Myelin under stress
Abstract
The capacity to fold proteins properly is fundamental for cell survival. Secreted and transmembrane proteins are synthesized in the endoplasmic reticulum (ER), an organelle that has the ability to discriminate between native and nonnative proteins, in a process called protein quality control. When folding is not properly achieved, misfolded proteins can accumulate. The terminally misfolded proteins are typically retrotranslocated into the cytoplasm for degradation by the proteasome, in a process known as endoplasmic reticulum-associated degradation. However, if the degradation is insufficient, accumulation of abnormal proteins in the ER activates the unfolded protein response (UPR), a complex set of new signals aimed to reduce further the load of abnormal protein in the ER. Massive synthesis of myelin lipids and proteins is necessary to support myelinogenesis. Not surprisingly, therefore, ER stress (including the UPR), the proteasome, and autophagy (lysosomes) have been implicated in myelin disorders, such as Pelizaeus-Merzbacher disease and vanishing white matter disease in the central nervous system and Charcot-Marie-Tooth neuropathies in the peripheral nervous system. Here we discuss recent evidence supporting an important role for ER stress in myelin disorders. © 2009 Wiley-Liss, Inc.
The endoplasmic reticulum (ER) is a highly specialized organelle, continuous with the nuclear membrane. Proteins destined for secretion or insertion into the cell membranes are synthesized on ER-bound ribosomes and cotranslationally translocated into the ER (Gilmore and Blobel,1985; Kleizen and Braakman,2004; Anelli and Sitia,2008). In addition to the optimal redox conditions, the ER also provides resident chaperones and folding enzymes that exhibit different biochemical properties, necessary for correct protein folding, oligomerization, and posttranslational modifications (Schroder and Kaufman,2005; Zhang and Kaufman,2006). For example, some chaperones, such as BiP (GRP78), do not actively catalyze protein folding but rather keep proteins in a folding-competent state while preventing aggregation (Bertolotti et al.,2000); others, such as calnexin (CNX) and calreticulin (CRT), recognize and assist the folding of nascent chains of N-glycosylated proteins. Finally, enzymes, such as disulfide isomerase (PDI), catalyze protein-folding reactions, accelerating the rate of correct disulfide bond formation (Kaufman,1999). Proteins that fail to fold are eventually retrotranslocated across the ER membrane for proteasomal degradation.
ER homeostasis is vital for cell function and survival. Alterations in calcium storage or in the redox status, sugar/glucose deprivation, or the expression of misfolded proteins compromise the folding capacity of the ER, resulting in the accumulation of unfolded proteins in the lumen. An imbalance between the unfolded protein load and the ability to process that load causes ER stress. To maintain homeostasis, the mammalian ER has evolved molecular transducers that sense the stress in the lumen and signal to the cytoplasm and the nucleus, triggering a series of responses collectively termed the unfolded protein respose (UPR; Harding et al.,2002; Rutkowski et al.,2006; Ron and Walter,2007).
The mammalian UPR exhibits an integrated signaling network of three ER transmembrane sensors: pancreatic ER kinase (PERK), inositol-requiring kinase 1 (IRE1), and activating transcription factor 6 (ATF6). These factors coordinate the cell response to ER stress via transcriptional and translational control (Kaufman,1999; Mori et al.,2000; Harding et al.,2002). PERK phosphorylates eIF2alpha to attenuate translation (Harding et al.,2000). Paradoxically, eIF2alpha phosphorylation enhances the translation of some proteins, including the transcription factor ATF4, which in turn induces genes involved in amino acid metabolism, protein secretion, and the antioxidant response (Rutkowski and Kaufman,2003). Notably, eIF2alpha can also be phosphorylated by other kinases, such as PKR and GCN2, independently of ER stress. For this reason, this arm of the UPR is called the integrated stress response (ISR; Ron and Walter,2007).
Slightly after translational repression, IRE1 and ATF6 activate the transcription of genes encoding components that increase ER folding capacity, protein export, and degradation. In particular, IRE1 has an endoribonuclease activity that elicits the unconventional splicing of the mRNA for the transcription factor Xbp-1. The spliced form, sXbp-1, encodes a potent transcription factor that activates UPR target genes that promote ER-associated degradation (ERAD) and ER biogenesis (Acosta-Alvear et al.,2007).
ATF6 is instead translocated to the Golgi, where it is proteolytically cleaved by the site-1 and site-2 proteases (SP1 and SP2). This cleavage generates an ∼60-kDa bZip-containing fragment that migrates to the nucleus, where it activates the transcription of chaperones (such as BiP) and ERAD proteins (Wu et al.,2007; Yamamoto et al.,2007).
However, if the cell cannot cope with the unfolded protein overload, these transducers induce programmed cell death. At present, three main apoptotic pathways, emanating from the UPR, have been identified: the IRE1/c-Jun-N-terminal protein kinase (JNK) pathway, the caspase-12 pathway, and the PERK/CHOP pathway. Still, what determines the balance between anti- and proapoptotic signals remains a crucial question (Rutkowski and Kaufman,2007).
The intensity and the duration of the UPR appear to be important in determining cell fate. In fact, recent work has indicated that survival is favored during mild stress, a condition in which proapoptotic mRNAs and proteins are intrinsically less stable compared with those that promote adaptation (Rutkowski et al.,2006). Moreover, in human cells, during persistent ER stress, the IRE1 and ATF-6 arms of the UPR are attenuated, whereas the PERK/CHOP arm appears to be maintained. Artificially prolonged IRE1 activity promotes cell survival, indicating a possible link between this arm of the UPR and cell surivival (Lin et al.,2007). These observations may also provide a basis for context-dependent cell death; different cells may activate the three arms of the UPR differently or be able to maintain all of the arms of the UPR active for longer periods, favoring survival.
Myelinogenesis requires massive cellular synthesis of myelin lipids and proteins. Not surprisingly, therefore, ER stress (including UPR and ISR), autophagy, and proteasome inefficiency have been implicated in myelin disorders in both the central nervous system (CNS) and the peripheral nervous system (PNS; Fig. 1). For example, UPR and ISR activation was recently associated with central myelin damage in mouse models of Pelizaeus-Merzbacher disease (PMD), attributable to mutations of proteolipid protein, or in mice with enforced expression of interferon-γ in the CNS, respectively. Genetic modulation of the UPR or ISR altered the severity of the disease in each of these mouse models (Southwood et al.,2002; Lin et al.,2005). Moreover, various mutations in subunits of eukaryotic translation factor EIF2B (its substrate, EIF2α, is an important component of ER stress response) produce vanishing white matter disease (VWM; Proud,2001; van der Knaap et al.,2006).
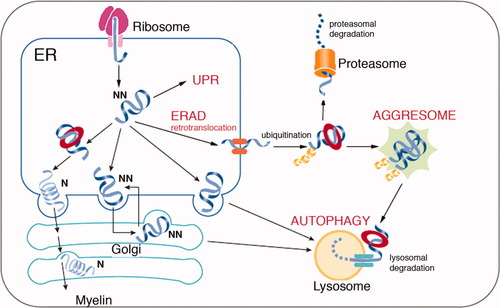
The possible outcomes of protein misfolding in the endoplasmic reticulum (ER) of glial cells. In the ER, nonnative proteins are assisted in their folding by various chaperones. If the native conformation is correctly achieved, the protein transits through the Golgi and reaches the myelin sheath. Terminally misfolded (or nonnative) proteins are usually retrotranslocated for ER-associated degradation (ERAD) by the proteasome. If the degradative machinery is unable to prevent the accumulation of misfolded protein in the ER, an unfolded protein response (UPR) can be elicited. In some circumstances, misfolded proteins are retrotranslocated but accumulate in the cytoplasm, forming the so-called aggresomes. This accumulation can in turn lead to the activation of autophagy and lysosomal degradation. N, native; NN, nonnative. Modified with permission from Yerbury et al. (2005).
In the PNS, some mutant P0 and PMP22 proteins, causing Charcot-Marie-Tooth type 1B (CMT1B) and CMT1A disease in humans, respectively, may be retained in the ER. Whereas P0 mutant elicits a pathogenetic UPR that can be genetically modulated (Wrabetz et al.,2006; Pennuto et al.,2008), PMP22 mutants are retrotranslocated for degradation, then aggregate and lead to inefficient proteasome function, toxic to the cell (Notterpek et al.,1999; Fortun et al.,2005).
PLP AND PMD
Proteolipid protein (PLP) is the major myelin protein in the CNS (Griffiths et al.,1998; Hudson,2004). The PLP gene is composed of seven exons and encodes for two tetraspan proteins, PLP and its smaller isoform DM20, generated from alternative splicing of exon 3B (Vouyiouklis et al.,2000). Various mutations in the X-linked gene for PLP1 give rise to PMD and spastic paraplegia type II (SPG2) in humans (Hudson et al.,2004). PMD is a neurodegenerative disorder characterized by diffuse hypomyelination in the CNS, and the phenotype can vary from mild to extremely severe, depending on the mutation. The most severe cases of PMD are due either to duplication of the PLP locus or to point mutations that can act through a gain of function (Hudson et al.,2004).
Several natural and transgenic mouse models are currently available with which to study PLP function and the pathogenesis of PMD. Notably, some mutations found in patients are also naturally occurring in mice. For example, the A242V amino acid substitution that causes a severe form of PMD in humans is found in the myelin synthesis-deficient mouse (msd), whereas the I186T mutation, which in humans results in a milder disease, is found in the rumpshacker (rsh) mouse (Gow et al.,1994b; Nave and Griffiths,2004). The molecular mechanisms underlying the diverse phenotypes are poorly understood.
Accumulating data show that in most of the severe forms of PMD resulting from point mutations, the PLP/DM20 protein is localized abnormally in the ER rather than being transported to the forming myelin membrane, suggesting that the altered trafficking of the protein is involved in the pathogenesis of the disease (Gow et al.,1994a; Gow and Lazzarini,1996). In some cases, the misfolding of the mutant protein leads to exposure of unpaired cysteine residues that engage in intramolecular cross-links and may cause ER retention (Dhaunchak and Nave,2007). ER retention is associated with up-regulation of the mRNA for BiP and other molecular chaperones in several PLP mutant animals, including msd and rsh mice (Hudson and Nadon,1992; Gow et al.,1998; Southwood and Gow,2001). These observations led to the analysis of the role of the UPR in PLP mutant mice. In msd, rsh, and jimpy (jp) mice, the mutant PLP protein triggered the up-regulation of chaperones such as BiP and Erp72 and of the transcription factors CHOP and ATF3, suggesting the activation of a UPR (Southwood et al.,2002; Gow,2004). This indicates a similarity in the molecular pathogenesis of the disease in the three models, despite the different mutations and disease severity. Strikingly, genetic removal of CHOP worsened the disease phenotype dramatically in rsh (Southwood et al.,2002). Although rsh mice have a normal life span, rsh/Chop null mice die as early as 5 weeks of age and exhibit frequent seizures following handling or sudden noises. This extreme exacerbation of the phenotype is accompanied by an increase in oligodendrocyte apoptosis in the spinal cord and in the optic nerve, from threefold in rsh to fivefold in rsh/Chop null, compared with wt (Southwood et al.,2002; Sharma and Gow,2007). However, whether this mild increase in cell death is enough to explain the dramatic worsening of the phenotype is not clear. Still, these data indicate that the UPR can modulate disease severity but that CHOP has a protective role in oligodendrocytes.
These observations raise an important issue related to the role of CHOP during the UPR. Although CHOP is proapoptotic in various cell types in vitro and in vivo (Zinszner et al.,1998; Oyadomari et al.,2002a; Marciniak et al.,2004), it appears to be protective in oligodendrocytes. One explanation for this discrepancy could be genetic background. In fact, whereas rsh mice with a C3H background have a normal life span, with a C57BL/6 background they die at about postnatal day 30 (Al-Saktawi et al.,2003). The reason for this difference is still unknown, but it could reflect an alteration in the activation of CHOP relative to other UPR mediators such as XBP-1 (McLaughLin et al.,2007), or the influence of modifying genes. The rsh/Chop null mice analyzed in the work from Southwood and colleagues were on a mixed C3H-C57BL/6 background, raising the possibility that the worsening of the phenotype in the absence of Chop was due to the presence of C57BL/6 background rather than to a protective role of CHOP. However, rsh//Chop+/+ mice regenerated from the siblings of experimental animals maintained a mild phenotype even in the continued presence of C57BL/6 background, making the background hypothesis unlikely (Southwood et al.,2002). Another possibility is cell specificity. It is possible that the target genes of CHOP vary from cell to cell, and, in agreement with this, downstream-of-CHOP (Doc) genes (Wang et al.,1998) are not induced in PMD models, suggesting that the targets of CHOP in oligodendrocytes could be different from those in other cell types.
Interestingly, ablation of the transcription factor ATF3 or of caspase-12, both supposed to have proapoptotic functions in the UPR, has at best only a marginal effect on the disease severity in msd and rsh mice (Sharma and Gow,2007; Sharma et al.,2007), indicating that these genes are not related to cell survival in PLP mutants. Finally, no UPR could be identified in md rat brain, despite oligodendrocyte death and short life span (Hudson and Nadon,1992). Further studies will be required to understand the relationship among specific mutations, UPR, oligodendrocyte death, and overall phenotype in PLP mutant animals.
VWM DISEASE
Leukoencephalopathy with vanishing white matter is an autosomal recessive disease clinically characterized by progressive ataxia, spasticity, and seizures (van der Knaap et al.,2006). VWM affects mainly the oligodendrocytes in the brain, often described as “foamy” in appearance, with abundant cytoplasm and abnormal mitochondria. In the most severe cases, there is diffuse oligodendrocyte death by apoptosis. Astrocytes can also appear abnormal in morphology and in their differentiation (Scheper et al.,2006). The primary defect of VWM resides in mutations in any of the five subunits of the translation initiation factor eIF2B (Leegwater et al.,2001; van der Knaap et al.,2002; Richardson et al.,2004). The initiation of protein synthesis is a complex process that requires the interplay between mRNAs, tRNAs, ribosomes, and various eukaryotic initiation factors (eIFs; Pestova et al.,2001). The eIF2-GTP complex is necessary to deliver the initiator methionyl-tRNA (Met-tRNAi) to the ribosome when the proper AUG sequence is identified. This is followed by hydrolysis of the GTP to GDP, with consequent release of eIF2. The guanine nucleotide exchange factor (GEF) eIF2B catalyzes the exchange of GDP to GTP on eIF2, to allow its association to another Met-tRNAi and recruitment to the ribosomal preinitiation complex (Scheper et al.,2006). The eIf2 factor comprises three subunits, α, β, and γ. As previously mentioned, phosphorylation of eIF2α is involved in translational control during the UPR. The phosphorylated eIF2α converts eIF2 from a substrate of eIF2B to a strong competitor, thus reducing the formation of the eIF2-GTP complex and therefore inhibiting protein synthesis. Mutations in eIF2B reduce its activity, and VWM patients have residual eIF2B activity ranging from 30% to 80%, with a correlation between the reduction of eIF2B activity and the age of onset and severity of the disease (Fogli et al.,2004).
One of the peculiar features of VWM disease is sensitivity to stress. Fever, acute fright, or head trauma can lead to rapid neurological deterioration (van der Knaap et al.,2006). This observation, coupled with the interplay of eIF2B and eIF2α, had prompted the investigation of the role of the UPR in VWM. Analysis of brain tissue from VWM patients indicated that all three branches of the UPR are activated (van der Voorn et al.,2005; van Kollenburg et al.,2006b). This activation is exclusively in white matter and predominantly in oligodendrocytes and astrocytes. How does the activation of the UPR result from the reduced activity of eIF2B? One possibility is that mutations in eIF2B alter the global protein synthesis rate. Surprisingly, in lymphoblasts from VWM patients, no differences in the regulation of protein synthesis were found (van Kollenburg et al.,2006a). However there was a slight increase in ATF4 expression (Li et al.,2004). This in turn was associated with an increase in CHOP levels that in some circumstances could further sensitize the cells to ER stress (McCullough et al.,2001), predisposing the cells to apoptosis. Another possibility is that UPR activation occurs normally during life, for example, in the case of fever, and that mutations in eIF2B impair the adaptive response of such UPR.
However, if eIF2 is such a vital component of protein synthesis in all cell types, why are only glial cells in the CNS affected? Oligodendrocytes have to synthesize vast amount of proteins and lipids necessary for the formation of the myelin sheath, which may render them more sensitive to ER homeostasis alterations. Currently, there are no animal models with which to study VWM, but they appear to be necessary to understand the pathophysiology of this complex disease properly.
INTERFERON-γ IN THE CNS: ER STRESS MODULATES THE RESPONSE OF THE OLIGODENDROCYTES TO INTERFERON
Interferon-γ is a proinflammatory cytokine secreted by activated T lymphocytes. Though not normally present in the CNS, it plays complex roles in immune-mediated demyelinating disorders, such as multiple sclerosis (MS) in humans and experimental autoimmune encephalomyelitis (EAE) in animals (Popko et al.,1997). For example, treatment of MS patients with interferon-γ exacerbates the disease (Panitch et al.,1987; Panitch,1992), and transgenic mice expressing this cytokine in the CNS display some myelin abnormalities (Corbin et al.,1996), whereas low levels of ectopically expressed interferon-γ appear to protect mouse CNS from cuprizone (a copper chelator)-induced demyelination (Gao et al.,2000). In vitro, differentiating oligodendrocytes undergo death by apoptosis after treatment with interferon-γ (Baerwald and Popko,1998). This process is accompanied by the up-regulation of some ER-stress markers, such as BiP, CHOP, caspase-12, and phosphorylated eIF2α (Lin et al.,2005). Similarly, these ER-stress markers correlated with hypomyelination and oligodendrocyte loss in a mouse model in which interferon-γ was ectopically expressed by astrocytes in the CNS from embryonic day 14 (Lin et al.,2005). When these mice were crossed into a PERK+/– background, the phenotype worsened dramatically, indicating that the stress response activated through the PERK pathway could have a protective role in immune mediated hypomyelinating disorders (Lin et al.,2005). This is further supported by the observation that, after cuprizone treatment, PERK+/– mice displayed a significantly reduced number of remyelinated axons, suggesting that the ER stress also modulates the response of remyelinating oligodendrocytes to interferon-γ (Lin et al.,2006).
Why does interferon-γ activate a stress response in oligodendrocytes? It has been shown that this cytokine increases the expression of MHC class I molecules and that accumulation of these molecules in oligodendrocytes causes hypomyelination accompanied by tremors and seizures (Baerwald et al.,2000). Developing and remyelinating oligodendrocytes are already synthesizing huge amounts of proteins and could develop a physiological UPR, similar to that observed in plasma cells and pancreatic β-cells (Kaufman,1999). This would make them more sensitive to the accumulation of MHC class I molecules and more prone to ER stress. Accordingly, mature oligodendrocytes, which are synthesizing only low, steady-state levels of myelin proteins, are much less sensitive to the detrimental effects of interferon-γ (Lin et al.,2005,2006).
PMP22 OVEREXPRESSION AND POINT MUTATIONS IN THE PNS
PMP22 (peripheral myelin protein 22) is a 22-kD glycoprotein that is a minor component of the myelin sheath of peripheral nerve, localized mainly to compact myelin (Snipes et al.,1992; Suter,2004). Most of the knowledge about the role of PMP22 in peripheral nerve comes from genetics, because PMP22 is the most common cause of neuropathies in humans and rodents. In humans, duplications of a chromosomal region containing the PMP22 gene account for most cases of CMT1A, although point mutations also exist (Snipes et al.,1999; Suter,2004). Some mutations described in human families are also naturally occurring in mice, such as the G150D (DSS in humans) and the L16P (CMT1 in humans) found in trembler (Tr) and Tr-j mice, respectively (Suter,2004). The trafficking of the Tr and Tr-j mutant protein is different from that of wt PMP22, with Tr-PMP22 mainly retained in the ER (Naef et al.,1997; Colby et al.,2000) and Tr-j-PMP22 retained in the intermediate compartment between the ER and the Golgi (Tobler et al.,1999). Interestingly, the ER-retained Tr-PMP22 associates with the chaperone calnexin, but not with BiP, with the consequence that the UPR is not activated in these mutants (Dickson et al.,2002).
One of the characteristics of PMP22 is its rapid turnover, with the majority of the newly synthesized protein rapidly degraded via the proteasome (Pareek et al.,1997; Notterpek et al.,1999). Overexpressed and mutated PMP22 accumulate in aggregates in the cytoplasm, and this correlates with a reduced activity of the ubiquitin-proteasome system (Notterpek et al.,1999; Ryan et al.,2002; Fortun et al.,2005). These protein aggregates are termed aggresomes (Johnston et al.,1998). Aggresomes are membrane-free cellular inclusions, rich in ubiquitinated proteins, usually localized near the centrosome, an area enriched in proteasomal subunits and heat shock proteins (HSPs; Kopito,2000). Accordingly, PMP22 in aggresomes appears to be ubiquitinated and associates with proteasomal components and with molecular chaperones such as HSP-70 (Notterpek et al.,1999; Fortun et al.,2005). Whether the formation of aggresomes is harmful or protective in various disease paradigms is still a matter of debate, but accumulating data point toward a protective role at least in PMP22 disease models. For example, cell transfections with a series of three PMP22 mutant proteins has shown that the larger aggregates correlate with the less severe phenotypes (Isaacs et al.,2002). Moreover, if the aggregates were toxic, cell death could be the expected outcome of the toxicity, whereas very little cell death is observed in PMP22-associated neuropathies (Sancho et al.,2001).
The accumulation of PMP22 in aggresomes results instead in a concomitant activation of autophagy (Fortun et al.,2003). Autophagy is defined as the process by which a portion of cytosol or organelle is sequestered in a double membrane structure called the autophagosome, which then fuses with lysosomes (Ohsumi,2001). PMP22 aggreagates are indeed surrounded by autophagosomes and lysosomes, which appear to be involved in the clearance of the aggresomes (Fortun et al.,2003). Stimulation of autophagy hinders the accumulation of proteasome substrates and correlates with a reduction in the formation of aggresomes (Fortun et al.,2007). In parallel to autophagy, an increase in the expression of molecular chaperones aids the cell in disposing of misfolded proteins (Fortun et al.,2007). These studies support a model in which the proteasome is inefficient, and misfolded proteins aggregate in the cytoplasm and are degraded via the autophagic-lysosomal pathway, further aided by cytoplasmic chaperones. These observations suggest that the development of pharmacological agents to stimulate these pathways would be a promising therapeutic approach to eliminate protein aggregates, at least in PMP22-related CMT neuropathies (Rangaraju et al.,2008).
P0S63del AND UPR MODULATION IN SCHWANN CELLS
We might have thought that the main problem with PMP22 was the lack of adaptive UPR; instead, myelin protein zero (MPZ, P0) mutants elicit a UPR, and this also causes myelin abnormalities (Table I). P0 is the major protein in peripheral nerve myelin, where it accounts for up to 50% of the total protein (Kirschner et al.,2004). Analysis of homozygous and heterozygous Mpz-null mice has shown that P0 is required in myelin compaction during development and for long-term maintenance of the myelin sheath (Giese et al.,1992). In humans, diverse mutations in P0 cause a range of hereditary neuropathies, suggesting diverse gain of function mechanisms (Wrabetz et al.,2004). For example, deletion (S63del) or conversion of serine 63 to cysteine (S63C) results in CMT1B or Dèjèrine-Sòttas syndrome, respectively. Consistent with toxic gain of function, when expressed in mouse together with wt P0, the mutant P0s produce a demyelinating or hypomyelinating neuropathy that mimics the corresponding human disease. In particular, S63del never reaches the myelin sheath and is instead retained in the ER (Wrabetz et al.,2006).
| Parameter | Mutant P0 S63del | Mutant PMP22 (Tr and Tr-j) |
|---|---|---|
| ER retention | +++ | + |
| UPR | +++ | − |
| Proteasome inefficiency | ? | ++ |
| Aggresome formation | − | +++ |
| Autophagy (lysosomes) | ? | +++ |
| Cell death | + | + |
Accumulation of S63del in the ER triggers a canonical UPR indicating a toxic gain of function (Wrabetz et al.,2006; Pennuto et al.,2008). Notably, in contrast to what happens in the CNS of PLP mutants (Southwood et al.,2002), in S63del mice the genetic ablation of the transcription factor CHOP ameliorates the demyelinating phenotype and completely rescues the motor deficit, indicating that the UPR is pathogenetic (Pennuto et al.,2008). How does this UPR produce demyelination, and why does the removal of CHOP improve the phenotype? One possibility is that CHOP induction activates Schwann cell apoptosis and, therefore, demyelination. However, the level of cell death in S63del mice is very low compared with that in a number of misfolded protein disorders with primary cell death, including diabetes (Oyadomari et al.,2002b), GM1 gangliosidosis (Tessitore et al.,2004), and PMD (Southwood et al.,2002). Accordingly, S63del nerves do not show an obvious reduction in numbers of Schwann cells or a phenotype typical of extensive Schwann cell death (Messing et al.,1992). Moreover, the rise in apoptosis in S63del mice is delayed by weeks relative to CHOP induction and parallels better the rise in demyelinated fibers (Pennuto et al.,2008). Finally, CHOP is usually detected in nonpyknotic Schwann cell nuclei, associated with normal myelin sheaths (Pennuto et al.,2008), suggesting that UPR and CHOP induce demyelination with limited secondary Schwann cell death and not vice versa. In fact, other demyelinating neuropathies not associated with UPR manifest similar low levels of apoptosis (Sancho et al.,2001). This provides further support for the idea that CHOP induction in the context of UPR does not necessarily imply cell death (Rutkowski et al.,2006).
Another appealing hypothesis is that CHOP could perturb myelin stoichiometry, either transcriptionally or at the translational level; GADD34, a target gene of CHOP, regulates the dephosphorylation of eIF2α and thereby stress-induced changes in translation of specific mRNAs (Marciniak et al.,2004). As we have seen in the case of VWM, subtle changes in translational control may exert dramatic effects on myelin homeostasis in the CNS, and this is likely also to be true in the PNS. Finally, the reason why CHOP appears to have a maladaptive role in Schwann cells as opposed to the protective role in oligodendrocytes (Southwood et al.,2002) remains unclear. A comparison of CHOP target genes, as well as the relative level of activation of the arms of the UPR, in the two pathological cells, could illuminate the different responses (Fig. 2).
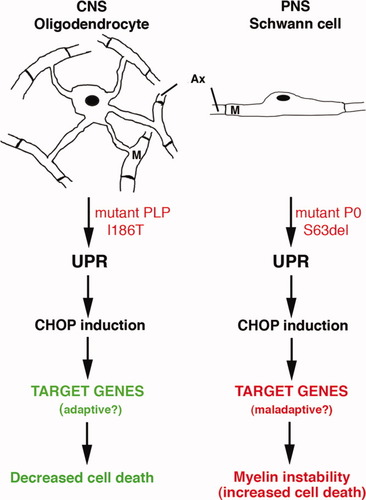
The transcription factor CHOP has different roles in oligodendrocytes and in Schwann cell. Accumulation of misfolded PLP and P0 proteins triggers an unfolded protein response (UPR) in oligodendrocytes and Schwann cells, respectively. However, whereas the genetic ablation of CHOP exacerbates the phenotype in the CNS, it improves it in the PNS. This suggests that the target genes of CHOP could be different in the two cell types and that they have an adaptive role in oligodendrocytes and a maladaptive one in Schwann cells. Ax, axon; M, myelin.
CONCLUSIONS
ER stress has been implicated in various diseases, ranging from cancer to diabetes (Zhao and Ackerman, 2006). Glial cells also appear to be vulnerable to ER stress, and the UPR, or alterations to some of its mediators, can perturb myelination by both oligodendrocytes and Schwann cells. This susceptibility may have various causes. Myelination is a complex process that requires a precise stoichiometry in gene dosage and in protein and lipid synthesis. It is possible that induction of the UPR alters this tightly controlled mechanism, with detrimental effects. The UPR in fact attenuates translation; broadly alters the transcription and stability of specific mRNAs (Hollien and Weissman,2006); and has global effects on the dosage, folding, and function of other metastable proteins (Gidalevitz et al.,2006). For example, in the PNS, PMP22 could be considered a metastable protein, with much of the newly synthesized PMP22 rapidly degraded under physiological conditions (Pareek et al.,1997). In this context, it is worth noting that alteration of the dosage of PMP22 causes CMT1A, the most common hereditary demyelinating neuropathy.
Another possibility is that some of the mechanisms activated during the UPR overlap with those necessary for myelin membrane production, creating a sort of competition. For example, Xbp-1 increases the expression of genes related to phospholipid biosynthesis and ER membrane expansion (Sriburi et al.,2004; Acosta-Alvear et al.,2007), and ATF6 is cleaved in the Golgi by the SP-1 and SP-2 proteases, which are also responsible for the cleavage and activation of the sterol regulatory element-binding proteins 1 and 2 (SREBPs), which are involved in the expression of cholesterol/lipid biosynthesis genes during myelination (Verheijen et al.,2003; Leblanc et al.,2005).
The mouse models reviewed here also indicate that the effects of the UPR in vivo are not easily predicted. The cell context appears to be important in determining whether the response will be adaptive or maladaptive. For example, the CHOP arm of the UPR is adaptive in oligodendrocytes of rsh mice (Southwood et al.,2002) and maladaptive in Schwann cells of P0 S63del mice (Pennuto et al.,2008). Notably, the maladaptive effect of CHOP in S63del Schwann cells results primarily in hypomyelination and demyelination and not in cell death, suggesting that the cell context may also be important to determine the form that the maladaptive response takes.
Finally, these observations raise a corollary point: most of the studies on ER stress and UPR have been conducted in cell cultures with pharmacological induction of ER stress. However, these studies have not always predicted the range of effects in stressed cells in vivo, where different mechanisms cause ER stress. Further studies will define the range of response to ER stress in vivo.
Acknowledgements
We dedicate this review to the memory of Steve Pfeiffer, teacher, colleague, and friend.




