Differential clustering of Caspr by oligodendrocytes and Schwann cells
Abstract
Formation of the paranodal axoglial junction (PNJ) requires the presence of three cell adhesion molecules: the 155-kDa isoform of neurofascin (NF155) on the glial membrane and a complex of Caspr and contactin found on the axolemma. Here we report that the clustering of Caspr along myelinated axons during development differs fundamentally between the central (CNS) and peripheral (PNS) nervous systems. In cultures of Schwann cells (SC) and dorsal root ganglion (DRG) neurons, membrane accumulation of Caspr was detected only after myelination. In contrast, in oligodendrocytes (OL)/DRG neurons cocultures, Caspr was clustered upon initial glial cell contact already before myelination had begun. Premyelination clustering of Caspr was detected in cultures of oligodendrocytes and retinal ganglion cells, motor neurons, and DRG neurons as well as in mixed cell cultures of rat forebrain and spinal cords. Cocultures of oligodendrocyte precursor cells isolated from contactin- or neurofascin-deficient mice with wild-type DRG neurons showed that clustering of Caspr at initial contact sites between OL processes and the axon requires glial expression of NF155 but not of contactin. These results demonstrate that the expression of membrane proteins along the axolemma is determined by the type of the contacting glial cells and is not an intrinsic characteristic of the axon. © 2009 Wiley-Liss, Inc.
The nodes of Ranvier are bordered by specialized junctions that are formed between the axon and the paranodal loops of myelinating glia (Rosenbluth, 1995). In this region, the compact myelin lamellae open up into a series of cytoplasmic loops that spiral around the axon, forming a series of septate-like junctions with the axolemma. The paranodal junction (PNJ) appears relatively late during myelination, first being generated closer to the nodes by the outer most paranodal loop and then forming gradually as additional loops are attached to the axon (Tao-Cheng and Rosenbluth, 1983; Wiggins et al., 1988). The PNJ attaches the myelin sheath to the axon, separates the electrical activity at the nodes of Ranvier from the internodal region that lies under the compact myelin sheath, and provides a boundary that limits the lateral diffusion of membrane components (Rosenbluth, 1995; Poliak and Peles, 2003; Salzer et al., 2008).
The axonal membrane at the paranodes contains a complex of two cell adhesion molecules: Caspr, a member of the neurexin family (Einheber et al., 1997; Menegoz et al., 1997; Peles et al., 1997), and contactin, a GPI-linked protein of the immunoglobulin superfamily (Rios et al., 2000). The interaction between Caspr and contactin is required for the delivery of Caspr to the cell surface through a Golgi-independent pathway (Faivre-Sarrailh et al., 2000; Bonnon et al., 2003) as well as for its axonal targeting (Boyle et al., 2001). In addition, the association of Caspr and contactin regulates the glycosylation of the latter and affects its ability to interact with other cell adhesion molecules (CAMs; Gollan et al., 2003; Bonnon et al., 2007). The glial membrane at the PNJ contains neurofascin 155 (NF155), a splice isoform of the cell adhesion molecule neurofascin that is found specifically at the glial loops (Tait et al., 2000). All three CAMs (i.e., Caspr, contactin, and NF155) are essential for the formation of the PNJ, and, in their absence, the ultrastructure of the paranodes is severely altered: the gap between the glial and axonal membranes is increased, and the electron-dense material forming the septa, the hallmark of the PNJ in wild-type mice, is absent (Bhat et al., 2001; Boyle et al., 2001; Sherman et al., 2005; Zonta et al., 2008; Pillai et al., 2009). The localization of Caspr, contactin, and NF155 at the PNJ is interdependent, suggesting that they form an axoglial adhesion complex. However, although the extracellular domain of NF155 can pull-down Caspr and contactin from rat brain lysate (Charles et al., 2002), further experiments suggested that this interaction might have occurred indirectly through an additional, though yet to be discovered, PNJ component (Gollan et al., 2003). Developmental analyses (Rasband et al., 1999a; Rios et al., 2000; Schafer et al., 2006) as well as observations in several myelin mutants (Dupree et al., 1999; Rasband et al., 1999b; Poliak et al., 2001; Arroyo et al., 2002, 2004; Ishibashi et al., 2002; Jenkins and Bennett, 2002) have demonstrated that myelinating glial cells regulate the localization of Caspr along the axon. Paranodal accumulation of Caspr is composed of a number of rings representing each turn of the myelin warp and thus does not represent a uniform domain (Arroyo et al., 1999; Rios et al., 2000; Poliak et al., 2001). During myelination of DRG neurons by SC, Caspr is detected in a spiral corresponding to the overlying turn of the forming paranodal loop, which later consolidates into a tight helical coil (Pedraza et al., 2001, 2009). We report here that, in contrast to the case in PNS axons, membrane accumulation of Caspr is induced by oligodendrocyte contact prior to myelination, a process that is mediated by NF155.
MATERIALS AND METHODS
Cell Culture
DRG neurons, dissociated rat DRGs, SC/DRG neurons cocultures, and OL/RGC neuron cultures were previously described (Eshed et al., 2005; Spiegel et al., 2007; Watkins et al., 2008; Spiegel and Peles, 2009). Dissociated spinal cord cells were prepared from mice embryos at day 13.5 of gestation as described recently (Golan et al., 2008; Thomson et al., 2008). OL precursor cells (OPCs) were isolated and maintained essentially as described by McCarthy and de Vellis (1980). For OLs/DRG neurons coculture (Chan et al., 2004), 8 × 105 cells/ml OPCs were seeded on 3-week-old cultures of DRG neurons.
Mouse OLs/DRG Neuron Cultures
OPCs were obtained form mice lacking contactin (Berglund and Ranscht, 1994) or neurofascin (Sherman et al., 2005) using a modification of the procedure described for the isolation of mature mouse oligodendrocytes (Vitry et al., 1999). Briefly, cerebral hemispheres of postnatal day 0–3 animals (P0–P3) were minced in HBSS supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 0.15% sodium bicarbonate, and 10 mM Hepes buffer; digested for 25 min at 37°C with 0.0025% trypsin; and mechanically dissociated with a fire- polished Pasteur pipette. The cell suspension was then plated at high density (2–3 × 106 cells per cm2) on uncoated tissue culture plastic flasks and kept at 37°C in a humidified 9% CO2. Cells were grown in 2% FBS DMEM supplemented with 10 ng/ml PDGF-AA and 10 ng/ml βFGF for 1 week. Cells were then digested with trypsin and plated on DRG neurons in concentration of 50,000–70,000 cells/slide. Mixed DRG-OPC culture was grown in DMEM/Sato medium (2 mM glutamine, 100 μg/ml apotransferrin, 0.0286% BSA, 0.2 μM progesterone, 100 μM putrescine, 0.45 μM thyroxine, 00.224 μM sodium selenite, 0.5 μM trioiodothyronine, and 5 μg/ml insulin) until it was fixed with 4% paraformaldehyde (PFA).
Mixed Forebrain Cultures
CNS mixed cultures were prepared from either E-15 ICR mouse embryos or E-18 Wistar rat embryos. Brains were carefully dissected into HBSS (Sigma, St. Louis, MO) supplemented with 100 μg/ml penicillin and 100 μg/ml streptomycin (Gibco, Grand Island, NY). Meninges-free cortices were collected and minced using a sterile scalpel blade. Cortices were then trypsinized for 30 min with 0.025% trypsin (Sigma) at 37°C 5%CO2 and triturated. The cell suspension was then centrifuged at 1,200 RPM for 5 min and passed through a 30-μm cell strainer. Cells were then centrifuged for 10 min at 1,200 RPM and resuspended to a density of 2.5 × 106 cells/ml in a medium containing DMEM, 2 mM glutamine, 10% FCS, and pen/strep (all from Gibco). Cells (20 μl) were plated on each 13-mm glass coverslip coated with 100 μg/ml poly-L-lysine (PLL; Sigma) and placed in 24-well tissue culture dishes. Slides were incubated for 90 min, followed by the addition of 0.5 ml/well of N/D Sato medium containing 50%/50% DMEM/Neurobasal medium (Gibco), 2 mM glutamine, pen/strep, 1 mM pyruvate (Gibco), 5 μg/ml insulin (Sigma), 1× B-27 (Gibco), 10 ng/ml biotin (Sigma), 1× trace elements (Cellgro), 100 μg/ml transferring, 100 μg/ml crystalline BSA, 60 ng/ml progesterone, 16 μg/ml putrescine, 40ng/ml sodium selenite, 40 ng/ml triiodo-thyronine, 5 μg/ml N-acetyl cysteine (all from Sigma), and 10 ng/ml PDGF-AA (Chemicon, Temecula, CA). Medium was replaced the following week (without PDGF-AA) and every other day thereafter.
Immunofluorescence
Cells were fixed with 4% PFA for 10 min, washed three times with PBS, and kept at 4°C in PBS containing sodium-azide. Before staining, cells were postfixed with methanol at −20°C for 10 min or with Bouin's fixative (Sigma) for 1 min. Immunofluorescence was carried out as previously described (Eshed et al., 2005; Brockschnieder et al., 2006; Golan et al., 2008). The following monoclonal antibodies were used: MAG (1:300; Boehringer, Mannheim, Germany), RIP (1:1,000; The Developmental Studies Hybridoma Bank), gliomedin (1:250; Eshed et al., 2005), βIII tubulin (TUJ 1; 1:1,000; Convance, Berkeley, CA), Caspr (1:300; Poliak et al., 1999), and rat anti-MBP (1:250; Chemicon). Polyclonal antibodies used included Caspr (1:1,000; Peles et al., 1997) and NF-155 (1:100; Tait et al., 2000). Labeling of cultures for contactin was done with βC-Fc as previously described (Peles et al., 1995). Cy3-, Cy5-, or Alexa488-coupled secondary antibodies were obtained from Jackson Immunoresearch (West Grove, PA) and Invitrogen (Carlsbad, CA). Fluorescence images were obtained with an Axioskop 2 microscope equipped with Apotom imaging system (Carl Zeiss), or a Nikon eclipse E1000 microscope fitted with a Hamamatsu ORCA-ER CCD camera. Final minimal figure adjustment was performed in Adobe Photoshop.
RESULTS
Caspr Accumulates at Axon–OL Contact Sites
To follow the distribution of Caspr during CNS myelination, we employed mixed cell cultures isolated from embryonic rat forebrains (Charles et al., 2000). In 15-day-old cultures (15 DIV), Caspr accumulated in discrete areas along axons, which were contacted by processes of premyelinating OL expressing myelin-associated glycoprotein (MAG; Fig. 1A) and 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase; Fig. 1D). This pattern of Caspr immunoreactivity became even more apparent after 22 DIV, when ensheathment had already progressed, but compact myelin internodes had not yet appeared (Fig. 1B). After the generation of compact myelin, as observed in more mature myelinating cultures at 28 DIV, Caspr was excluded from the internodes and was concentrated at the PNJs (Fig. 1C). At 15 DIV, whenever premyelinating OLs were located on a dense bed of axons, membrane clusters of Caspr formed at axon–glial contacts, resulting in a pattern that mirrored the contour of the OL (Fig. 1E,F). Immunolabeling of cultures with antibodies against Caspr, MBP, and the neuronal specific βIII-tubulin demonstrated that Caspr colocalized with βIII-tubulin-positive axons (Fig. 1H–J). Remarkably, the accumulation of Caspr along the axonal membrane was detected only at sites that were aligned with OL processes (Fig. 1J–L). As depicted in Figure 1K,L, axonal expression of Caspr was markedly reduced in regions extending beyond filopodia that were present at the edge of the OL processes. Taken together, these results demonstrate that Caspr accumulates on the axolemma at contact sites between the axon and differentiating OL prior to the formation of compact myelin.
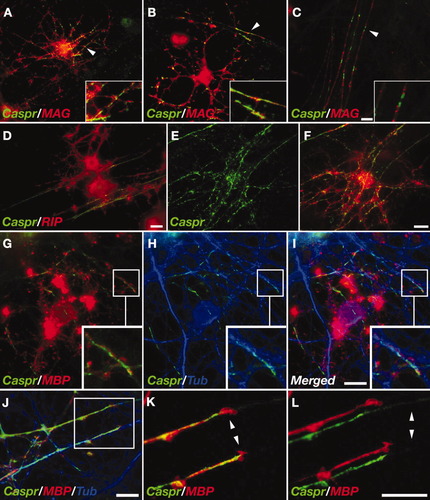
Clustering of Caspr during CNS myelination. A–C: Myelinating rat forebrain cultures 15 (A), 22 (B), and 28 (C) days in vitro (DIV) were immunolabeled with antibodies to Caspr and MAG. Arrowheads point to the areas enlarged in the insets. D: Cultures (22 DIV) were labeled with antibodies to Caspr and RIP. Note the strong Caspr immunoreactivity dispersed over the axolemma. E,F: Labeling of 22 DIV culture with antibodies to Caspr (green) and RIP (red). Caspr immunoreactivity is detected under the contacting oligodendrocyte membrane. G–I: A culture (15 DIV) labeled with antibodies to Caspr, MBP, and βIII-tubulin. Caspr and MBP (G), Caspr and βIII tubulin (H), and the merged image (I) are shown. J: 22 DIV culture labeled with antibodies to Caspr, MBP, and βIII-tubulin. K,L: Higher magnifications of the boxed area in J, showing Caspr and MBP immunoreactivity as merged (K) or shifted (L) images. Arrowheads mark the presence of filopodia at the edge of the ensheathing OLs process (K) and the absence of Caspr at axonal segments devoid of OLs (L). Scale bars = 10 μm.
Membrane Accumulation of Caspr in the PNS Requires Myelination
To study the expression of Caspr during myelination by Schwann cells (SC), we used mouse embryonic DRG mixed cultures, which can be induced to myelinate by the addition of ascorbic acid and serum to the culture medium. Prior to the induction of myelination (including the first day of induction), weak and uniform Caspr immunoreactivity was detected along all neurons (Fig. 2A,E). This was reminiscent of the Caspr expression observed in mixed forebrain cultures during their first week in culture (data not shown). Expression of neither the early myelin marker MAG nor the later myelin marker MBP was observed in SC/DRG cultures prior to the induction of myelination. Caspr remained homogenously scattered in axons that were ensheathed by MAG-positive SCs (data not shown). At 9 days postinduction (DPI), Caspr immunoreactivity was detected as a spiral coil or as a uniform domain at the edge of myelin internodes, a site that represents the forming PNJs (Fig. 2B,F). At 16 DPI, as myelination progressed, Caspr was already present at the PNJs that bordered most of the compact myelin internodes (Fig. 2C,G). It was previously reported that OLs originating from satellite cells rarely appear in mixed SC/DRG cultures (Svenningsen et al., 2004). Interestingly, in all cases in which such OLs were present in the cultures, Caspr accumulated in axons that were contacted by premyelinating OLs (Fig. 2D,H). These results indicate that, in DRG neurons myelinated by SCs, the accumulation of Caspr on the axolemma requires myelination. This is in marked contrast to OLs, which induce the clustering of Caspr at axon–glia contact sites.
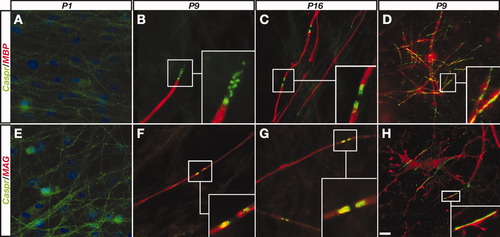
Localization of Caspr during PNS myelination. SC/DRG neurons cultures collected at different days following the induction of myelination (P1–P16), were immunolabeled with antibodies to Caspr and MBP (A–D) or MAG (E–H). At P1 (A,E), Caspr is weakly detected and is dispersed along the axon; DAPI staining is shown in blue. Higher magnifications of the forming PNJs are shown in the insets in B,C and F,G. D,H: Nonparanodal clustering of Caspr is observed when OLs are present in the culture. Scale bar = 10 μm.
Premyelination Clustering of Caspr by OLs Occurs in Various CNS Neurons
To determine whether premyelination clustering of Caspr depends on the type of neuron that is being contacted by OLs, we cocultured OLs with retinal ganglion cells (RGC), motor neurons (MN), and a mixed population of spinal cord neurons. In RGC/OL cocultures (Watkins et al., 2008), strong Caspr immunoreactivity was detected along axonal regions contacted by MBP-positive nonmyelinating OLs (Fig. 3A–C). Upon myelin formation, Caspr began concentrating at the edges of the internodes (Fig. 3D–F). Premyelination clustering of Caspr was also detected in MN/OL cocultures (Fig. 3G–I). Notably, strong Caspr immunoreactivity was occasionally detected in the absence of contacting MBP-positive processes. A similar observation was made in mixed forebrain cultures at an early developmental stage in which OL processes express MAG and CNPase, but not MBP (data not shown), further indicating that Caspr clustering is induced by premyelinating cells. In mixed spinal cord cultures (Thomson et al., 2008), when OL made initial contact with an axon, Caspr clustered at the contact sites, as shown by costaining of Caspr and CNPase (Fig. 3J–L). These results demonstrate that contact-mediated clustering of Caspr by OLs is not restricted to a specific type of neurons.
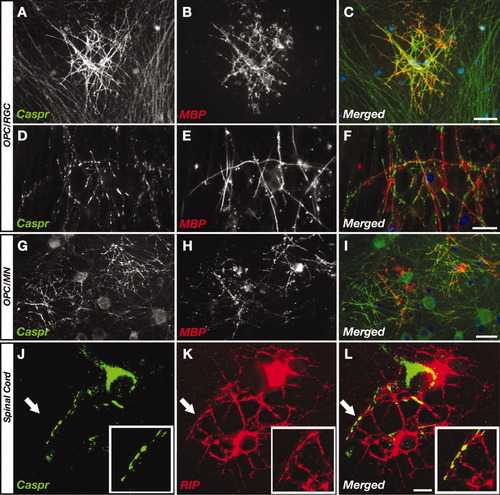
OL-mediated clustering of Caspr in different types of neurons. Distribution of Caspr in cocultures of OLs with retinal ganglion cells (A–F; OPC/RGC), in motor neurons (G–I; OPC/MN), and in mixed spinal cord cultures (J–L). Cultures were immunolabeled with antibodies to Caspr and MBP, or RIP as indicated. Arrows point to the area enlarged in the insets, respectively. Premyelinating (6 DIV; A–C,G–I,J–L) and myelinating (14 DIV; D–F) cocultures are shown. Scale bars = 50 μm in C (applies to A–C); 50 μm in I (applies to G–I); 25 μm in F (applies to D–F); 25 μm in L (applies to J–L).
Premyelination Clustering of Caspr is Induced by OLs and Not by SCs
Our results so far indicate that Caspr clustering is not an intrinsic characteristic of the neuron but is determined by the myelinating glia. To determine directly whether this is the case, we compared the distribution of Caspr when OLs or SCs were cocultured with the same type of neurons. In 4 DIV OL/DRG neurons cocultures, Caspr was clustered along axons that were contacted by OL processes (Fig. 4A,E). At 12 DIV, Caspr expression was more pronounced, and it appeared as elongated lines that were associated with ensheathing OLs processes (Fig. 4B,F). At 16 DIV, some fibers were already at a progressive state of myelination, as indicated by the expression of MBP and its confinement to the myelin sheath and not to the cell body. At this stage, Caspr was often detected in a line that spiraled around the internodes, a spiral that most likely represents each turn of the myelin sheath (Fig. 4G, inset). This spiral coil began to compress into a uniform domain at the forming PNJ. At 27 DIV, when many mature internodes had formed, Caspr was confined mainly to the PNJ (Fig. 4D,H). In contrast to the case in OL/DRG neuron cultures, in early SC/DRG neuron cocultures Caspr was diffusely distributed along the axons and was not clustered at the membrane before myelination (Fig. 4I). After 9 additional days, weak Caspr expression was detected at the edges of MBP-positive myelin internodes (Fig. 4J). These sites were found adjacent to gliomedin-labeled heminodes, which are assembled in the PNS prior to the formation of the PNJs (Ching et al., 1999; Schafer et al., 2006). The accumulation of Caspr at the PNJs became more pronounced as of 14 and 20 DPI, concomitant with the progression of myelination (Fig. 4K,L). The distinct distribution of Caspr along axons that were contacted by OLs and SCs was detected in all areas of the cultures that were examined. Thus, insofar as both the OLs and SCs were plated on the same populations of neurons, these results indicate that the type of the myelinating glial cell differentially determines the distribution of Caspr on the axolemma.
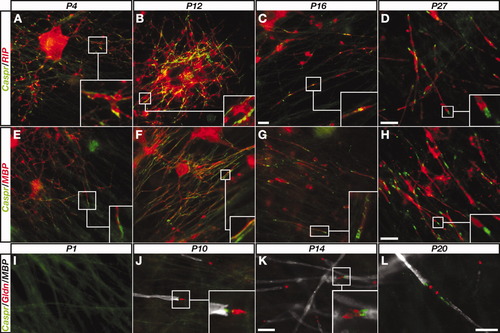
OLs, but not SCs, cause premyelination clustering of Caspr. A–H: DRG neurons cultured with OLs were collected at different days after plating (P1–P27) and immunolabeled using antibodies to Caspr and CNPase (RIP; A–D) or MBP (E–H). Boxes point to the area enlarged in the insets. At P4 and P12, Caspr accumulates along axons at nonparanodal sites, whereas, afterward (P16 and P27), it is detected mainly at the forming PNJs. I–L: DRG neurons/SC cultures collected at P1–P20 were immunolabeled with antibodies to Caspr, gliomedin (Gldn), and MBP. Scale bars = 10 μm.
Contactin and NF-155 Cocluster With Caspr Upon OL Contact
The localization of Caspr at the PNJ depends on the presence of contactin and NF155 (Boyle et al., 2001; Pillai et al., 2009), both of which are expressed by OLs (Koch et al., 1997; Tait et al., 2000). To examine whether these proteins are also localized with Caspr at premyelinating OL–axon contact sites, we immunolabeled OL/DRG neuron cocultures with an antibody to NF155 or with a human Fc fusion protein containing the carbonic anhydrase of RPTPβ, which specifically binds contactin (Peles et al., 1995). In nonmyelinating cultures at 16 DIV, Caspr immunoreactivity was always present with contactin (Fig. 5A–C). However, contactin was also present on the OL cell bodies and processes that were devoid of Caspr. It was not possible to determine whether contactin, which colocalized with Caspr, was expressed by neurons, OLs, or both. Given that Caspr and contactin form an axonal adhesion complex (Peles et al., 1997; Rios et al., 2000) and that the axonal targeting of Caspr requires contactin (Faivre-Sarrailh et al., 2000), it is likely that contactin of neuronal origin is present along with Caspr at early OL–axon contact sites. Immunolabeling with antibodies to Caspr and NF155 revealed that Caspr was invariably associated with NF155-positive OL processes (Fig. 5D–F). Caspr was not present with NF155 in the OL cell bodies, further suggesting that these two proteins colocalized only at OL–axon contact sites. These results show that both contactin and NF155 localize with Caspr upon OL contact and suggest that they may both be responsible for axonal accumulation of Caspr at these sites.
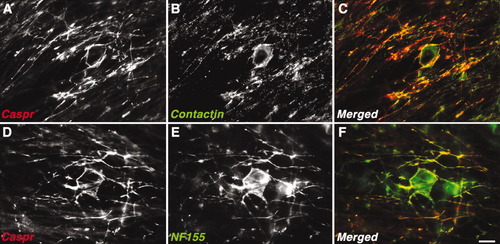
Distribution of NF155 and contactin in OLs/DRG cultures. Contactin and NF-155 colocalize with Caspr in OLs/DRG neurons cocultures. Cells were immunolabeled with antibodies to Caspr and contactin (A–C) or Caspr and NF155 (D–F). Single channels and the merged images are shown. Both contactin and NF155 accumulate with Caspr at OLs–axon contact sites. Scale bar = 10 μm.
Premyelination Clustering of Caspr Is Mediated by NF155
To examine directly whether NF155 or contactin is necessary for the induction of Caspr clustering by OL contact, we cocultured OL precursor cells isolated from neurofascin (Sherman et al., 2005) or contactin (Berglund and Ranscht, 1994) null mice with wild-type DRG neurons. As depicted in Figure 6, Caspr clustered on axons that were in direct contact with differentiating OLs lacking contactin in a manner that was indistinguishable from wild-type OLs (Fig. 6A–C). After myelination, Caspr was excluded from the internodes and was present at the PNJs (Fig. 6A–C, insets), demonstrating that contactin of glial origin is mediating neither the clustering of Caspr by premyelinating OLs nor the localization of Caspr at the PNJ. In contrast, Caspr immunoreactivity was barely detected along axons that were contacted by OLs isolated from nfasc–/– mice (Fig. 6D–H). In agreement with a recent report showing that the formation of PNJs depends on the expression of glial NF155 (Pillai et al., 2009), in these cultures Caspr never accumulated at the paranodes (data not shown). These results demonstrate that NF155 mediates the axolemmal clustering of Caspr when axons are being contacted by OLs prior to myelination.
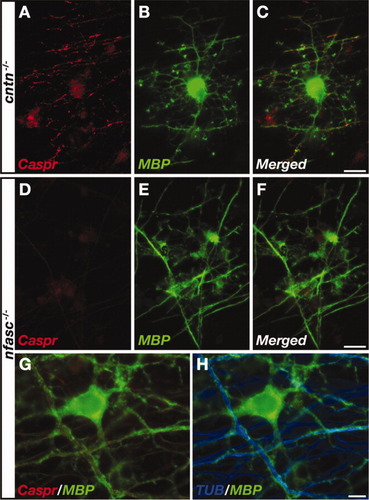
NF-155 is required for myelin-independent clustering of Caspr. OLs isolated from contactin (cntn–/–; A–C) or neurofascin (nfasc–/–; D–H) null mice cocultured with wild-type DRG neurons. A–F: 7 DIV cultures immunolabeled with antibodies to Caspr and MBP. In mature myelinated cultures, Caspr is present at the PNJ. G,H: Immunolabeling of Caspr, MBP, and neuronal tubulin (TUB) showing an OL making contact with multiple axons (H). Caspr does not accumulate at axon–glia contact sites in the absence of NF155. Scale bars = 15 μm.
DISCUSSION
Previous studies have shown that formation of the PNJ requires an adhesion complex composed of glial NF155 and axonal Caspr and contactin (Bhat et al., 2001; Boyle et al., 2001; Sherman et al., 2005; Zonta et al., 2008; Pillai et al., 2009). Here we report that, although all of these adhesion molecules are common to both CNS and PNS PNJs, their spatiotemporal expression markedly differs when axons are myelinated by SC vs. OLs. In agreement with previous studies (Einheber et al., 1997; Schafer et al., 2006), during myelination of DRG neurons by SC, Caspr is initially diffusely distributed along the axon and only later accumulates on the axolemma, first being detected as a spiral coil that is further consolidated at the PNJs. This spiral coil represents contact sites between the paranodal loops and the axons, thus corresponding to each turn of the myelin sheath (Pedraza et al., 2001). In adult peripheral nerves, Caspr is also located along the internodal region in a strand apposing the inner mesaxon of the myelin sheath and in circumferential rings just below the inner aspect of the Schmidt-Lanterman incisures (Arroyo et al., 1999). In contrast, during myelination of axons by OLs, membrane localization of Caspr is more complex and could be divided into several stages: 1) in the absence of OLs, weak and uniform expression of Caspr along axons, 2) clustering of Caspr at initial contact sites between OL processes and the axon, 3) increased membrane expression of Caspr in axonal segments ensheathed by OL, and 4) exclusion of Caspr from the internodes and its accumulation at the paranodes as myelination proceeds and more lamella are wrapped around the axon. In adult nerves, Caspr is confined to the PNJ and is completely excluded from the internodes (Arroyo et al., 2001). Using several in vitro myelination systems, we detected premyelination clustering of Caspr at contact sites between OLs and various neurons, including RGC, MN, DRG, as well as in less well characterized neurons present in mixed spinal cord and forebrain cultures. Hence, we deduce that membrane accumulation of Caspr along the axolemma is dictated by the type of the contacting glial cells and is not an intrinsic characteristic of the axon.
The distinct membrane accumulation of Caspr most likely reflects differences between PNS and CNS myelination. Before myelination in the PNS, SC are aligned along the axon in a one-to-one relationship, making contact with the axon well before ensheathment begins (Jessen and Mirsky, 2005). The CNS environment is much more complex and thus requires OL processes to navigate between different axons, contact the correct axons, and extend along them before they ensheath internodes, as well as retracting the nonsucceeding processes. Thus, axons receptive to myelination are likely to express cell surface molecules that signal the OL process to contact and ensheath the axon, whereas OL processes that do not encounter these molecules ultimately retract (Butt et al., 1997). Our results raise the possibility that the Caspr/contactin complex and NF155 might contribute to one of the axon–glia recognition signals required for proper myelination. Notably, although compact myelin is produced in the absence of Caspr and contactin (Bhat et al., 2001; Boyle et al., 2001), it is yet to be determined whether other parameters, such as internodal length, remain normal in these mutants. This notion is reinforced by recent observations demonstrating that, in contrast to PNS myelination, which is normal in mice lacking all neurofascin isoforms (Sherman et al., 2005), the extent of myelination in the spinal cord at P6 is impaired in mice lacking NF155 (Zonta et al., 2008). This was attributed to a delay in the longitudinal extension of the myelin internodes, indicating that the PNJ adhesion complex functions in promoting the extension and convergence of oligodendrocyte processes during early CNS myelination (Zonta et al., 2008).
The recruitment of Caspr to OL–axon contact sites may occur by lateral diffusion of existing proteins on the membrane, as well as by specific axonal transport mechanisms, which are regulated by OLs (Edgar et al., 2004; Nave and Trapp, 2008). However, in both cases, it is likely that membrane accumulation of Caspr is mediated by direct interaction with molecules located on the OL process. The presence of such ligands in OLs, but not in SC, may account for the difference in the ability of these two cell types to induce contact-dependent clustering of Caspr. Two candidates are NF155 and contactin, both of which are expressed by OLs (Koch et al., 1997) and are required for the formation of the PNJ (Bhat et al., 2001; Boyle et al., 2001; Sherman et al., 2005; Zonta et al., 2008; Pillai et al., 2009). Contactin is not expressed by SC (Rios et al., 2000), and, although NF155 is expressed by both SC and OLs, its expression in OLs already exists at the premyelinating stage (Collinson et al., 1998), whereas in SC it can be detected only during myelination (Basak et al., 2007). In the absence of glial NF155, Caspr is distributed along the axon, further suggesting that NF155 regulates the localization of Caspr in myelinated axons (Sherman et al., 2005; Zonta et al., 2008; Pillai et al., 2009). In agreement, both Caspr and NF155 are mislocalized in myelin mutant mice (Dupree et al., 1999; Rasband et al., 1999b; Poliak et al., 2001; Arroyo et al., 2002, 2004; Ishibashi et al., 2002; Jenkins and Bennett, 2002) as well as in human demyelinating diseases (Wolswijk and Balesar, 2003; Coman et al., 2006; Howell et al., 2006). As previously shown (Zonta et al., 2008), genetic ablation of nfasc does not change the level of expression of Caspr, making it likely that the effect we see is indeed a result of reduced cell surface clustering of Caspr in nfasc–/– axons. At present, we cannot exclude the possibility that, in addition, NF155 affects the degradation and turnover of Caspr along axons. NF155 is considered as a glial ligand for the axonal Caspr–contactin complex (Charles et al., 2002), although some evidence suggests that this interaction may require additional proteins (Gollan et al., 2003). By coculturing OL precursors cells isolated from nfasc–/– or cntn–/– mice with wild-type DRG neurons, we show that premyelinating clustering of Caspr, as well as its accumulation at the PNJ, requires glial expression of NF155 but not of contactin. Notably, premyelination axonal clustering of Caspr was not detected when DRG neurons were cocultured with SC, which ectopically expressed NF155 (data not shown), indicating that, although required, it is not sufficient to induce clustering of Caspr. This result is in line with earlier observations demonstrating that, although Caspr and contactin are present on DRG axons (Rios et al., 2000), they do not bind to the extracellular domain of NF155 (Gollan et al., 2003). It further suggests that OLs contain yet another, unidentified ligand for the Caspr–contactin complex, which is absent in SCs.




