BDNF confers resistance of cultured cerebellar cell to Aβ toxicity (Kawamoto et al.,2008) as well as of PC12 cell to cocaine-induced cell death (apoptosis and necrosis; Lepsch et al.,2009).
Other studies have suggested that NF-κB mediates at least in part the neuroprotective effects of NMDA receptor activation in the central nervous system. We suggest that the interaction between Na,K-ATPase and NMDA signaling cascade can modulate NF-κB as well as neuron depolarization in rat hippocampus, both of which are important to cell survival and differentiation. This pathway could be associated with biological mechanisms that may underlie the basal homeostatic state linked to inflammatory responses in the brain, because OUA induced an increase of Bdnf, iNos, Tnf-α, and Bcl2 mRNA levels. The present work suggests that OUA-induced NF-κB activation could provide new targets for the development of novel medications for neurodegenerative diseases.
Acknowledgements
E.M.K. and F.G.A. are supported by postdoctoral fellowships from FAPESP; L.M.Y., R.R.F.P., and A.M.M.O. are PhD students and research fellows from FAPESP and CAPES; P.F.K. is an undergraduate student fellow from FAPESP; and L.S.L. is supported by a grant from the Universidade de São Paulo. C.S., J.C.N, L.R.G.B., M.C.W.A. and L.V.R. are research fellow from CNPq. The authors declare that, except for income received from a primary employer, no financial support or compensation has been received from any individual or corporate entity over the past 34 years for research or professional service and that no personal financial holdings that could be perceived as constituting a potential conflict of interest.




