Tissue kallikrein alleviates glutamate-induced neurotoxicity by activating ERK1
Abstract
Glutamate-induced neurotoxicity consequent to N-methyl-D-aspartic acid (NMDA) and 2-amino-3-(3-hydroxy-5-methyl-isoxazol-4-yl) propionic acid (AMPA) receptor activation underlies the pathogenesis of a wide range of central nervous system disorders, including brain ischemia. Prevention of ischemia/reperfusion (I/R)-induced neuronal injury has long been regarded as an effective therapeutic strategy for ischemia. Human tissue kallikrein (TK) gene transfer has been shown to protect neurons against cerebral I/R-induced apoptosis and oxidative stress, via activation of the brandykinin B2 receptor (B2R). However, little is known about the role of TK on glutamate-induced neurotoxicity. Here we report that pretreatment of cultured cortical neurons with TK largely prevented glutamate-induced morphological changes and cell death. We found that TK pretreatment alleviated glutamate-induced oxidative stress by inhibiting neuronal nitric oxide synthase (nNOS) activity, thereby reducing the generation of nitric oxide (NO) and reactive oxygen species (ROS). Blockage of NMDA and AMPA receptors by their specific antagonists MK801 and CNQX had effects similar to those of TK administration. Furthermore, we found that the extracellular signal-regulated kinase 1/2 cascade (ERK1/2), particularly ERK1, and nuclear factor-κB (NF-κB) were involved in TK neuroprotection against glutamate-induced neurotoxicity. TK pretreatment activated ERK1 and NF-κB, leading to enhanced expression of brain-derived neurotrophic factor (BDNF) mRNA and antiapoptotic gene Bcl-2 protein. Collectively, these findings demonstrate that TK attenuates glutamate-induced apoptosis through an intracellular signaling pathway including activation of B2R, ERK1/2, and NF-κB and up-regulation of BDNF and Bcl-2 expression. Thus, TK represents a promising therapeutic strategy for ischemic stroke. © 2009 Wiley-Liss, Inc.
Glutamate is a major excitatory neurotransmitter in the central nervous system. However, the presence of excessive glutamate has been shown to induce neurotoxicity (Choi,1988; Choi and Rothman,1990). Glutamate-induced neurotoxicity has been shown to play a critical role in the neuronal damage and death underlying a wide range of central nervous system disorders, including ischemic stroke and some neurodegenerative disorders, such as Alzheimer's disease. Activation of ionotropic glutamate receptors by high levels of glutamate can trigger dysregulated homeostasis of calcium and other cellular ions (Bano and Nicotera,2007). Among the ionotropic glutamate-gated receptors, overstimulation of N-methyl-D-aspartic acid (NMDA) and α-amino-3-hydroxy-5-methyl-isoxsazole-4-propionic acid (AMPA) receptors is predominantly responsible for triggering glutamate excitotoxic death, because of their high permeability for calcium. Calcium overload has been shown to participate in glutamate-induced neurotoxicity by activating calcium-regulated enzymes such as neuronal nitric oxide synthase (nNOS), ATPases, proteases, and lipases (Aarts et al.,2002; Chinopoulos and Adam-Vizi,2006). Despite abundant evidence from animal studies implicating NMDA receptor (NMDAR) activity in neuronal loss following ischemia, a growing body of evidence suggests that physiological synaptic NMDAR activity is essential for neuronal survival. For example, clinical trials that evaluated NMDAR antagonists in the treatment of stroke did not achieve the expected effect because of poor tolerance and efficacy, which was likely caused by the inhibition of physiological, NMDAR-mediated prosurvival signaling (Ikonomidou and Turski,2002; Muir,2006). These results suggest that NMDAR signaling has the capability of promoting both cell survival and cell death under different conditions. Therapeutic agents able to block selectively the prodeath pathway triggered by glutamate, while preserving prosurvival signals, may represent a more promising strategy (Papadia et al.,2007).
Tissue kallikrein (TK), an important component of the kallikrein/kinin system (KKS), is a serine proteinase capable of cleaving low-molecular-weight kininogen to release vasoactive kinins, which in turn trigger a series of biological effects, primarily by activating bradykinin B1 and B2 receptors (B1R and B2R) (Emanueli et al.,2003; Chao et al.,2004). All components of the KKS are widely distributed throughout many mammalian tissues and are up-regulated by ischemic stroke (Walker et al.,1995; Wagner et al.,2002). Several studies have demonstrated that systemic or local delivery of the TK gene protects against mouse cerebral ischemia/reperfusion (I/R) injury by inhibiting oxidative stress and apoptosis through stimulation of B2R (Xia et al.,2004,2006). Moreover, bradykinin (BK), a specific B2R agonist, was reported to protect against both hypoxia/reoxygenation-induced injury in cultured rat cortical neurons (Tang et al.,2009) and glutamate-induced neurotoxicity in cultured rat retinal neurons (Yasuyoshi et al.,2000). Based on these observations, we hypothesized that antagonizing glutamate-mediated neurotoxicity may be involved in TK neuroprotection, because large amounts of glutamate accumulate in the extracellular space after the onset of ischemic stroke owing to loss of cerebral blood flow and subsequent energy deficits and neuronal depolarization. In the present study, we tested this hypothesis by investigating the potential role of TK in glutamate-induced neurotoxicity, using primary cultures of cortical neurons.
To investigate the neuroprotective mechanism of TK, we evaluated the effects of TK on glutamate-induced production of reactive oxygen species (ROS) and nitric oxide (NO), activity of ERK cascade and nuclear factor-κB (NF-κB), and expression of BDNF and Bcl-2. TK affected glutamate-induced neurotoxicity via multiple distinct biochemical pathways.
MATERIALS AND METHODS
Materials
Neurobasal A medium, B27 supplement, fetal bovine serum, horse serum, Mg2+-free Earle's balanced salt solution (EBSS), and L-glutamine were obtained from Invitrogen (Grand Island, NY). Antiactin primary antibody and Cy3-conjugated anti-mouse secondary antibody were purchased from Sigma (St. Louis, MO). Mouse anti-rat MAP2 monoclonal antibodies were purchased from Chemicon (Temecula, CA). Rabbit antiphospho-ERK1/2, mouse monoclonal anti-ERK1/2, antihospho-IKKα/β, anti-p65, and anti-Bcl-2 antibodies were from Cell Signaling Technology (Beverly, MA). Horseradish peroxidase (HRP)-conjugated with anti-mouse and rabbit secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The Cell Counting Kit-8 was obtained from Dojindo Molecular Technologies (Gaithersburg, MD). CytoTox96 Non-Radioactive Cytotoxicity Assay was purchased from Promega Corp. (Madison, WI). FragELTM DNA Fragmentation Detection Kit was from Calbiochem (La Jolla, CA). The enhanced chemifluorescence reagent and the BCA Protein Assay Kit were obtained from Pierce Manufacturing Inc. (Appleton, WI). The real-time PCR Master Mix Kit and the ReverTra Ace Kit were purchased from Toyobo (Osaka, Japan). The fluorescent probes DCFH-DA and DAF-AM, as well as all other reagents, were purchased from Sigma.
Primary Culture of Cortical Neurons
Newborn Sprague-Dawley rats, less than 24 hr old, were purchased from the Shanghai Institute of the Chinese Academy of Science. All experimental procedures were carried out in accordance with Fudan University experimental standards as well as the NIH Guide for the care and use of laboratory animals. Primary cortical neuron cultures were prepared as described previously (Brewer et al.,1993). Briefly, cerebral cortices were excised from neonatal Sprague-Dawley rats, stripped of meninges and blood vessels, and minced. The tissue was dissociated by digestion in 0.125% trypsin for 10 min at 37°C with gentle trituration; plated at a density of 3–10 × 105 cell/ml on poly-L-lysine-coated glass coverslips, 96-well plates, or 100-mm dishes; and maintained at 37°C in a humidified 5% CO2 incubator. Neurons were cultured in Dulbecco's modified Eagle's medium with L-glutamine plus 10% fetal bovine serum and 10% horse serum, which was replaced with Neurobasal A medium supplemented with 2% B27 and 0.5 mM L-glutamine every 3–4 days. Cultures were treated with 5-fluoro-2-deoxyuridine and uridine on the third day after plating to suppress glial growth. Under these conditions, neuronal purity exceeded 90%, as estimated by immunocytochemical staining with antibodies against neurofilament proteins and glial fibrillary acidic protein (data not shown). The cultured neurons were used for studies between in vitro days 8 and 10 (DIV 8–10).
Drug Treatment
Neurotoxicity was induced by exposure to 100 μM glutamate and 10 μM glycine for different times (1 hr, 2 hr, 4 hr, 6 hr, and 24 hr), in a modified Mg2+-free Earle's balanced salt solution (EBSS). For restoration or reperfusion, cultures at the end of glutamate exposure were rinsed twice with EBSS, changed to the original feeding medium, and placed at 37°C in a 5% CO2 cell culture incubator for the desired time. For drug treatments, various concentrations of TK (10–1,000 nM) or BK (1 μM) were added to the culture medium 12 or 24 hr before glutamate exposure, whereas the NMDA receptor antagonist (5S,10R)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]-cyclohepten-5,10-imine maleate (MK801) and the AMPA receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) were applied 30 min before exposure to glutamate. All drug treatments were continued through the duration of glutamate exposure. To study the effect of B2R inhibition on the TK effect, the B2R antagonist HOE140 (500 nM) was added 1 hr prior to TK treatment. Addition of PD98059 (10 μM) 30 min before glutamate exposure was used to confirm the involvement of the ERK1/2 cascade in glutamate neurotoxicity. Control neurons received no drug treatment.
Cell Viability Assay
Cell viability was assessed by WST-8 assay using the Cell Counting Kit-8, in which colored formazan is formed in viable cells in response to cellular dehydrogenase activity. Briefly, cortical cells (5 × 104 cells/100 ml/well) were cultured on 96-well plates until DIV 10, at which point they were exposed to glutamate for 1 hr. After 24 hr of reperfusion, 10 μl of the Cell Counting Kit-8 solution was added to each well, and the cultures were incubated for another 1–2 hr at 37°C. The results are expressed as the percentage of viable cells relative to untreated controls, with absorbance at 450 nm read with a reference wavelength of 630 nm on a microplate reader (SpectraMax M5; Molecular Devices, Sunnyvale, CA). Each experiment, comprising eight readings for each experimental condition, was performed at least three times.
Cell Injury Assay: Lactate Dehydrogenase Measurement
Cellular injury was determined by measuring the amount of lactate dehydrogenase (LDH) released into the medium. In brief, cortical cells grown on 96-well plates to DIV 8 were randomly divided into various treatment groups. After drug treatment, the medium was removed, and cells were washed three times with EBSS, then exposed to glutamate for 1 hr. After 24 hr of reperfusion, the amounts of LDH released into the medium and total cellular LDH were determined by using the CytoTox 96 Non-Radioactive Cytotoxicity Assay. Medium (50 μl) was transferred from the culture wells to 96-well plates and mixed with 50 μl of the reaction solution provided with the kit. The mixtures were incubated at room temperature in the dark for 30 min, and then 50 μl of the stop solution provided by the supplier was added to each well. Thirty minutes later, the absorbance was read at 492 nm with a microplate reader. At the end of each experiment, cells were repeatedly frozen and thawed in order to determine maximal LDH release for each well. Each experimental condition was repeated in triplicate, with each experiment containing eight readings. Results are expressed as a percentage of the maximal LDH release, after the subtraction of background levels determined from analysis of the medium alone.
Immunocytochemistry
Immunohistochemistry was performed to detect the p65 unit of NF-κB, p-ERK1/2, or MAP2 immediately following glutamate exposure or at 1 hr or 2 hr reperfusion, respectively. After various treatments, cells grown on glass coverslips were fixed for 10 min in 0.1 M PBS containing 4% paraformaldehyde. After washing in PBS, cells were permeabilized for 15 min in PBS-T (0.3% Triton X-100 in PBS), blocked in PBS-T containing 3% fetal bovine serum (FBS), and then incubated overnight at 4°C with mouse anti-MAP2 polyclonal antibody (1:400), anti-p-ERK1/2 monoclonal antibody (1:1,000), or anti-p65 (1:25), diluted in PBS-T containing 3% FBS. After three washes in PBS, cells were incubated for 1 hr with Cy3-conjugated secondary antibodies at room temperature in the dark. Cell nuclei were stained using by incubation with the membrane-permeable dye Hoechst 33342 (1:100) for 10 min, followed by three washes in PBS. Fluorescent labeling was then visualized under an inverted fluorescence microscope (Olympus, Tokyo, Japan).
TUNEL Staining
Neuronal apoptosis was detected by the TUNEL [terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling] method at 24 hr after 1 hr of glutamate exposure. In this assay, TdT binds to exposed 3′-OH ends of the DNA fragments generated in response to apoptotic signals, where it catalyzes the addition of biotin-labeled and unlabeled deoxynucleotides. Biotinylated nucleotides are detected with a streptavidin-horseradish peroxidase (HRP) conjugate. The labeled samples were treated with diaminobenzidine (DAB) to generate an insoluble colored substrate at the site of DNA fragmentation. Counterstaining with methyl green aided in the morphological evaluation and characterization of normal and apoptotic cells. Briefly, after they had been treated as described above, cortical cell cultures (DIV 8) plated on glass coverslips were fixed in 4% paraformaldehyde in PBS and then subjected to TUNEL staining using the FragELTM DNA Fragmentation Detection Kit, according to the manufacturer's instructions. The cells were examined under a light microscope (Olympus). The number of TUNEL-positive (apoptotic) cells, which appeared dark brown, and the total number of cells, appearing as a mixture of dark brown and blue-green, were determined in eight randomly chosen microscopic fields, each at ×200 magnification. Data were expressed as the ratio of apoptotic neurons to total neurons.
Measurement of ROS
Intracellular ROS were detected by using the fluorescent probe 2,7-dichlorofluorescin diacetate (DCFH-DA; Possel et al.,1997). DCFH-DA is converted by intracellular esterases,which is oxidized into the highly fluorescent dichlorofluorescein (DCF) in the presence of a proper oxidant. After various drug pretreatments, cortical cell cultures grown on 96-well plates or glass-bottom dishes were loaded with DCFH-DA (5 μM) at 37°C in the dark for 10 min, followed by three washes with PBS (pH 7.8), and then exposed to 100 μM glutamate and 10 μM glycine. Fluorescence photomicrographs of cortical neurons on glass-bottom dishes were obtained with an Olympus fluorescence microscope after 2, 4, and 6 hr of glutamate exposure. The DCF fluorescence intensity of cells on 96-well plates was quantified by using a fluorescence microplate reader (SpectraMax M5) at excitation and emission wavelengths of 485 and 538 nm, respectively. Intracellular ROS production was expressed as -fold relative to normal control (untreated) cells after background fluorescence obtained from the medium alone was subtracted. Eight readings were obtained from each of three separate experiments.
Measurement of NO Production and nNOS Activity
NO production in neurons was monitored by the NO-sensitive dye 4-amino-5-methylamino-2,7-difluorofluorescein (DAF-FM-DA; Kojima et al.,1999). DAF-FM-DA is converted by intracellular esterases to DAF-FM, which is transformed to its corresponding highly fluorescent triazoles in the presence of NO. After various drug treatments, neurons were loaded with 5 μM DAF-FM-DA at 37°C in the dark for 30 min, then washed twice with PBS. After 2 hr of glutamate exposure, the cellular DAF-FM fluorescence intensity was obtained with a fluorescence microplate reader, with excitation at 495 nm and emission at 519 nm. NO production was expressed as -fold relative to the fluorescence intensity of untreated control cells after the background fluorescence signal of the medium alone was subtracted.
The assay for NOS activity was based on the ability of the synthase to catalyze arginine (Arg) to form NO, which was detected by using the fluorogenic probe DAF-FM, according to a protocol similar to that described above. Briefly, after 2 hr of glutamate stimulation, cells were loaded with 5 μM DAF-FM-DA in the presence or absence of 1.8 mM free calcium. The intensity of DAF-FM fluorescence was measured by a fluorescence microplate reader at excitation and emission wavelengths of 495 and 519 nm, respectively. The nNOS activity was expressed as a ratio of the differential DAF-FM fluorescence intensity in the presence vs. absence of calcium, divided by the fluorescence intensity in the presence of calcium.
Western Blot Analysis
Western blotting was performed to analyze the expression of phospho-ERK1/2 (p-ERK1/2), total ERK1/2 (t-ERK1/2), phospho-IKK (p-IKK), and Bcl-2. After various treatments, cells grown on 100-mm dishes (DIV 8) were harvested by scraping in ice-cold lysis buffer containing 1% Triton, 0.1% SDS, 0.5%deoxycholate, 1 mmol/liter EDTA, 20 mmol/liter Tris (pH 7.4), 150 mmol/liter NaCl, and 10 mmol/liter NaF, supplemented with a cocktail of phosphatase and protease inhibitors (KangChen Bio-tech, Shanghai, China), and then clarified by centrifugation at 13,000g for 20 min. The protein concentration of each supernatant was determined by using the BCA kit. A total of 30 μg protein was electrophoretically separated on 10% SDS-PAGE and transferred onto nitrocellulose membranes. The membranes were soaked in blocking buffer [1× Tris-buffered saline (TBS), pH 7.6, containing 3% BSA] for 1 hr at room temperature, then incubated overnight at 4°C with primary phospho-ERK1/2 (diluted 1:1,000), t-ERK1/2 (diluted 1:1,000), p-IKK (diluted 1:500), or Bcl-2 (diluted 1:500) antibodies. After washing three times with TBS-Tween (0.1% Tween 20), membranes were probed with corresponding secondary HRP-conjugated anti-mouse IgG antibodies (diluted 1:1,000) for 1 hr at room temperature. Equal loading was confirmed by probing the membranes with antiactin antibody (1:10,000 dilution). Detection was carried out with the enhanced chemiluminescence assay. Negative controls, in which primary antibodies were omitted, had no visible staining. Quantification of protein bands was achieved by densitometric analysis in Chemimage 5500 software.
Relative Real-Time PCR
Cortical neurons on DIV 8–10 were treated with 100 nM TK for 12 hr or the inhibitors of NMDA and AMPA receptors (10 μM MK801 and 20 μM CNQX) for 30 min before and during subsequent glutamate stimulation. At 7 hr or 12 hr of glutamate exposure, the cultures were rinsed with cold PBS, immediately followed by total mRNA purification with Trizol, and reverse transcribed into cDNA at 42°C for 1.5 hr and at 72°C for 15 min using the ReverTra Ace Kit. SYBER Green Master Mix (Toyobo) was used as a RT-PCR solution. The real-time PCR was performed with a 5-min incubation at 95°C and 40 amplification cycles (15 sec at 94°C, 15 sec at 63°C, and 45 sec at 72°C). The following primers were used: for BDNF, forward 5′-CCC ATG GGT TAC ACG AAG GA-3′ and reverse 5′-CCC GAA CAT ACG ATT GGG TAG T-3′; for Bcl-2, forward 5′-CCC CAG AAG AAA CTG AAC C-3′ and reverse 5′-GCA TCT CCT TGT CTA CGC-3′; and, for GAPDH, forward 5′-GCC TTC CGT GTT CCT ACC-3′ and reverse 5′-GCC CCT CCT GTT GTT ATG-3′. The amplification and data acquisition were run on a real-time PCR system (ABI Prism 7700; Applied Biosystems, Foster City, CA).
Data Analysis
Data are shown as mean ± SD based on three separate experiments. Statistical analyses were performed by one-way ANOVA, followed by Bonferroni post hoc tests for multiple-comparisons tests. Nonparametric data were analyzed by the Kruskal-Wallis test, followed by Mann-Whitney test. Unpaired Student's t-tests were used for comparisons of two groups only. Differences were considered significant at P < 0.05.
RESULTS
TK Protects Cortical Neurons Against Glutamate Neurotoxicity
We used two different methods to detect neuronal injury and death, both measuring the amount of LDH released into the medium (Koh et al.,1987) and carrying out an assay of cell viability. As shown in Figure 1A, cortical neurons exposed to 1 hr of glutamate (100 μM) combined with glycine (10 μM), followed by 24 hr of reperfusion, displayed a significant increase in LDH release, from 16.32% ± 1.76% by the normal control to 63.86% ± 4.51% (n = 5, P < 0.01). However, a 30-min pretreatment with NMDAR and AMPAR antagonists (10 μM MK801 and 20 μM CNQX), continued throughout the subsequent glutamate exposure, was able to suppress the increase in LDH release to 20.31% ± 2.53% (n = 5, P < 0.01). Notably, compared with glutamate exposure alone, addition of TK (1.0 nM, 100 nM, and 1.0 μM) 24 hr before and during the glutamate insult elicited a concentration-dependent suppression of LDH release to 50.45% ± 3.56%, 40.67% ± 3.13%, and 31.67% ± 2.98%, respectively (n = 5, P < 0.01; Fig. 1A). A specific B2R agonist, BK (1.0 μM ), was able to simulate the effect of 100 nM TK on LDH release induced by glutamate exposure, whereas addition of the specific B2R antagonist HOE140 before TK treatment counteracted the protective effect of TK.
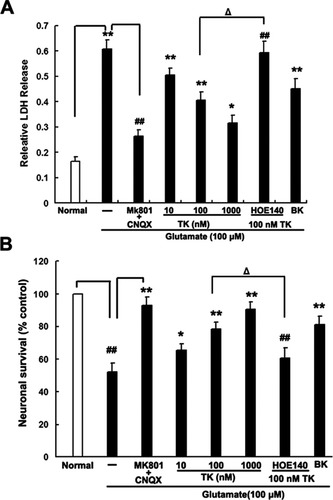
TK protects against glutamate-induced neurotoxicity in cultured cortical neurons. Cells were pretreated for 24 hr with TK (10nM, 100 nM, 1 μM), BK (1 μM), or HOE140 (500 nM) or for 30 min with MK801 (10 μM) or CNQX (20 μM). After 24 hr of reperfusion following a 1-hr glutamate (100 μM) exposure, pretreatment had a significant effect on LDH release (A) and cell viability (B; n = 8 for each group). ☆P < 0.05, ☆☆P < 0.01 vs. normal control, ##P < 0.01 vs. glutamate exposure alone, ΔP < 0.05 for HOE140 + 100 nM TK + glutamate vs. 100 nM TK + glutamate.
Consistently with the results of LDH measurement, at 24 hr of reperfusion after glutamate exposure, the cell viability assay demonstrated a decrease in cell survival, with 52.05% ± 5.43% cells surviving relative to the normal control. Neuronal survival was elevated by treatment with NMDAR and AMPAR inhibitors, to 90.15% ± 4.83% (n = 5, P < 0.01; Fig. 1B). Similarly, glutamate-induced cell death was significantly reduced, in a dose-dependent manner, by a 24-hr pretreatment with TK (1.0 nM to 1.0 μM), which significantly increased the neuronal survival rate to 65.3% ± 3.93%, 77.27% ± 4.61%, and 85.67% ± 5.33%, respectively. The effect of 100 nM TK treatment was abolished by HOE140 but simulated by BK. These findings demonstrate that TK can alleviate glutamate-induced neurotoxicity in cultured cortical neurons by inhibition of LDH release and promotion of cell viability, primarily via B2R.
To characterize the changes in neuron morphological induced by glutamate and to determine whether these were also subject to the apparent protective effects of TK, immunofluorescent labeling was performed with antibodies against the cytoskeletal protein MAP2, a neuron-specific somatodendritic marker. As shown in Figure 2, after 2 hr of reperfusion following glutamate exposure, cortical neurons showed obvious morphological changes compared with untreated cells. These included prominent somatic swelling, dendritic beading and disappearance, and a loss of cellular MAP2 immunoreactivity. Pretreatment with 100 nM TK or 10 μM MK801 and 20 μM CNQX had a dramatic effect in preventing these changes. These results further indicated that TK can exert protective effects against glutamate-induced neurotoxicity in cortical neurons.
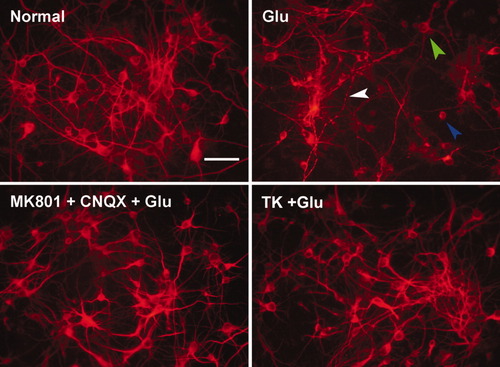
Effect of TK and blockers of NMDAR and AMPAR on morphological changes induced by glutamate in cultured cortical neurons. Neuronal cells were immunostained for the cytoskeletal protein MAP2 (in red). At 2 hr of reperfusion following glutamate insult, somatic swelling (green arrow) and scattered dendritic breakdown (blue arrow) or dendritic beading (white arrow) were observed. Pretreatment with TK (100 nM) for 24 hr or with MK801 and CNQX for 30 min extended throughout the glutamate exposure interval, caused cortical neurons to show only subtle morphological changes, with mild body swelling and areas of dendritic breakdown. Glu, glutamate (100 μM). Scale bar = 20 μm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
TK Attenuates Glutamate-Induced Apoptosis in Cultured Cortical Neurons
Neuronal cells exposed to glutamate insult have been observed to undergo apoptotic-like death (Bonfoco et al., 1995; Ikeda et al.,1996). Here, we investigated the effect of TK on glutamate-induced apoptosis in cortical neuronal cells by staining DNA with the membrane-permeabledye Hoechst 33342, as well as by assessing DNA strand breakage using TUNEL staining. At 24 hr following glutamate exposure, Hoechst staining of neurons demonstrated clear signs of apoptotic morphology, characterized by nuclear pyknosis (Fig. 3A). After exposure to glutamate (100 μM) and glycine (10 μM), 80.25% ± 7.76% of cells displayed nuclear condensation, compared with 18.32% ± 3.45% of normal control cells (Fig. 3B). Blockade of NMDAR and AMPAR prevented neurons from glutamate-induced nuclear morphological changes, with only 25.02% ± 5.71% of cells exhibiting nuclear condensation. Pretreatment with TK (100 nM) or BK (1 μM) markedly reduced the rate of glutamate-induced nuclear condensation, to 33.40% ± 4.69% and 35.54% ± 5.65%, respectively (Fig. 3B).
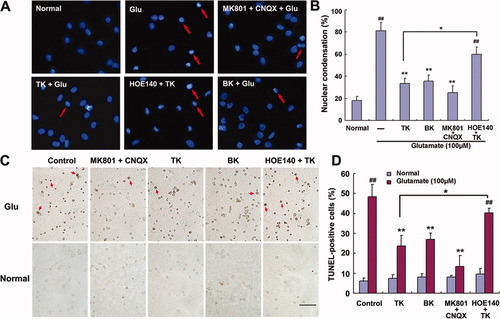
Hoechst 33342 staining and TUNEL assay in cultured rat cortical neurons at 24 hr of reperfusion following glutamate stimulation. TK, BK, or MK801 and CNQX pretreatment largely prevented nuclear pyknosis induced by glutamate exposure. Addition of HOE140 1 hr before TK treatment abolished the effect of TK. A: Nuclear morphology was determined by staining with Hoechst 33342. Most nuclei exposed to glutamate alone showed pyknotic changes (red arrow) and irregular shapes. B: Percentage of nuclear condensation in total cultured cortical neurons. C: Morphological apoptosis was determined by TUNEL assay. TUNEL-negative (nonapoptotic) cells appear blue-green, whereas TUNEL-positive (apoptotic) cells appear dark brown (red arrow). D: Percentage of TUNEL-positive cells in total cultured cortical neurons. ##P < 0.01 vs. normal control. ☆☆P < 0.01 vs. glutamate stimulation alone. ☆P < 0.05 HOE140 + TK + glutamate vs. TK + glutamate. Glu, glutamate (100 μM). Scale bar = 100 μm in C; 20 μm for A. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
To confirm these results, we also conducted TUNEL staining, which indicated that the apoptotic DNA fraction (TUNEL-positive cells) was significantly increased after the exposure of neurons to glutamate and that this was dramatically prevented by pretreatment with combination of 10 μM MK801 and 20 μM CNQX, 100 nM TK, or 1 μM BK (Fig. 3C). Quantitation of TUNEL staining demonstrated that exposure to glutamate alone yielded 48.45% ± 6.09% TUNEL-positive cells, which was significantly higher than the 6.21% ± 1.56% of TUNEL-positive cells for the normal untreated control. Moreover, pretreatment of cells with MK801 combined with CNQX, TK, or BK significantly decreased the ratio of TUNEL-positive cells to total cells to 13.46% ± 2.37%, 23.67% ± 3.21%, and 26.96% ± 5.43%, respectively (Fig. 3D). However, blockade of B2R with HOE140 prior to TK treatment abolished the effect of TK on glutamate-induced apoptosis. Each agent had very little effect on the basal apoptotic death of normal cultured neurons.
TK Inhibits ROS Production Induced by Glutamate Exposure
ROS play important roles in neuronal injury in many pathological conditions. Oxidative injury has been proved to be one of the major death pathways induced by glutamate insult, and attenuation of oxidative stress has been shown to have a neuroprotective effect against glutamate-induced neurotoxicity (Nagy et al.,2004; Ha et al.,2006). Human TK gene transfer has been reported to protect rats against cerebral ischemic injury by suppression of oxidative stress (Xia et al.,2004). To determine whether, and the extent to which, TK affects ROS generation in cultured cortical neurons exposed to glutamate, we examined changes in ROS levels in neurons subjected to glutamate alone or combined with TK pretreatment. When observed under the fluorescence microscope, neurons exposed to glutamate alone exhibited a marked, time-dependent increase in DCF fluorescence intensity, whereas neurons pretreated with TK before glutamate exposure showed dramatically less fluorescence intensity at the same time points (Fig. 4A). A quantitative analysis of the images indicated that, compared with the normal control, the fluorescence intensity was 14.97-, 26.75-, 46.09-, and 57.76-fold higher after 1, 2, 5, and 6 hr of glutamate exposure, respectively. Pretreatment with 10 μM MK801 and 20 μM CNQX was effective in attenuating the increase in fluorescence intensity at all time points (Fig. 4B). Notably, pretreatment of cells with TK significantly attenuated the increase in DCF fluorescence intensity induced by glutamate exposure, to 9.47-, 16.47-, 28.44-, and 33.75-fold relative to the normal control at the same time points, respectively (Fig. 4B). No fluorescence was apparent when neurons in normal culture conditions were stimulated with TK. These results indicate that TK treatment can inhibit glutamate-induced oxidative stress, which suggests that TK has antioxidant properties, as previously observed in vivo (Xia et al.,2004).
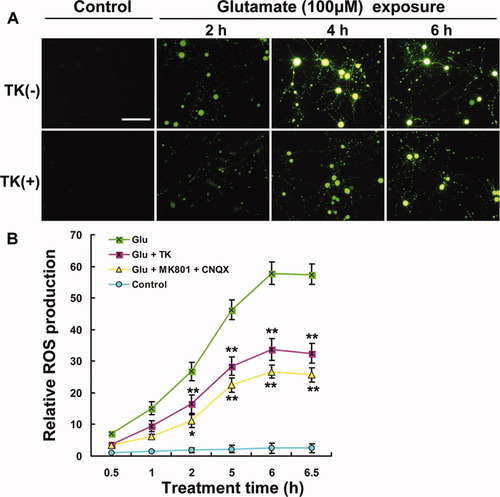
Detection of intracellular ROS production in cultured rat cortical neurons. Pretreatment with TK (100 nM), MK801 (10 μM), or CNQX (20 μM) suppressed the ROS production induced by glutamate exposure. A: Micrographs from representative experiments showing the time course of ROS production induced by glutamate exposure (for 2, 4, and 6 hr) and suppression, by TK, of glutamate-induced ROS at the same time points. B: Quantitative assay of DCF fluorescent intensity. ☆P < 0.05, ☆☆P < 0.01 vs. glutamate exposure alone at the corresponding time point. Glu, glutamate (100 μM). Scale bar = 100 μm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Effects of TK on NO Production and nNOS Activity in Primary Cortical Neurons
NO, a gas, readily diffuses from its site of synthesis and regulates adjacent cells to participate in many pathological processes, including mitochondrial dysfunction. Studies (Black et al.,1995; Ferriero et al.,1995) reported that NO derived from nNOS plays a detrimental role in many pathogenic processes arising from glutamate neurotoxicity and that selective inhibition of nNOS with 7-nitroindazole (7-NI) has a neuroprotective effect against glutamate neurotoxicity. To determine whether the protective effects of TK against glutamate-induced neuronal injury involved a mechanism driven by nNOS-NO, we used the NO-sensitive probe DAF-FM-DA to measure NO production relative nNOS activity. Compared with untreated normal cultures, 2 hr of glutamate stimulation induced a 6.66- and 14.77-fold increase in relative nNOS activity and NO production, respectively (Fig. 5). Blockade of NMDA and AMPA receptor prior to glutamate insult suppressed both the increased nNOS activity and the NO production to approximately the level of normal control. Importantly, TK pretreatment markedly reduced the elevated nNOS activity and NO production to 0.81- and 2.71-fold relative to the control, respectively. Pretreatment with BK produced similar effects (1.36- and 6.56-fold compared with the control). However, prior to TK administration, addition of the B2R inhibitor HOE140 to the culture medium for 1 hr reversed the inhibition of TK on the nNOS activity and NO production. These data suggest that TK, via activation of B2R, may attenuate cultured neurons against glutamate-induced nitrosative stress by inhibiting nNOS activity and NO production.
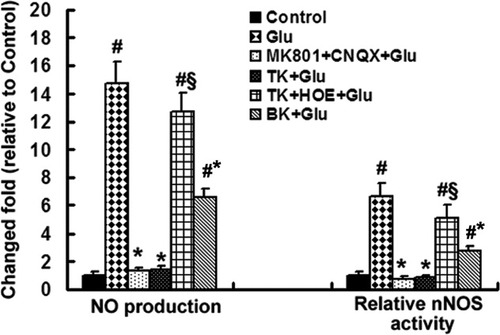
Measurement of nNOS activity and NO production in cultured cortical neurons. After 2 hr of glutamate exposure, cells pretreated with TK or BK or blockers of NMDAR or AMPAR showed an attenuation of the normal glutamate-induced increase in nNOS activity and NO production (n = 8, #P < 0.01 for normal control, §P < 0.01 compared with TK + Glu. Relative nNOS activity is expressed as -fold relative to untreated normal control, and NO production is shown as -fold relative to DAF-FM fluorescence intensity of the untreated control. Glu, glutamate (100 μM).
TK Activates the ERK1/2 Signaling Pathway
To explore the possible mechanism responsible for neurprotective effects of TK, we investigated the ability of TK to stimulate intracellular antiapoptotic signaling pathways. Previous studies have shown that several signaling pathways, including PI3K/Akt and the p44/42 mitogen-activated protein kinases (p44/42MAPKs, ERK1/2) signaling pathways, are involved in NMDA neuroprotection against glutamate excitotoxicity (Kaltschmidt et al.,1995; Sutton et al.,2002; Wang et al.,2007). The PI3K pathway is well known to promote survival in many systems. However, in our pilot study, we found that TK pretreatment did not significantly alter the level of Akt phosphorlyation in glutamate-treated cultured cortical neurons (data not shown), suggesting that the PI3K pathway is not a requirement for the neuroprotective activity of TK. This observation was similar to a previous report on the neuroprotective activity of NMDA (Zhu et al.,2005). Therefore, in the following study, our focus was to determine whether the neuroprotective effect of TK involves activation of the ERK1/2 signaling pathway.
ERK1/2 is a prominent member of the MAPKs superfamily. The ERK1/2 cascade is also a key component of pathways that regulate cell survival. Therefore, we used immunocytochemistry and Western blot to examine the expression of total ERK1/2 (t-ERK) as well as the level of activated ERK1/2 (p-ERK) in our model.
Immunocytochemical staining was performed 1 hr after glutamate exposure. We observed that cultured cortical neurons exposed to glutamate alone showed stronger red fluorescence in their cytoplasm than those under normal conditions, whereas neurons treated with TK prior to glutamate insult exhibited even stronger fluorescence than those challenged by glutamate alone (Fig. 6).
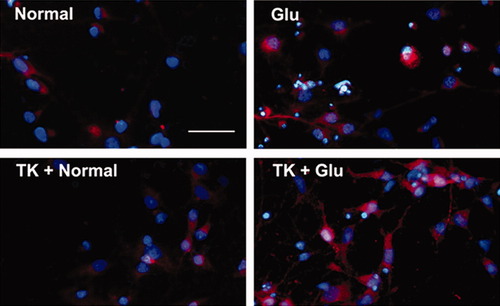
Immunocytochemistry shows expression of p-ERK in cultured cortical neurons. Cells were immunostained for p-ERK1/2 (in red). Nuclear was stained by Hoechst 33342 (in blue). Cells under normal conditions express p-ERK at a low level. Glutamate exposure for 1 hr induced a significant increase in expression of p-ERK. TK (100 nM) pretreatment further increased the expression of p-ERK induced by glutamate exposure. Glu, glutamate (100 μM). Scale bar = 20 μm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
We used Western blot to examine quantitatively the level of p-ERK1/2 and t-ERK1/2 expression. Despite the absence of any marked change in the protein level of t-ERK (Fig. 7A,B), and consistently with the previous results immunocytochemical results, a brief exposure to glutamate immediately increased the level of p44-ERK1 by 153.7-fold and the level of p42-ERK2 by 82.33-fold relative to control levels (Fig. 7A,B). This activation of ERK1/2 was almost totally blocked by antagonists of NMDA and AMPA receptors (10 μM MK801 and 20 μM CNQX) or the ERK-protein-kinase inhibitor PD98059 (10 μM). In addition to its ability to increase basal activitiy of ERK1/2 in cultured cortical neurons, compared with glutamate exposure alone, 24 hr of TK pretreatment before glutamate insult induced a much greater increase in the level of p44-ERK1, to 566-fold relative to the normal control (P < 0.01, n = 3). In contrast, BK, the selective agonist of B2R, had no significant effect on the activation of ERK1 by glutamate exposure (P > 0.05, n = 3; Fig. 7A,D). After 1 hr of reperfusion, the increased phosphorylation of ERK1/2 that was otherwise seen immediately following glutamate exposure significantly declined, to 6.79- and 20.39-fold that of the normal control. However, compared with glutamate insult, TK pretreatment for 24 hr before glutamate insult elevated the level of p44-ERK1, but not p42-ERK2, to 51.05-fold (P < 0.01, n = 3), and BK pretreatment for 24 hr induced another 12.05-fold increase in the level of p-ERK1. Furthermore, the B2R antagonist HOE140 had a dramatic effect in preventing the activation of both ERK1 and -2 induced by TK pretreatment, reducing the level of p-ERK1/2 approximately to that with glutamate exposure alone (P < 0.05, n = 3; Fig. 7B,D). Neither TK nor BK pretreatment significantly affected the level of p42-ERK2 induced by glutamate stimulation (Fig. 7A–D). These results suggest that preconditioning of neurons with TK requires activation of the ERK1/2 pathway to protect neurons against glutamate neurotoxicity.
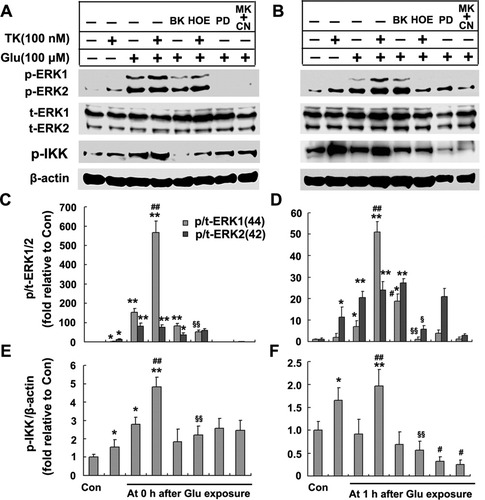
Western blotting analyses of expression of ERK1/2 and p-IKK in cultured cortical neurons. TK (100 nM) pretreatment significantly enhanced the levels of p-ERK1/2, particually p-ERK1, and p-IKK but did not significantly alter t-ERK expression levels. HOE140 (500 nM) was able to abolish the TK effect, and PD98059 (10 μM) or MK801 (10 μM) and CNQX (20 μM) largely inhibited the elevated level of p-ERK1/2 and p-IKK induced by glutamate exposure. Representative Western blots of p-ERK1/2, t-ERK1/2, and p-IKK immediately after glutamate exposure without reperfusion (A) or followed by 1 hr reperfusion (B). The relative density of phospho-ERK1/2 to that of total-ERK1/2 without reperfusion (D) or with 1 hr of reperfusion (C). The relative density of p-IKK to that of β-actin without reperfusion (E) or with 1 hr reperfusion (F). Each data point represents the mean ± SD from four independent experiments. ☆P < 0.05, ☆☆P < 0.01 for normal control. #P < 0.05, ##P < 0.01 compared with glutamate exposure alone. §P < 0.05, §§P < 0.01 for HOE140 + TK + glutamate vs. TK + glutamate. Glu, glutamate (100 μM); HOE, HOE140; PD, PD98059; MK + CN, MK801 + CNQX.
TK Increases NF-κB Activity Through Phosphorylation of IκB Kinase
NF-κB is a critical transcription factor in the suppression of apoptosis through its transactivation of antiapoptotic genes. Cruise et al. (2000) found that glutamate stimulated NF-κB through the ERK1/2-mediated pathway in striatal neurons. NF-κB activation is also induced by Aβ in primary cultured cerebellar cells (Kawamoto et al.,2008) through phosphorylated ERK1/2. Might TK-induced ERK1/2 phosphoryaltion also enhance NF-κB activity? We next analyzed NF-κB activity in TK-pretreated cultured cortical neurons. In its silent state, NF-κB exists as a cytoplasmic complex together with its inhibitory protein IκB. IκB kinase (IKK) is the major kinase responsible for phosphorylation of IκBα. The phosphorylation and degradation of IκBα is necessary for NF-κB activation and subsequent translocation into the nucleus (Beg and Baldwin,1993). In the present study, we chose to analyze NF-κB activity indirectly by Western blot detection of IKK phosphorylation and to investigate NF-κB activation at the cellular level directly through immuostaining the DNA-binding subunit p65 NF-κB (RelA).
Western blot analysis showed (Fig. 7) that 1 hr of glutamate stimulation immediately induced a 2.8-fold increase in the relative expression of p-IKK compred with the control (Fig. 7A,E). The elevated p-IKK decreased to the basal level in 1 hr after glutamate stimulation (Fig. 7B,F). TK by itself induced a modest but significant increase in basal IKK activity in cultured rat cortical neurons (Fig. 7A,B). A 24-hr TK pretreatment resulted in a significantly elevated level of IKK phosphorylation at 0 and 1 hr after glutamate exposure to 4.84-fold and 1.98-fold relative to the control, respectively (compared with glutamate exposure alone, P < 0.01, n = 3; Fig. 7E,F). Although the B2R agonist BK did not significantly alter the p-IKK levels in glutamate-exposed neurons, blockade of B2R with HOE140 prior to TK treatment completely abolished the increases in IKK phosphorylation induced by TK. The antagonists of the NMDA and AMPA receptors (10 μM MK801 and 20 μM CNQX) or the ERK inhibitor PD98059 (10 μM) partially reduced NF-κB activity at 0 hr and 1 hr after glutamate exposure. Immunofluorescence staining revealed a faint immunostaining nuclear p65 (pink) in rat cortical neurons under normal cultured conditions. After glutamate stimulation for 1 hr, p65 immunoreactivity was strongly enhanced in nuclei of cortical neurons. Neurons pretreated with TK followed by glutamate stimulation showed even stronger nuclear p65 immunostaining than those exposed to glutamate alone (Fig. 8). Taken together, these observations show that the IKK-NF-κB signaling pathway was involved in the neuroprotective activity of TK, in part, through activation of ERK1/2.
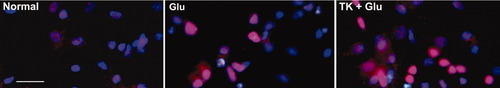
Immunocytochemistry reveals effects of TK on p65 immunoreactivity in nuclei of cultured cortical neurons. Cell was immuostained for p65 NF-κB (in red) with specific antibodies and for nuclear Hoechst 333242 (in blue) after 1 hr of glutamate exposure alone or following 24 hr of TK pretreatment. The normal cultured cells revealed a faint immunostaining nuclear p65 (in pink). Neurons stimulated by glutamate for 1 hr showed a strong immunostaining p65 in nuclei. Neurons pretreated with TK (100 nM) prior to glutamate exposure exhibited even stronger nuclear p65 immunostaining than those exposed to glutamate alone. Glu, glutamate (100 μM). Scale bar = 20 μm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
TK Promotes Expression of BDNF and Bcl-2
Previous studies have showed that glutamate injury decreases gene expression of BDNF and Bcl-2; however, neuroprotective concentrations of NMDA and BDNF exerts their survival-promoting and antiapoptotic effects against glutamate-mediated excitotoxicity via increasing Bcl-2 and BDNF gene expression (Lipsky et al.,2001; Zhu et al.,2005). To elucidate the relationship between expression of Bcl-2 and BDNF and the neuroprotection of TK, we analyzed the Bcl-2 and BDNF expression levels in glutamate-challenged cortical cultures with real-time PCR.
Neurons pretreated with TK (100 nM) or BK (1 μM) for 12 hr increased BDNF mRNA levels to 2.21- and 1.99-fold, respectively, compared with the control group (Fig. 9A,C). Glutamate (100 μM) stimulation alone for 7 hr and 12 hr reduced the level of BDNF mRNA to 0.70- and 0.44-fold that of the control, respectively. Pretreatment with TK or BK for 12 hr prior to glutamate stimulation effectively prevented the reduction in BDNF mRNA levels at 7 hr and 12 hr. Pretreatment with ionotropic glutamate receptor inhibitors (10 μM MK801 and 20 μM CNQX) for 30 min also attenuated the decreases in BDNF mRNA levels induced by glutamate (Fig. 9A,C).
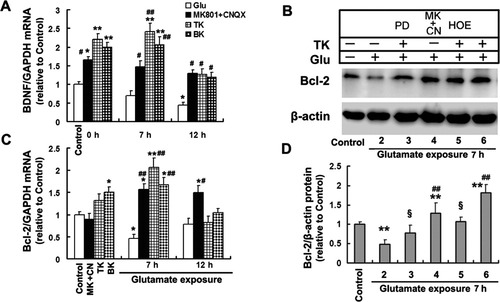
TK promotes expression of BDNF and Bcl-2. Primary cortical neurons pretreated with TK (100 nM) or BK (1 μM) for 12 hr or MK801 (10 μM) and CNQX (20 μM) for 30 min without or with glutamate stimulation for 7–12 hr. Real-time PCR was performed to analyze the mRNA levels of BDNF (A) and Bcl-2 (C). Representative Western blots of Bcl-2 protein at 7 hr of glutamate exposure (B). D: The relative density of Bcl-2 to that of β-actin. Pretreatment with TK, BK, or MK801 and CNQX increased basal expression of BDNF mRNA, but slightly altered basal levels of Bcl-2 mRNA, and significantly attenuated the reduction in the levels of BDNF (by 7 hr and 12 hr) and Bcl-2 mRNA (by 7 hr) induced by glutamate exposure. Pretreatment with TK or MK801 and CNQX reversed the decreases in the levels of Bcl-2 protein induced by glutamate stimulation for 7 hr. PD98059 or HOE140 greatly suppressed the increases in Bcl-2 protein levels induced by TK. The values are presented as mean ± SD of three samples.☆P < 0.05, ☆☆P < 0.01 for normal control. #P < 0.05, ##P < 0.01 compared with glutamate exposure alone. §P < 0.05, for TK + glutamate. Glu, glutamate (100 μM); HOE, HOE140; PD, PD98059; MK + CN, MK801 + CNQX.
Exposure to glutamate for 7 hr strikingly decreased Bcl-2 mRNA levels to 0.46-fold relative to the control, wherease 12 hr of exposure gradually restored the Bcl-2 mRNA level to 0.79-fold of the control. Pretreatment with NMDA and AMPA receptor inhibitors for 30 min with subsequent glutamate exposure up-regulated the Bcl-2 mRNA levels to 1.57-fold and 1.49-fold of the control at 7 hr and 12 hr, respectively. Pretreatment with TK or BK significantly attenuated the decline in the Bcl-2 mRNA levels induced by glutamate exposure for 7 hr (Fig. 9A,C). The protein levels of Bcl-2 were further determined by Western blot analysis (Fig. 9B). After glutamate exposure for 7 hr, the protein levels of Bcl-2 in cultured cortical neurons were 0.48-fold lower than the level of the control group. Pretreatment with TK or MK801 and CNQX before glutamate exposure elicted a striking increase in expression of Bcl-2 protein by 1.82- and 1.28-fold, repectively. Addition of PD98059 or HOE140 30 min prior to TK treatment partially attenuated the effects of TK on the Bcl-2 protein levels (Fig. 9D).
DISCUSSION
A substantial body of previous evidence indicates that neurons of many different types suffer extensive cellular damage after exposure to high concentrations of glutamate (Choi,1988; Portera-Cailliau et al.,1997; Li et al.,2007). In the current study, we found that primary cortical neurons had a similar response to glutamate exposure, which triggered a dramatic loss of cell viability, a sharp increase in LDH release, and distinct morphological changes. It is also well documented that, during the interval of reperfusion or restoration that follows detrimental stimuli such as ischemic, hypoxic, or excitotoxic insults, neurons often undergo delayed apoptotic cell death (Cheung et al.,1998; Zhou et al.,2008). To characterize fully the features of neuronal death in our model, we assayed both nuclear morphology, by staining DNA with Hoechst 33343, and DNA strand fragmentation, by using the TUNEL assay. We confirmed previous observations that, during reperfusion after exposure to glutamate, primary cortical neurons undergo extensive apoptotic-like death, characterized by nuclear condensation and DNA strand breakage. Glutamate-induced damage, including apoptosis, was effectively prevented by selective blockage of NMDAR and AMPAR, a result in accordance with previous findings that activation of these, and perhaps other, ionotropic glutamate receptors contributes to glutamate-induced neurotoxicity (During and Spencer,1993; Ueda and Tsuru,1994).
The kallikrein/kinin system, including TK, has been shown to play an important role in many physiological and pathological processes. Several animal studies have suggested that TK, in the form of either exogenous protein or the transferred human gene, plays a protective role against I/R-induced cell apoptosis. However, few published studies have examined the potential in vivo or in vitro role of TK in glutamate-induced neurotoxicity. Here, we examined the effect of TK on glutamate-induced neurotoxic damage, with particular focus on the signaling mechanisms involved. Our findings show that treatment of primary cortical neurons with TK before glutamate exposure reduces, in a concentration-dependent manner, the consequent LDH release and loss of cell viability. Similarly, TK pretreatment was effective in attenuating both the morphological changes and the apoptotic features in cultured cortical neurons that otherwise follow glutamate insult. A previous study on cultured rat retinal neurons demonstrated that the selective B2R agonist BK was able to protect retinal neurons against glutamate neurotoxicity through activation of B2R (Yasuyoshi et al.,2000). In the present experimental model, we confirmed the observation that BK could stimulate the neuroprotective effects of TK, whereas blockage of B2R with its specific antagonist HOE140 could counteract TK action. This suggests that the protective effect of TK against glutamate neurotoxicity is likely mediated by B2R.
Oxidative stress has been implicated in the pathogenesis of a variety of neurological disorders, including ischemic stroke. Numerous studies have suggested that, in addition to the excitotoxic pathway mediated by ionotropic glutamate receptors, the oxidative pathway plays a critical role in glutamate-induced neuronal injury (Coyle et al.,1993). Glutamate-induced oxidative stress, which has been attributed to dysfunction of the glutamate/cystine antiporter and elevated intracellular Ca2+ influx, leads to accumulation of intracellular ROS. Excessive production of ROS may contribute to cell damage either directly, through interacting with and destroying cellular structural molecules, or indirectly, by affecting normal cellular signaling pathways and gene regulation (Lievre et al.,2000; Chan,2001; Sugawara et al,2003). Antioxidants and ROS scavengers can reduce neuronal damage following ischemic and excitotoxic injury (Cuzzocrea et al.,2000). Systemic or local delivery of human TK gene transfer was reported to protect brain tissue from oxidative stress damage following cerebral ischemia/reperfusion (Xia et al.,2004,2006). In the present study, continuous exposure to glutamate elicited a time-dependent increase in ROS production, which was significantly attenuated by either blockade of NMDAR or AMPAR or TK pretreatment. This suggests that TK may have the capacity to be antioxidative and thereby protect primary cortical neurons from glutamate-induced oxidative injury and death.
NO is not only a gaseous free radical but also an important and ubiquitous neurotransmitter. It is synthesized from arginine by NOS, of which three isoforms have been identified, the constitutive calcium-dependent neuronal NOS (nNOS) and endothelial NOS (eNOS) isoforms and the inducible calcium-independent (iNOS) isoform (Griffith et al.,1995; Iadecola et al.,1997). Excessive NO rapidly reacts with the superoxide radical to form peroxynitrite. Peroxynitrite and other, related reactive nitrogen species (RNS) induce nitration of lipids, DNA, and proteins, leading to irreversible modification of biological membrane constituents and destruction of the cell membrane. Neuronal NOS has also been implicated in playing a detrimental role in ischemic damage and glutamate neurotoxicity and selective inhibition of nNOS with 7-NI has been reported to attenuate both ischemic injury and the excitotoxic lesions produced by NMDA (Dawson et al.,1991; Yoshida et al.,1994; Huang et al.,1994; Schulz et al.,1995). Cortical cultures from nNOS-deficient mice have been shown to be resistant to both NMDA and hypoxic neurotoxicity. In the present study, we first confirmed the observation that glutamate exposure enhances the activity of nNOS, with increased production of NO, and that selective NMDAR and AMPAR antagonists inhibit each of these. Most notably, we observed that TK had effects comparable to those of NMDAR and AMPAR inhibitors, which suggests that TK may resist glutamate-induced neuronal damage via an NO-mediated mechanism.
Several signaling pathways have been implicated in the protective abilities of TK in ischemic injury, including PI3K/Akt and the MAPKs (Irving and Bamford,2002; Hetman and Gozdz,2004). The PI3K pathway is well known to promote cell survival in many other systems. However, our pilot study suggested that the PI3K pathway is not a requirement for the neuroprotective activity of TK in our experiment.
ERK1/2 is activated by glutamate or NMDA and is involved in the neuroprotective activity of NMDA against glutamate neurotoxicity (Lipsky et al.,2001; Zhu et al.,2005). Our previous in vitro studies on I/R-induced injury showed that B2R activation lessened neuronal apoptosis through a mechanism involving the ERK1/2 pathway (Tang et al.,2009). Thus, we investigated here the effects of TK treatment on phosphorylation of ERK1/2. Consistently with previous studies (Jiang et al.2000), our results showed that the exposure of primary cortical neurons to glutamate transiently activated the ERK1/2 pathway. However, during reperfusion after glutamate exposure, the elevated level of p-ERK rapidly declined to approach baseline values. TK pretreatment greatly enhanced expression of p-ERK1/2, particularly p-ERK1, not only at the stage of glutamate stimulation but also during subsequent reperfusion, and this was abolished by blockage of B2R. A recent study suggests that ERK1/2 activation, especially ERK1 activation in Müller cells, plays a protective role against NMDA-induced retinal cell death, whereas ERK1-deficient mice show greater susceptibility to NMDA injury (Nakazawa et al.,2008). Therefore, we propose, based on our findings, that ERK1/2 activation may to some extent mediate TK neuroprotection.
Accumulating evidence has revealed that activation of the ERK1/2 pathway is involved in NF-κB activation induced by glutamate (Cruise et al.,2000; Lipsky et al.,2001). To elucidate further the signaling pathways involved in the neuroprotective activity of TK, we adopted two methods to analyze the activity of NF-κB indirectly and directly. Results from Western blot analysis demonstrated that glutamate stimulation induces a brief but significant IKK phosphorylation. TK pretreatment not only increases basal level of p-IKK but also significantly promotes the p-IKK level in glutamate-treated neurons. Although MK-801 at a concentration of 10 μM is known to block completely NMDA- and Aβ1–40-induced NF-κB activation in cerebellar granule cells (Lipsky et al.,2001; Kawamoto et al.,2008), under our experimental conditions, NMDA and AMPA receptor antagonists MK-801 (10 μM) and CNQX (20 μM) or the ERK inhibitor PD98059 (10 μM) partially attenuated IKK phosphorylation in cultured cortical neurons stimulated by glutamate. The B2R inhibitor HOE140 blocks completely the increases in the level of p-IKK induced by TK pretreatment. Immunohistochemical staining revealed that cultured cortical neurons pretreated with TK prior to glutamate exposure exhibited much a stronger nuclear p65 immunostaining than those exposed to glutamate alone, which suggests that TK promotes the nuclear translocation of NF-κB in glutamate-treated neurons. These findings reveal that TK, through B2R, activates the IKK-NF-κB signaling pathway through the ERK1/2 signaling pathway. Our data are consistent with previous reports (Cruise et al.,2000; Lipsky et al.,2001).
Several previous studies have demonstrated that ERK1/2 and NF-κB activation induces expression of genes involved in cell proliferation, growth factor regulation, and apoptosis. BDNF and Bcl-2 are key targets of NF-κB involved in NMDA receptor-mediated survival-promoting and antiapoptotic signaling (Zhu et al.,2005). Our observations demonstrate that a 12-hr pretreatment with TK or BK promotes the levels of BDNF and Bcl-2 mRNA in cortical neurons with or without glutamate exposure, which is comparable to the effects produced by ionotropic glutamate receptor inhibitors. TK application or blockade of ionotropic glutamate receptors also up-regulates the decreased expression level of Bcl-2 protein induced by glutamate, whereas the specific B2R or ERK inhibitor largely abolishes the up-regulation. These results indicate that up-regulation of BDNF and Bcl-2 expression is involved in the neuroprotective activity of TK, which is similar to previous reports on protective effects of NMDA against glutamate toxicity (Lipsky et al.,2001; Zhu et al.,2005). In summary, TK, acting mainly through B2R, plays a protective role against glutamate-induced neurotoxicity in cultured cortical neurons, by conferring resistance to oxidative stress and suppressing neuronal apoptosis, in part through phosphorylation of the ERK1/2, in particular ERK1, and subsequent activation of the IKK-NF-κB signaling pathway, which in turn up-regulates the expression of antiapoptotic and survival genes, including Bcl-2 and BDNF.




