Adult CST-null mice maintain an increased number of oligodendrocytes
Abstract
The galactolipids galactocerebroside and sulfatide have been implicated in oligodendrocyte (OL) development and myelin formation. Much of the early evidence for myelin galactolipid function has been derived from antibody and chemical perturbation of OLs in vitro. To determine the role of these lipids in vivo, we previously characterized mice lacking galactocerebroside and sulfatide and observed abundant, unstable myelin and an increased number of OLs. We have also reported that mice incapable of synthesizing sulfatide (CST-null) while maintaining normal levels of galactocerebroside generate relatively stable myelin with unstable paranodes. Additionally, Hirahara et al. (2004; Glia 45:269–277) reported that these CST-null mice also contain an increased number of OLs in the forebrain, medulla, and cerebellum at 7 days of age. Here, we further the findings of Hirahara et al. by demonstrating that the number of OLs in the CST-null mice is also increased in the spinal cord and that this elevated OL population is maintained through, at least, 7 months of age. Moreover, we show that the enhanced OL population is accompanied by increased proliferation and decreased apoptosis of oligodendrocytic-lineage cells. Finally, through ultrastructural analysis, we show that the CST-null OLs exhibit decreased morphological complexity, a feature that may result in decreased OL competition and increased OL survival. © 2009 Wiley-Liss, Inc.
The combination of early appearance and abundance of the galactolipids, galactocerebroside and sulfatide, has resulted in intense interest in the significance of these lipids in oligodendrocyte (OL) development and myelin structure (Pfeiffer et al., 1993; Taylor et al., 2004). Much of the initial work designed to elucidate the roles of the galactolipids was conducted in vitro by a variety of immunological and chemical perturbations (for review see Dupree and Popko, 1999). For example, Dubois-Dalcq et al. (1970) reported that the addition of cerebroside antisera inhibited myelin formation in culture. These findings were subsequently confirmed by several groups who demonstrated that antibody perturbation not only inhibited myelin formation (Ranscht et al., 1987; Owens and Bunge, 1990) but also compromised myelin integrity in vitro (Fry et al., 1974; Saida et al., 1979; Roth et al., 1985; Saito et al., 1986; Bansal and Pfeiffer, 1994) and in vivo (Sergott et al., 1986; Rosenbluth et al., 1994, 1995).
Steve Pfeiffer and colleagues published a series of seminal articles that provided the foundation for our understanding of the roles that the galactolipids play in OL development. In 1989, Bansal and Pfeiffer demonstrated that the addition of an antibody that recognizes both galactocerebroside and sulfatide (Ranscht monoclonal antibody; Ranscht et al., 1982) inhibited OL progenitors from terminal differentiation; however, antibody removal resulted in continued differentiation and morphological changes consistent with OL maturation. Although these results were exciting, this antibody, which recognized both galactocerebroside and sulfatide, did not allow a distinction between the functions of these two closely related lipids. Thus, Pfeiffer and colleagues conducted subsequent studies designed to perturb specifically the function of galactocerebroside or sulfatide (Bansal et al., 1988, 1989; Bansal and Pfeiffer, 1989). The culmination of these works suggests that sulfatide is the primary galactolipid regulatory molecule in OL development (Bansal et al., 1999).
Genetically engineered mice deficient in galactolipid synthesis (Bosio et al., 1996; Coetzee et al., 1996; Honke et al., 2002) have also been employed to further our understanding of the role that these lipids play in OL development and myelin structure. In mice that lack sulfatide (disruption in the cerebroside sulfotransferase gene; referred to as CST-null mice) or both sulfatide and galactocerebroside (disruption in the ceramide galactosyltransferase gene; referred to as the CGT-null mice), OLs express mature myelin markers and form abundant myelin sheaths (Bosio et al., 1996; Coetzee et al., 1996; Honke et al., 2002; Marcus et al., 2006). Although OLs in these mice terminally differentiate and form myelin, the regulatory mechanisms that control OL numbers are compromised in that both mutants exhibit enhanced numbers of terminally differentiated OLs in young animals (Bansal et al., 1999; Marcus et al., 2000; Hirahara et al., 2004). At present it is not clear why the OL populations in these mice are increased or whether these increased populations are maintained throughout life. Here, we have explored these questions using the CST-null animals. Our results show that the increased OL population is maintained in the spinal cords of adult CST-null mice to at least 7 months of age. We also report increased proliferation in the central nervous system (CNS) of young mutant mice, an increase that is at least partially related to cells of the OL lineage. In addition, significantly fewer CST-null OL-lineage cells undergo apoptosis, suggesting that increased survival plays a role in establishing and maintaining the enhanced OL population. Finally, we provide ultrastructural evidence that the CST-null OLs extend fewer myelin-forming processes, a feature that may allow more OLs to establish axonal contact, resulting in an increase in OL survival. Together, these in vivo data confirm the findings of Pfeiffer and colleagues that sulfatide plays a role OL development.
MATERIALS AND METHODS
Animals
All animals used in this study were generated and housed in the Virginia Commonwealth University Division of Animal Resources. Mice heterozygous for the gene that encodes cerebroside sulfotransferase (CST) were mated, and offspring were genotyped as previously described (Honke et al., 2002), with slight modifications. Briefly, genotypes of the CST mice were determined by PCR with primers specific to the CST gene (CSTFL 5′-CTA TTG GAC AAC TAC CCA CTA CCA CCT GC-3′ and CSTRL 5′-GCA CTT ATG TCC GTG TGA GAG TGT CAG GTC-3′) and to the neo cassette (CSTNEOF 5′-CAT TCG ACC ACC AAG CGA AAC ATC G-3′ and CSTNEOR 5′-GCA CGA GGA AGC GGT CAG CCC AAT-3′). PCR cycles were as follows: 95°C for 5 min, 95°C for 10 sec, 60°C for 10 sec, 68°C for 20 sec, for a total of 30 cycles with Klentaq DV Ready Mix (Sigma, St. Louis, MO). Wild-type (WT) and CST-null mice yield only a single PCR product of 548 base pairs and 332 base pairs, respectively. Heterozygous mice yield both products.
Immunocytochemistry
WT and littermate CST-null mice 15 days, 30 days, and 7 months of age were perfused with 4% paraformaldehyde in 0.1 M Millonigs phosphate buffer (pH 7.3); brains and spinal cords were harvested intact to preserve spinal cord orientation. The tissues were cryoprotected, frozen, transversely or longitudinally sectioned, and immunolabeled as previously described (Dupree and Popko, 1999; Dupree et al., 2004). Primary antibodies required for these studies included CC1, anti-olig2, antiglial fibrillary acidic protein (GFAP), IBA1, and antibromodeoxyuridine (BrdU). The CC1 antibody (Calbiochem, Cambridge, MA; mouse monoclonal, 1:100), recognizes mature OL cell bodies without labeling the myelin sheath (Bhat et al., 1996). We and others have successfully used the CC1 marker to label OLs in developing, mature, and remyelinating systems in vivo (Fuss et al., 2000; Messersmith et al., 2000; Dupree et al., 2005; Murtrie et al., 2005; Sohn et al., 2006; Vana et al., 2007). The olig2 antibody (rabbit polyclonal, 1:10,000), which was kindly provided by Drs. John Alberta and Chuck Stiles (Dana-Farber Cancer Institute, Boston, MA), recognizes both immature and mature OLs (Ligon et al., 2004). The GFAP antibody (Thermo/Fisher Scientific, Pittsburgh, PA; 1:200) was used to identify astrocytes, and the IBA1 antibody (Wako Chemical, Richmond, VA; 1:500) was used to label microglia.
Prior to anti-BrdU labeling, the sections were pretreated with 3 N HCl to enhance epitope availability as previously described (McGinn et al., 2004; Sun et al., 2005). Three BrdU antibodies were used (Dako Cytomation, Carpinteria, CA, 1:100; Serotec, Raleigh, NC, 1:200; B-D Biosciences, San Jose, CA, 1:200) to confirm the results and to facilitate double labeling. With the exception of the B-D Biosciences anti-BrdU, which was directly conjugated to FITC, all primary antibodies were visualized by indirect labeling with a fluorescently tagged secondary antibody (Vector Laboratories, Burlingame, CA). All fluorescently labeled sections were also labeled with the nuclear marker DAPI (see Fig. 6) and were imaged with either a Leica TCS-SP2 AOBS confocal laser scanning microscope (inverted) with a spectrophotometer scan head (Leica Microsystems Inc., Bannockburn, IL) or a Nikon Eclipse E800M microscope (Nikon Instruments, Melville, NY) equipped with a Diagnostic Instruments Spot RT Camera (Diagnostic Instruments Inc., Sterling Heights, MI).
Cell Type Quantitation
All quantitative analyses were limited to the ventral columns of the cervical spinal cord. Ventral columns were delineated centrally by neuronal cell bodies of the gray matter and by exit points of the ventral roots. To compare mature and immature OL populations quantitatively, four 1,600- × 1,200-pixel images per transversely sectioned spinal cord (two images per side) were collected using the Nikon Eclipse E800M microscope with a Plan Fluor ×40/0.75 NA objective lens. For this objective lens, the image depth of field is approximately 1 μm thick. Each pixel, based on the pixel parameters and objective used for image collection, equaled 0.183 μm per side; hence, the microscopic field represented 0.064 mm2.
To compare mature OL populations, the numbers of DAPI+/CC1+ cells per microscopic field were determined for each genotype at 15 days, 30 days, and 7 months of age. The relative number of immature OLs was determined by employing an antibody directed against olig2, a transcription factor expressed by cells in the OL lineage (Lu et al., 2000; Zhou et al., 2000) that is used to identify immature OLs (Pernet et al., 2008). Because olig2 is also present in differentiated OLs (Ligon et al., 2004; Gokhan et al., 2005), we triply labeled spinal cord sections with the olig2 antibody, the CC1 antibody, and DAPI. DAPI+/olig2+/CC1+ were considered mature OLs; DAPI+/olig2+/CC1− cells were identified as immature OLs. For both mature and immature counts, three sections per animal from four WT and four CST-null littermate pairs were analyzed per labeling combination. To account for variability among litters, the data are presented as mean number of cells per microscopic field expressed as a percentage of littermate WT ± percentage SD.
Proliferative Analysis: BrdU Studies
BrdU (Sigma) was prepared according to the manufacturer's directions. Briefly, 20 mg/ml of BrdU was prepared in 0.9% saline containing 0.007 N sodium hydroxide. Fifteen-day-old WT and CST-null littermates were intraperitoneally injected with 200 mg/kg body weight using the 20 mg/ml stock BrdU solution. Two or seventy-two hours after injection, the animals were perfused and spinal cords were processed for immunohistochemistry as described above. To label for only BrdU and DAPI, the sections were incubated in 3 N HCl for 30 min at room temperature. To label with BrdU, DAPI, and a cell identification marker (GFAP, olig2, or Iba1), the immunocytochemical labeling protocol was modified. Briefly, WT and CST-null mice were perfused with 0.9% saline, frozen, and sectioned. The sections were incubated in the olig2 antibody overnight, followed by labeling with a goat anti-rabbit secondary antibody conjugated with Texas red. The olig2-labeled sections were incubated in 4% paraformaldehyde for 10 min at room temperature, rinsed in 0.1 M phosphate-buffered saline, and exposed to 2 N HCl for 5 min at room temperature to facilitate BrdU labeling. A brief exposure to a more dilute acid was employed to reduce olig2 labeling disruption. A minimum of three 10-μm frozen transverse spinal cord sections from a total of five CST-null mice and seven littermate WT animals from three litters were used for quantitative analysis. The data are presented as mean percentage of WT ± percentage SD.
Because the acid treatment dramatically reduced the fluorescent intensity of the secondary antibodies used for cell type identification and the BrdU labeling was very strong, all quantitative analyses of dual-labeled sections involving BrdU labeling were conducted by confocal microscopy. To facilitate accurate recognition of the fluorescent signals, all images were collected with a ×63 PL Apo/NA 1.4 oil immersion objective lens, a scan resolution of 1,024 × 1,024 pixels, a line averaging of 8, sequential scanning between green and red channels (to avoid signal cross-talk), and a narrow detection window (20 nm; 510–530 nm for FITC) for the BrdU secondary antibody. Images were collected as z-stacks of eight optical sections through a depth of 3.17 μm, which were then compiled as average pixel intensity projections. For the dual labeling with BrdU, values are presented as mean percentage of total DAPI+/BrdU+ cells.
Apoptosis Analysis: Terminal Uridine Deoxynucleotidyl Transferase dUTP Nick End (TUNEL) Labeling
Transverse cryosections (10 μm thick) of cervical spinal cords (prepared as described above) from 15-day-old WT and CST-null mice were immersed in –20°C acetone and further permeabilized by incubation in 0.1% Triton X-100 in 0.1% sodium citrate. The sections were then incubated in the TUNEL reagent as described by the manufacturer (Roche, Boulder, CO; In Situ Cell Death Detection Kit), and labeled cells were visualized as described above. For quantification of TUNEL labeling, at least three 10-μm frozen, transverse spinal cord sections per mouse from six CST-null and eight littermate WT animals from three litters were triply labeled with DAPI, the olig2 antibody, and the TUNEL reagent. Counts for the TUNEL analysis are expressed as mean cell number per field expressed as a percentage of WTs ± percentage SD.
Light and Electron Microscopy
Spinal cord tissue from WT and CST-null animals was prepared as previously described, with slight modifications (Dupree et al., 1998; Marcus et al., 2006). Animals were perfused with 0.1 M Millonigs phosphate buffer (pH 7.3) containing 4% paraformaldehyde and 5% glutaraldehyde. Spinal cord samples were postfixed in 1% osmium tetroxide, dehydrated in serial dilutions of ethanol, and embedded in PolyBed 812 resin (Polysciences, Warrington, PA). Thick (1 μm) and thin (90 nm) sections were stained with toluidine blue or uranyl acetate and lead citrate, respectively. Thick sections were qualitatively assessed with a Nikon Eclipse E800 upright compound microscope equipped with a Spot camera, using an oil immersion Plan Fluor ×100/1.3 N.A. objective lens. The ultrathin sections were analyzed with a JEOL 1230 transmission electron microscope (JEOL Ltd., Tokyo, Japan) equipped with a Gatan UltraScan CCD camera (Gatan Inc., Pleasanton, CA).
Quantification of OL Processes
Cells in the WT and CST-null spinal cord ventral columns with dense cytoplasm yet containing neither intermediate filaments nor glycogen granules were identified as OLs and were imaged by transmission electron microscopy at ×3,000. The number of primary OL processes, defined as processes that branched directly from the cell body (see Figs. 1A, 7), was determined for each soma. Analysis was conducted on presumptive OLs from 2- and 4-day-old mice, because the processes are more reliably delineated than in older animals. The data are presented as the mean number of primary processes per cell ± SD.
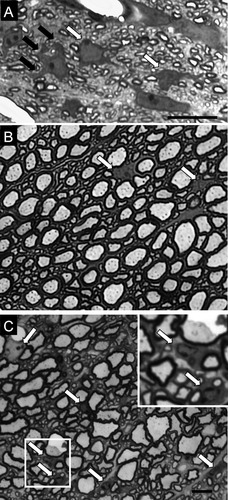
Cervical spinal cord white matter tracts of CST-null mice show increased cellularity. A: As observed in 1-μm-thick Epon sections, the ventral column of the cervical spinal cord from a 4-day-old WT mouse reveals numerous cells with morphologies that are consistent with immature OLs (white arrows), insofar as each cell contains a prominent nucleus and abundant and dense cytoplasm and extends multiple processes (black arrows) that contact axons and presumably form myelin sheaths. By 7 months of age, cells (white arrows with black border) in the ventral column of the cervical spinal cord of both WT (B) and CST-null (C) mice are much less prominent compared with the cells in the 4-day-old spinal cord. Although cells are less conspicuous in both WT and CST-null animals, the number of observed cells remained elevated in the CST-null mice compared with age-matched WT mice. A representative field taken from a 7-month-old WT spinal cord displays two cells. In contrast a comparable field from an age-matched CST-null animal reveals six cells. The inset in C provides a higher magnification of two of the cells in C. Both cells shown in the inset maintain a close association with a myelinated axon, suggesting that these cells are OLs. Scale bars = 10 μm in A; 20 μm in C (applies to B,C).
Statistical Analyses
All data were statistically compared by two-tailed t-tests. Statistical significance was accepted at P ≤ 0.05.
RESULTS
Adult CST-Null Mice Maintain a Larger Population of Mature OLs Than Their WT Littermates
Consistent with our previous analysis of mice that lack both galactocerebroside and sulfatide (Marcus et al., 2000), qualitative assessment of the thick sections indicated that young CST-null mice and WT littermates exhibit numerous putative OLs in the ventral column of the spinal cord (Fig. 1A). Although the apparent cellularity is dramatically reduced in both WT and mutant animals by 7 months of age, dramatically more cells are observed in the spinal cord of the CST-null mice compared with their littermate controls (Fig. 1B,C). From these 1-μm sections, we were unable to determine whether the increased cellularity in the null mice represents an increase in cell number or whether the cells are merely more apparent as a result of enlarged cell bodies, increased interaxonal spacing, and reduced myelination. Although many of the cells display morphologic characteristics consistent with OLs (Fig. 1C, inset), the identity of the cells cannot be determined from the 1-μm sections. Therefore, we employed immunocytochemical analyses to determine cell identity and to compare quantitatively the numbers of these cells in the WT and CST-null mice.
The number of CC1+ cells was significantly increased at 15 days of age, yielding a population that was 123.4% ± 16.6% of the WT population (85.0 ± 9.9 cells per field compared with 68.9 ± 5.2 cells per field in the WT mice; P < 0.05; Fig. 2). Similarly, a significant increase was also observed in 30-day-old CST-null mice, which exhibited a population that was 152.5% ± 10.9% of the WT population of mature OLs (100.2 ± 6.3 CC1+ cells per field compared with 65.7 ± 8.1 CC1+ cells per field in WT littermates; P < 0.05). Because the mature OL population was enhanced at both 15 and 30 days of age, we analyzed the OL population in 7-month-old animals to determine whether the enhanced number of OLs was maintained in adult animals. Interestingly, by 7 months of age, the number of CC1+ cells per field in both the WT and the CST-null mice was reduced compared with the number in 30-day-old mice (79.2 ± 8.4 CC1+ cells per field compared with 44.7 ± 5.3 CC1+ cells per field in 7-month-old mutant and WT animals, respectively). However, the percentage difference between the mutants and the WT littermates at this advanced age was greater (CC1 population in the mutant mice was 177.2% of the WT population). Thus the CST-null mice also reveal a significantly increased population of CC1-positive cells at 7 months of age (P < 0.05).
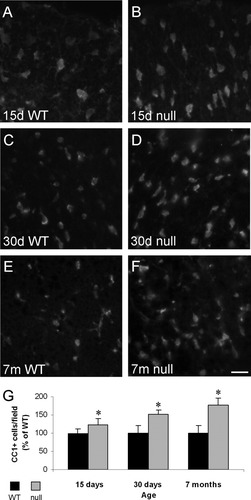
CST-null mice maintain significantly more mature OLs than their WT littermates through at least 7 months of age. Fluorescent images of 10-μm frozen sections, collected from the ventral column of the cervical spinal cord immunolabeled with the mature OL marker CC1, reveal more mature OLs in the CST-null mice (B,D,F) than their WT littermates (A,C,E) at 15 days, 30 days, and 7 months of age. Based on statistical analyses with a two-tailed t-test, the number of mature OLs in the spinal cord was significantly greater in the CST-null mice than the WT mice at all ages studied (G). Values indicate mean number of cells per microscopic field ± SD; n = 4 for 15-day-old WT and CST-null; n = 3 for 30-day-old WT and CST-null mice; n = 3 for 7-month-old WT and CST-null mice. ☆P < 0.05. Scale bar = 20 μm.
To determine whether the difference in cell density (number of cells per microscopic field) reflected an actual increase in OL number, we utilized 1-μm-thick sections to measure and compare the area of the ventral column white matter tracts at 15 days, 30 days, and 7 months of age. No difference in the size of the ventral column white matter tracts was observed between the WT and CST-null mice at 15 days. Thus, we conclude that, by 15 days of age, the CST-null mice maintain significantly more mature OLs than littermate WT animals.
Although the area of the white matter tracts was not different between the null and WT mice at 15 days of age, the ventral columns in both the 30-day-old and 7-month-old CST-null mice were 15% smaller than the ventral columns of the age-matched WT mice. To determine whether tighter packing of an equivalent number of cells resulted in the increased densities, we adjusted the CC1 counts accordingly. The adjusted densities of CC1+ cells in the 30-day-old and 7-month-old CST-null mice were 80.0 ± 11.1 and 67.2 ± 11.7, respectively, and both adjusted densities remained significantly greater than those in their age-matched WT counterparts (65.7 ± 8.1 CC1+ cells per field, P < 0.05 for 30-day-old mice; 44.7 ± 5.3 CC1+ cells per field, P < 0.05 for 7-month-old mice). It is important to note that, although the ventral white matter regions are smaller in the 30-day-old and 7-month-old CST-null mice, no axonal abnormalities, including axonal deterioration or loss, were observed until 7 months of age (Marcus et al., 2006). Even by 7 months of age, axonal loss was modest. Thus, we predict that the reduction in the white matter areas in the CST-null mice results from the significantly thinner myelin sheaths and reduced axonal caliber but not a reduction in the number of axons.
CST-Null Mice Exhibit Increased Cell Proliferation
To identify the mechanism responsible for the increased OL population in the CST-null mice, we assessed the extent of cellular proliferation via BrdU labeling. Proliferation in WT and CST-null mice was compared by quantifying the number of DAPI+/BrdU+ cells in the white matter of the cervical spinal cord. CST-null mice displayed a significant increase in the number of proliferative cells (165.9% ± 28.6%, P < 0.05) in the spinal cord white matter at PND 15 (Fig. 3). To determine the identity of these proliferating cells, we triply labeled spinal cord sections with DAPI, BrdU, and one of three cell specific markers, IBA1 (microglia), GFAP (astrocytes), or olig2 (oligodendrocytes). The percentages of BrdU-labeled cells did not differ for any of the three cell identifying markers when comparing the WT and CST-null mice. Approximately 4% of the BrdU+ cells were IBA1+ (not shown) in both the WT and the CST-null cords. Six and eight percent of the BrdU+ cells were GFAP+ in the WT and null tissues (Fig. 4A–C), respectively, whereas approximately 50% (49% and 45% in the WT and null samples, respectively) of the BrdU+ cells were immunolabeled with the olig2 antibody (Fig. 4D,E). Therefore, we conclude that the ventral columns of the CST-null mice exhibited significantly more proliferative activity and that the most frequently proliferating cell type was of the OL lineage.
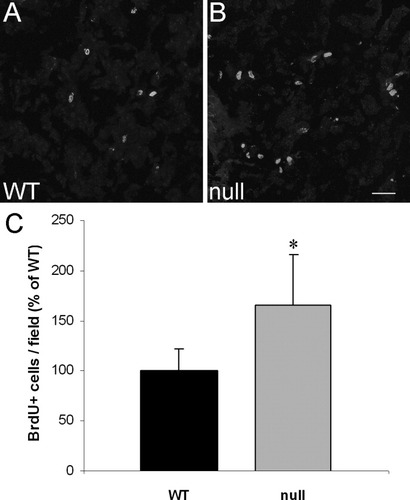
CST-null mice contain more proliferating cells in the cervical spinal cord than their WT littermates. Representative confocal images of spinal cord sections of 15-day-old WT (A) and CST-null (B) mice labeled in vivo with BrdU. Immunolabeling with anti-BrdU exhibited a similar distribution of BrdU+ cells in mice of the two genotypes; however, quantitative analysis revealed significantly more BrdU+ cells in the CST-null animals (C). Values indicate mean number of cells per microscopic field expressed as a percentage of WT ± percentage SD; n = 7 WT mice and 5 littermate CST-null mice. ☆P < 0.05. Scale bar = 50 μm.
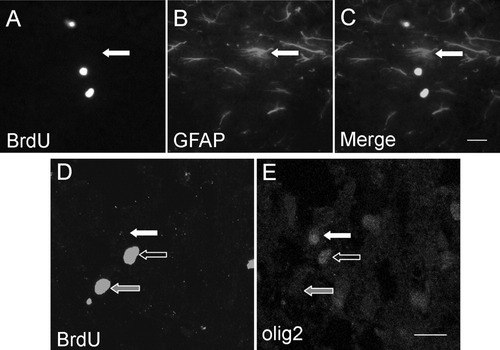
Oligodendrocyte-lineage cells, but not astrocytes, constitute the most prevalent proliferative cells in the spinal cord of the sulfatide-null and WT mice. A–C: Representative confocal image collected from a cervical spinal cord section of a 15-day-old CST-null mouse displays examples of BrdU+/GFAP− and BrdU−/GFAP+ cells. This labeling paradigm was used to calculate the percentage of BrdU+ cells that were astrocytes; 6% and 8% of the BrdU+ cells were GFAP+ in 15-day-old WT and CST-null mice, respectively. Although this is not shown, all analyzed sections were also labeled with DAPI to ensure accuracy of quantitation. D,E: Representative confocal image from a cryosectioned spinal cord of a 15-day-old CST-null mouse labeled for BrdU (D) and olig2 (E). Note that the black arrow outlined in white indicates a nucleus that is positively labeled for both BrdU and olig2. Such cells were identified as proliferating oligodendrocyte-lineage cells. The white arrows in D,E indicate a nucleus that is positively labeled for olig2 but lacks BrdU signal. These cells were identified as nonproliferating oligodendrocyte-lineage cells. Similarly, the gray arrow outlined in white signifies a cell classified as a proliferating, non-OL-lineage cell. With this scheme of cell classification, approximately 50% of the proliferating cells in the ventral columns of both the WT and the CST-null mice were identified as terminally differentiated OLs. Scale bars = 20 μm in C (applies to A–C); 20 μm in E (applies to D,E).
Olig2+ Cells Are More Abundant in CST-Null Mice
Because the percentage of proliferating cells that were in the OL lineage was similar between the genotypes but the population was significantly increased (Fig. 2), we reasoned that the CST-null mice would contain an increased population of immature OLs. For this study, the term immature OL is not used to identify a specific stage of OL differentiation but is used to refer to olig2+/CC1− cells. To test our hypothesis that the CST-null mice would contain more immature OLs, we first quantified the relative number of olig2+ cells in WT and CST-null mice at 15 days of age, and our data revealed significantly more olig2+ cells (129.6% ± 8.3%; P < 0.05) than the WT mice at 15 days of age. It should be noted that olig2 is also expressed in motor neurons and astrocytes (Masahira et al., 2006). Insofar as our analysis is limited to white matter tracts, neuronal cell body labeling with the olig2 antibody does not present a problem, and only a subset of astrocytes expresses olig2 (Masahira et al., 2006). Additionally, single labeling with the olig2 antibody has been successfully used as a marker for immature OLs (Nait-Oumesmar et al., 2007; Pernet et al., 2008).
Olig2 is also expressed by mature OLs (Ligon et al., 2004), so we triply labeled spinal cord sections with DAPI and the olig2 and CC1 antibodies to distinguish between immature (olig2+/CC1−) and mature (olig2+/CC1+) OLs (Fig. 5A,B). Based on the criterion that DAPI+/olig2+/CC1− cells are immature OLs, the CST-null mice contained significantly more immature OLs (170.1% ± 62.1% per microscopic field; P < 0.05) than their WT littermates (Fig. 5C).
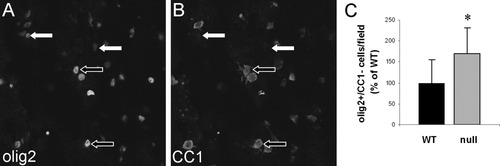
Fifteen-day-old CST-null mice contain more immature OLs than their WT littermates. A,B: Fluorescent images of the same microscopic field, with each panel displaying a single label of olig2 (A) or CC1 (B). Cells that are olig2+/CC1+ (black arrows outlined in white in A,B) were identified as mature OLs, whereas cells that were olig2+/CC1− were identified as immature OLs (white arrows in A,B). C: Significantly more immature OLs were observed in the ventral columns of the spinal cord in the CST-null mice than in the same region of the WT littermates (☆P = 0.038); n = 4 mice for both WT and CST-null.
Fewer OLs Undergo Apoptosis in the CST-Null Mice
Based on the above analyses, proliferation may play a role in initially establishing an enhanced population of OL lineage cells. However, Barres et al. (1992) reported that an excess of OL progenitors enters developing white matter tracts and that these “extra”, immature OLs are pruned through apoptosis. According to the axon contact-mediated survival paradigm (Barres et al., 1992, 1993a, b; Trapp et al., 1997), OLs that fail to establish sufficient axonal interactions undergo apoptosis, resulting in the survival of the precise number of myelinating cells required for adequate myelin formation. Because as many as 50% of the immature OLs that initially enter CNS white matter regions do not survive (Barres et al., 1992), the production of “extra” OLs may not be sufficient to maintain an increased population of mature OLs. This theme has previously been established by Calver et al. (1998), who showed that an overexpression of platelet-derived growth factor (PDGF) induced an increase in the OL population; however, these extra cells died, yielding the normal complement of OLs. From these findings, the authors concluded that survival controls were more influential than proliferation in determining the final OL population. Therefore, we reasoned that increased proliferative activity in the CST-null mutants must be accompanied by enhanced OL survival.
To test this hypothesis, we performed a TUNEL assay and subsequently stained spinal cord sections with DAPI. The number of DAPI+/TUNEL+ cells in the CST-null mice was 76.3% ± 15.7% of WT. Thus, at 15 days of age, the CST-null mice exhibited significantly fewer apoptotic cells than their WT littermates (P < 0.05). To compare the relative number of OLs that are TUNEL+, we triply labeled spinal cord sections with DAPI, the olig2 antibody, and the TUNEL reagent. The CST-null mice revealed 36.2% fewer (P < 0.05) TUNEL+/olig2+/DAPI+ cells than littermate WT mice (Fig. 6). Thus, in the absence of sulfatide, significantly fewer OL-lineage cells exhibited apoptotic activity, contributing to a sustained increase in the OL population.
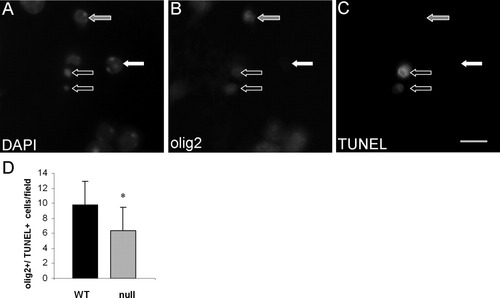
CST-null mice exhibit fewer apoptotic oligodendrocyte-lineage cells than littermate WT animals. Fluorescent images of the same microscopic field collected from the cervical spinal cord of a 15-day-old CST-null mouse reveal OL-lineage (olig2+; B) cells undergoing apoptosis (TUNEL reagent+; C). As for all labeling strategies employed in this study, DAPI (A) was used to counterstain nuclei. Note that the DAPI labeling of the two TUNEL+ cells (black arrows outlined in white) reveals condensed nuclei, a morphological characteristic consistent with apoptotic cells. Cells that were olig2+/TUNEL+ are indicated by black arrows with white outline; a cell that is olig2+/TUNEL− is indicated by a gray arrow with a white border, and a cell that is olig2−/TUNEL− is indicated by a white arrow. With this paradigm of triple labeling, the relative number of apoptotic oligodendrocytic cells was compared between CST-null and littermate WT mice. As shown in D, significantly fewer oligodendrocyte-lineage cells succumbed to apoptotic death in the CST-null mice, indicating increased OL survival in these mice. Values indicate the mean number of olig2+/TUNEL+ cells per microscopic field ± SD; n = 8 WT and 6 littermate CST-null mice. Scale bar = 10 μm.
CST-Null OLs Extend Fewer Processes In Vivo
As proposed by Barres et al. (1992, 1993a, b) and Trapp et al. (1997), immature OLs avoid developmental apoptosis by establishing axonal contact. The competition among these OLs plays an important role in determining the final number of myelin-forming cells maintained in the adult CNS. Thus, the number of processes extended per cell, which is presumably related to the number of axonal contacts that an OL can establish may be critical in finalizing the number of OLs in the mature CNS. Several studies have reported that sulfatide plays a role in regulating OL complexity with regard to process formation (Bansal et al., 1988; Dyer and Benjamins, 1990, 1991; Boggs and Wang, 2001), so we proposed that the CST-null OLs would extend fewer processes, thereby resulting in the observed significant increase in immature OLs capable of establishing axonal contact.
To determine whether the lack of sulfatide alters OL morphology, we employed electron microscopic analysis to compare the relative number of primary processes extended by 2-day-old WT and CST-null OLs in vivo. We determined that OLs from the CST-null mice extended significantly fewer primary processes (2.3 ± 1.0 primary processes per mutant OL; 89 cells collected from two mice) than OLs from littermate WT mice (3.4 ± 1.2 primary processes per WT OL; 91 cells collected from three mice; Fig. 7A,B; P < 0.05). Thus, as early as 2 days of age, the CST-null OLs extended approximately 33% fewer primary processes. By plotting a frequency distribution graph, we observed a dramatic leftward shift in the number of primary processes produced by the CST-null OLs compared with WT (Fig. 7C).
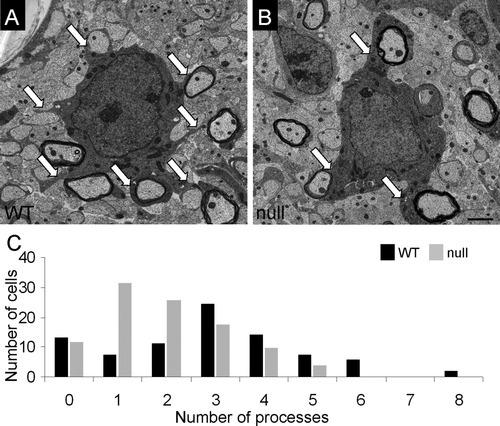
CST-null OLs extend fewer myelin-forming processes than their WT counterparts. Ultrastructural analysis of WT and CST-null OLs revealed that the CNS myelin-forming cells from the CST-null mice (B) extend significantly fewer primary processes than OLs from littermate WT animals (A; P = 0.05). C: Plotting process number against cell frequency for each genotype reveals a dramatic leftward shift for the CST-null OLs at PND 2. Note that the graph reveals that the mode of process number for the CST-null OLs is 1, which is substantially less than the mode for the WT OLs, which is 4; n = 91 cells collected from three WT mice and 89 cells collected from two CST-null mice. Scale bar = 2 μm.
To check the accuracy of these findings, we also analyzed OLs from 4-day-old mice. The data generated from the analysis of the 4-day-old mice confirmed our analysis of the 2-day-old mice. Ninety-six OLs from the three WT mice extended an average of 2.8 ± 1.0 primary processes per cell compared with 2.3 ± 1.0 primary processes extended by 74 OLs imaged from three CST-null mice (P = 0.05). By combining our findings from 2- and 4-day-old animals, 350 putative OLs from five CST-null and six WT mice were imaged and blindly assessed for primary process formation. Thus, our findings from both the 2- and the 4-day-old mice indicate that CST-null OLs extend significantly fewer primary processes. Unfortunately, our attempts to analyze older mice to determine whether process number per cell was altered with age were prevented by the presence of increasing numbers of myelinated axons abutting the OL somata, inhibiting accurate quantitation of process numbers. (Comparison of Fig. 1A with Fig. 1B depicts the difficulty described.)
DISCUSSION
Myelin galactolipids are early markers of terminal differentiation for OL-lineage cells (for review see Pfeiffer et al., 1993). Although expressed by other cell types (Pernber et al., 2002; Molander-Melin et al., 2004), these lipids are highly enriched in OL membranes, including the myelin sheath (Norton and Cammer, 1984). As a consequence of this early and abundant appearance, these lipids have been closely analyzed for possible roles in OL maturation and myelin formation (for review see Dupree and Popko, 1999). Here, we provide further evidence that sulfatide plays a role in regulating the OL population in vivo. We show that, in mice that lack the capacity to synthesize sulfatide, the number of mature OLs is increased and remains elevated through 7 months of age. We also demonstrate that this increased OL population is accompanied by both increased OL proliferation and decreased OL apoptosis. As a potential mechanistic explanation for the increased population, we also demonstrate that CST-null OLs extend fewer myelin-forming processes, facilitating the survival of more myelinating cells, which is required to meet CNS myelination demands.
Enhanced Number of OLs in Adult Mice Results From Increased Survival
Neonatal mice lacking galactolipids contain significantly more OLs than their WT littermates (Marcus et al., 2000; Hirahara et al., 2004); therefore, we concentrated our efforts on the OL populations in adolescent and adult mutant animals. Our findings demonstrate that the increased population of OLs persists through at least 7 months of age. The sustained increase in the number of OLs raises the question of how this enhanced number of OLs is achieved and maintained.
Previous studies have demonstrated that sulfatide is a negative regulator of OL development (Bansal et al., 1999; Hirahara et al., 2004), insofar as the absence of sulfatide results in an enhanced rate of cellular maturation, facilitating the increase in the number of adult OLs observed in immature galactolipid null animals. Here we provide complementary evidence that another factor that leads to an increased OL population in the CST-null mice is increased survival.
One method by which an OL population is expanded is through proliferation (Kierstead and Blakemore, 1999; Miller, 2002; Rowitch, 2004; Menn et al., 2006; Vana et al., 2007). We report that equivalent percentages of the proliferating cells in the WT and CST-null are OLs; however, the numbers of OLs, both mature and immature, are significantly increased in the CST-null mice compared with the littermate WT animals. Therefore, more OL-lineage cells are produced in the CST-null mice.
Because an excess of OLs normally enters white matter regions of the CNS only to die as a consequence of insufficient trophic support (Barres et al., 1992, 1993a, b), increased proliferation without a mechanisms to support these excess cells would not result in more OLs in adult animals. Support for this hypothesis has previously been presented by Calver et al. (1998) using mice that overexpress PDGF. Thus, we predicted that increased proliferation without corresponding increase in trophic factor availability would merely result in increased cell pruning through apoptosis and that any increase in OL population observed in immature animals would be transient, reminiscent of the PDGF-overexpressing mice (Calver et al., 1998). In contrast, if more cells are capable of acquiring the necessary trophic factors, then an increased OL population could persist. A current hypothesis is that trophic OL support is obtained through axonal contact (Barres et al., 1992, 1993a, b), so we proposed that more OL-lineage cells are able to establish and maintain axonal contact in the mutant mice. Our hypothesis is strongly supported by our ultrastructural analysis indicating that the CST-null OLs extend fewer processes per cell. With each cell extending fewer processes, more cells make sufficient axonal contact and avoid apoptosis. Because our previous work 1) did not demonstrate an increase in the number of unmyelinated axons in adult CST-null mice (Marcus et al., 2006), 2) revealed no axonal loss until 7 months of age (Marcus et al., 2006), and 3) showed normal numbers of nodal sodium channel clusters through 4 weeks of age (Ishibashi et al., 2002), we conclude that the CST-null mice do not form fewer myelin segments than their WT littermates. With equal demand for myelin formation combined with retarded capacity for process extension, more mutant cells are required to myelinate the CNS.
Is Lipid Production a Limiting Factor for Myelin-Forming Process Formation?
Although the CST-null OLs form fewer processes in vivo (Fig. 7), the pathologic mechanism responsible for this morphologic change is not known. The simplest explanation is that these lipid-deficient OLs are limited in their ability to produce membrane. Consistent with this possibility, we have shown that the CST-null mice synthesize significantly less (thinner) myelin (Marcus et al., 2006); however, sulfatide constitutes only between 4% and 7% of the dry weight of myelin lipids (Norton and Cammer, 1984). Thus, it is unlikely that the deficit in process formation results simply from a bulk loss of sulfatide. Instead, it is more probable that a sulfatide-mediated signaling event is compromised. This possibility has been previously presented by Bansal et al. (1999), who proposed that sulfatide facilitates a transmembrane signaling cascade required for proper regulation of OL development. Sulfatide is concentrated in the outer leaflet of the OL membrane (Cestaro et al., 1984) and thus is positioned to function as a ligand to “transmit environmental information” (Bansal et al., 1999). Although the precise cellular response that is induced by galactolipid activation is not known, these lipids have been implicated in playing a role in the regulation of microtubule organization within OLs through a second-messenger-mediated mechanism (Dyer and Benjamins, 1990, 1991). In the absence of sulfatide, microtubule organization may be compromised, which could account for not only the reduced number of OL processes but the altered morphology of the processes that are formed (i.e., thick processes that retain cytoplasm; see Fig. 7B).
Although our electron microscopic data suggest that the CST-null OLs extend fewer myelin-forming processes (Fig. 7), previous work by Bansal et al. (1999) and Hirahara et al. (2004) demonstrated that cultured OLs from the CST-null and CGT-null mice produce membrane networks that are at least comparable to the networks of their cultured WT counterparts. At present, the difference between the in vitro and in vivo data is difficult to reconcile. It is possible that the signals from other cell types negatively influence OL process formation and that this negative regulation is modulated by sulfatide. In the in vitro studies, which lack certain cell types, most notably neurons, the negative modulation is absent, and thus process or membrane formations are approximately equal with or without sulfatide. In contrast, in the in vivo studies, the negative influence from the other cell types is present and cannot be modulated in the absence of sulfatide.
Is Sulfatide Responsible for the Altered OL Population?
Because it is the most prominent lipid not synthesized by CST-null OLs, we have concentrated our discussion on sulfatide. However, the production of the prooligodendroblast antigen (POA; Bansal et al., 1992) is also CST dependent (Hirahara et al., 2004). Hence the increased cellular proliferation and survival observed in the CST-null mice could also be attributed to the lack of a lipid other than sulfatide. Little is known of the POA lipid other than it is sulfated, it is expressed prior to either sulfatide or galactocerebroside (Bansal et al., 1992), and it is not synthesized in either the CGT-null (Bansal et al., 1999) or the CST-null mice (Hirahara et al., 2004). However, because it is known to be missing in the CST-null animals, its potential role in the regulation of the OL population should not be dismissed.
Although a role for POA should not be ignored, the temporal expression of sulfatide coincides with the loss of mitotic activity of the developing OL (Gard and Pfeiffer, 1990; Pfeiffer et al., 1993). Thus, it is possible that the onset of sulfatide synthesis triggers the inhibition of OL proliferation. It is also possible that the link between the loss of sulfatide and the altered OL population is indirect. Axonal contact appears to play a role in regulating the OL population (Barres et al., 1992, 1993a, b; Trapp et al., 1997). Insofar as immature CST-null OLs form fewer processes, axonal demand for OL production may remain elevated, resulting in continued proliferation. In this scenario, the absence of sulfatide would result in altered cytoskeletal organization (Dyer and Benjamins, 1990, 1991) and reduced process formation (Fig. 7), which would stem the rate of myelination but would also facilitate sustained axonal influence responsible for continued OL proliferation. If this scenario is valid, then reduced myelin formation, independent of sulfatide, would also exhibit increased OL populations. Interestingly, Bu et al. (2004) reported that the number of immature and mature OLs is significantly increased in shiverer. Presently, the mechanisms and the molecules that regulate OL production, differentiation, survival, and myelin formation have not been fully delineated, and future studies are required to decipher these complex events.
In summary, our data demonstrate that the increased number of OLs reported by Hirahara et al. (2004) in 7-day-old CST-null mice is also maintained in the adult null animals. Furthermore, we provide evidence that increased proliferation contributes to the production of “extra” OLs in young CST-null mice. Although “extra” OLs are generated, we propose that enhanced survival is responsible for the sustained increased number of OL, because a greater proportion of these cells avoids pruning as a consequence of less complex cellular morphology. If our hypothesis is correct, the manipulation of sulfatide production may provide a means to enhance OL repopulation and ultimately optimize myelin repair following a demyelinating event.
Acknowledgements
The authors thank Ms. Judy Williamson for her technical assistance with the preparation of the light and electron microscopic samples and Drs. John Alberta and Chuck Stiles for the generous gift of the olig2 antibody. Microscopy was performed at the VCU Department of Anatomy and Neurobiology Microscopy Facility, which is supported in part with funding from NIH-NINDS center core grant 5P30NS047463.




