Myelin-associated glycoprotein and its axonal receptors
Abstract
Myelin-associated glycoprotein (MAG) is expressed on the innermost myelin membrane wrap, directly apposed to the axon surface. Although it is not required for myelination, MAG enhances long-term axon–myelin stability, helps to structure nodes of Ranvier, and regulates the axon cytoskeleton. In addition to its role in axon–myelin stabilization, MAG inhibits axon regeneration after injury; MAG and a discrete set of other molecules on residual myelin membranes at injury sites actively signal axons to halt elongation. Both the stabilizing and the axon outgrowth inhibitory effects of MAG are mediated by complementary MAG receptors on the axon surface. Two MAG receptor families have been described, sialoglycans (specifically gangliosides GD1a and GT1b) and Nogo receptors (NgRs). Controversies remain about which receptor(s) mediates which of MAG's biological effects. Here we review the findings and challenges in associating MAG's biological effects with specific receptors. © 2009 Wiley-Liss, Inc.
Myelination of axons provides for rapid nerve conduction that is essential to vertebrate nervous system function. In addition to providing segmental insulation, myelin enhances axon survival, regulates the axon cytoskeleton, directs the distribution of molecules at nodes of Ranvier, and inhibits axon regeneration after injury (Hsieh et al., 1994; Poliak and Peles, 2003; Edgar and Garbern, 2004; Sandvig et al., 2004). Molecules on the periaxonal surface of myelin, directly apposed to the axon surface, engage complementary receptors on the axon to initiate myelin–axon cell–cell interactions. Knowledge of the myelin molecules and axon receptors responsible for the nurturing and inhibiting effects of myelin on axons may provide insight into the basis of dysmyelinating disorders and provide lead molecules to enhance axon regeneration after injury or disease. The variety of molecules responsible for myelin–axon interactions must eventually be integrated into a unified cell–cell interaction model. Defining the structures, functions, and mechanisms of action of axon regulatory molecules on myelin and their receptors on axons is a necessary prerequisite to that larger goal.
One cell recognition molecule that regulates the interactions of myelin and axons is myelin-associated glycoprotein (MAG), which is expressed on periaxonal myelin membranes in both the central nervous system (CNS) and the peripheral nervous system (PNS). Mice lacking MAG express abundant myelin but suffer long-term axon degeneration, altered axon cytoskeletal structure, and altered distribution of channels and adhesion molecules at nodes of Ranvier (Fruttiger et al., 1995; Yin et al., 1998; Marcus et al., 2002). MAG also is a major inhibitor of axon regeneration after injury (Filbin, 2003; He and Koprivica, 2004; Sandvig et al., 2004). MAG's biological effects are initiated when MAG binds to complementary receptors on the apposing axon surface. Two major functional MAG receptor families have been identified: sialoglycans (particularly the major brain gangliosides GD1a and GT1b) and members of the Nogo receptor (NgR) family. Published studies conflict with regard to the roles of these receptor families in mediating MAG functions. Here we review the genetic, biochemical, and cell biological data that support the functional importance of each MAG receptor and the challenges that remain in integrating these data into a consistent model of MAG action.
MAG STRUCTURE AND FUNCTION
MAG is a cell surface member of the immunoglobulin-like (Ig) superfamily, with five extracellular Ig-like domains, a single transmembrane domain, and one of two alternatively spliced short cytoplasmic tails (Trapp, 1990). It is coded by a single gene that is conserved among vertebrates (Arquint et al., 1987); human and rodent MAGs are 95% identical at the amino acid level over the entire extracellular expressed domain (five Ig-like domains, ∼500 amino acids). MAG is produced only in myelinating glial cells: oligodendrocytes in the CNS and Schwann cells in the PNS. Although it is a quantitatively minor myelin protein (1% of CNS and 0.1% of PNS myelin protein), MAG is not expressed uniformly throughout myelin. In the CNS, MAG is located on the innermost (periaxonal) noncompacted myelin wrap (Bartsch et al., 1989). In the PNS, MAG is found on periaxonal myelin and on other noncompacted myelin (paranodal loops, Schmitt-Lanterman incisures, and the mesaxon; Trapp et al., 1989).
MAG supports axon–myelin stability. MAG was first hypothesized to regulate myelin–axon interactions based on its in vivo periaxonal location (Trapp et al., 1989) and its effects in cell culture (Johnson et al., 1989; Sadoul et al., 1990; McKerracher et al., 1994; Mukhopadhyay et al., 1994). Its role in supporting myelin–axon stability was established by using mice engineered to lack the Mag gene (Li et al., 1994; Montag et al., 1994). Mag null mice produce abundant myelin. However, they have more unmyelinated axons in the CNS (Bartsch et al., 1997), and older mice exhibit significant axon degeneration (Fruttiger et al., 1995; Fujita et al., 1998). Notably, MAG-deficient mice exhibit late-onset progressive axonal atrophy and increased Wallerian degeneration in both the CNS and the PNS (Bjartmar et al., 1999; Schachner and Bartsch, 2000; Pan et al., 2005).
MAG regulates the axon cytoskeleton. Myelinated segments of axons have increased intra-axonal neurofilament phosphorylation, increased neurofilament spacing, and increased axon caliber compared with unmyelinated segments of the same axons (Hsieh et al., 1994). In Mag null mice, however, myelinated axon segments have ultrastructural characteristics of unmyelinated segments, with less neurofilament phosphorylation, decreased neurofilament spacing, and decreased axon caliber compared with myelinated axons in wild-type mice (Yin et al., 1998; Dashiell et al., 2002; Kumar et al., 2002). The relevance of MAG regulation of the axon cytoskeleton in humans is apparent in nerve biopsy specimens from patients with anti-MAG neuropathy (Lunn et al., 2002), in which myelinated axons had decreased neurofilament spacing compared with unaffected individuals.
MAG participates in defining the distribution of axon molecules at nodes of Ranvier (Marcus et al., 2002). Wild-type nodes of Ranvier are distinguished by three distinct regions: the node, where sodium channels accumulate; the adjacent paranode, marked by the presence of the adhesion molecule Caspr; and the juxtaparanode, characterized by potassium channels and the adhesion molecule Caspr2 (Poliak and Peles, 2003). In MAG null mice, the proper distribution of these molecules is delayed, with clear overlap of the paranodal and juxtaparanodal regions (Marcus et al., 2002).
Interest in MAG intensified when it was found to be an axon regeneration inhibitor. At sites of CNS injury, molecules on residual myelin membranes direct axons to halt elongation. When proteins were solubilized from myelin and separated chromatographically, certain fractions inhibited neurite outgrowth in vitro (McKerracher et al., 1994). The major inhibitory peak coincided with the elution of MAG, and purified MAG was found to inhibit axon outgrowth. Neurons seeded on monolayers of fibroblasts engineered to express a recombinant form of MAG also had reduced neurite outgrowth (Mukhopadhyay et al., 1994). Studies using different types of nerve cells established MAG as an axon outgrowth inhibitor and implicated receptors, signaling pathways, and signaling pathway modulators (for review see Yiu and He, 2006). Studies in vivo demonstrate a modest but significant enhancement of axon regeneration in the absence of MAG; Mag null mice but not wild-type mice extended small numbers of long axons beyond an experimental lesion in the corticospinal tract (Li et al., 1996). Mice lacking MAG also demonstrate an increase in axon regeneration along residual peripheral myelin sheathes in mice that fail to clear myelin efficiently from PNS injury sites (Schäfer et al., 1996). The modest degree of axon regeneration in Mag null mice is similar to that in other animal models in which one of the several axon regeneration inhibitors is ablated or blocked. Application of recombinant soluble MAG to a transected peripheral nerve inhibited axon sprouting (Tomita et al., 2007). Models of axon regeneration after injury include MAG as a major contributor to the inhibitory environment in the CNS (Sandvig et al., 2004; Schwab, 2004; Yiu and He, 2006).
MAG RECEPTORS
MAG binds to complementary receptors on axons to initiate its stabilizing and regulatory effects. Although the number and nature of MAG receptors have yet to be fully established, two functional receptor families have emerged: sialoglycans and Nogo receptors.
Sialoglycans are glycoproteins and glycolipids bearing sugar chains capped with sialic acid (N-acetylneuraminic acid; NeuAc). MAG was first hypothesized to bind to sialoglycans based on its sequence similarity to a family of sialic acid binding proteins known as Siglecs (sialic acid binding Ig-family lectins; Kelm et al., 1994; Varki and Angata, 2006). In a confirmation of this hypothesis, recombinant MAG was found to bind to cells via their cell surface sialic acids. When sialic acids were removed from cells with the enzyme sialidase, MAG binding was lost, and, when sialic acids were reconstituted using linkage-specific sialyltransferases, MAG binding was restored (Kelm et al., 1994). These studies established MAG as a sialic acid binding protein and also hinted at the sialoglycan receptors for MAG, in that the preferred reconstituted binding target was the glycan sequence “NeuAc α2–3 Gal β1–3 GalNAc.” This terminal sequence is abundant on two prominent sialoglycans in the brain, gangliosides GD1a and GT1b (Fig. 1).
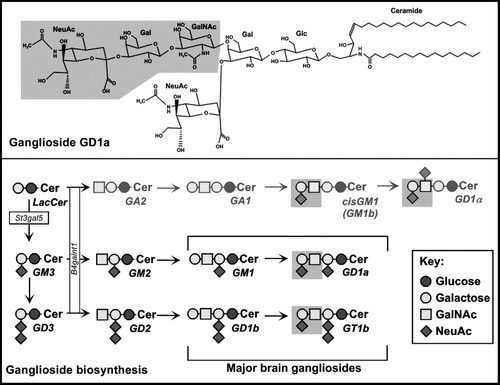
Ganglioside structure and metabolism. Top: The structure of GD1a is shown with the MAG-binding determinant (NeuAc α2–3 Gal β1–4 GalNAc) shaded. Bottom: Biosynthetic pathways to the major brain gangliosides. The MAG-binding determinant is shaded, and the two glycosyltransferases discussed in the text, St3gal5 and B4galnt1, are shown. St3gal5 null mice lack all major brain gangliosides but instead synthesize an equivalent amount of the rare gangliosides cisGM1 and GD1α, both of which carry the MAG-binding determinant.
Gangliosides are sialylated glycosphingolipids that are major cell surface determinants on vertebrate nerve cells (Schnaar, 2007). Mammals and birds express the same four major brain gangliosides (Fig. 1), which together make up 97% of gangliosides in the human brain. Direct tests of MAG binding to gangliosides revealed that GD1a and GT1b, two major brain gangliosides that bear the “NeuAc α2–3 Gal β1–3 GalNAc” terminal structure, are potent receptors for MAG (Yang et al., 1996). In contrast, GM1 and GD1b, gangliosides that lack that terminal structure, failed to bind MAG. This led to the hypothesis that GD1a and GT1b mediate MAG's axon stabilization and axon outgrowth inhibitory functions.
At the time gangliosides were being evaluated as potential MAG receptors, a new axon-regulatory receptor was discovered, the Nogo receptor (NgR1; McGee and Strittmatter, 2003). NgR1 was discovered as the high-affinity receptor for Nogo, another axon regeneration inhibitor (Fournier et al., 2001). Subsequently, NgR1 was discovered to bind to two other axon regeneration inhibitors, MAG and oligodendrocyte-myelin glycoprotein (OMgp), which have diverse structures unrelated to Nogo (Lauren et al., 2007). This led to the hypothesis that NgR1 was a common unifying receptor for myelin-derived axon regeneration inhibitors.
NgR1 is a glycosylphosphatidylinositol (GPI)-anchored cell surface glycoprotein with a structure dominated by a leucine-rich repeat protein–protein interaction domain (Fig. 2; Barton et al., 2003; He et al., 2003). Two additional NgR family members (NgR2 and NgR3) were subsequently discovered in mammals by sequence comparisons with NgR1 (Barton et al., 2003; Pignot et al., 2003). MAG binds to NgR1 and NgR2 with very high affinity (2–10 nM KD) but does not bind to NgR3 (Venkatesh et al., 2005; Lauren et al., 2007). The discovery of multiple binding partners for MAG naturally led to debate concerning which of these are functional receptors mediating MAG's effects on axon structure, axon stability, and inhibition of axon regeneration.
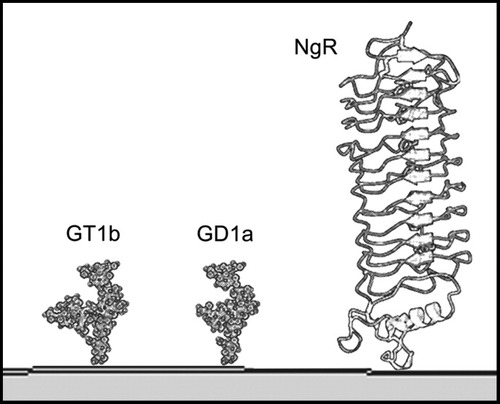
Structures of MAG receptors GT1b, GD1a, and NgR1. Only the portions of the molecules that extend from the membrane into the extracellular space are shown. Each MAG receptor is anchored to the cell membrane by a lipid moiety that is not shown (ceramide for GT1b/GD1a and phosphatidylinositol for NgR1). The relative sizes are accurate based on NMR structures of gangliosides (Sonnino et al., 2007) and crystallographic analyses of NgR (He et al., 2003). The gangliosides extend 2.4 nm from the cell surface, with their MAG-binding determinants at their outermost extent, whereas NgR is 8 nm in length, with centrally located MAG-binding residues (Barton et al., 2003; Lauren et al., 2007).
MAG RECEPTORS IN AXON STRUCTURE AND STABILITY
Although nervous system development and axon myelination are grossly normal in Mag null mice, the mice display signs of dysmyelination, including progressive axon degeneration, altered axon cytoskeletal architecture, and altered nodes of Ranvier (for review see Quarles, 2007). Although these long-term pathologies are difficult to model in vitro, examination of mice engineered to lack MAG receptors can provide correlative data to address the roles of those receptors in MAG's biological effects.
Studies of mice with altered ganglioside expression are consistent with a role of the MAG-binding ganglioside terminus (NeuAc α2–3 Gal β1–3 GalNAc), expressed on GD1a and GT1b, in axon–myelin interactions (Sheikh et al., 1999; Pan et al., 2005; Susuki et al., 2007). Ganglioside glycans are biosynthesized stepwise by specific glycosyltransferases (Fig. 1). Genetic ablation of a ganglioside glycosyltransferase results in loss of its downstream products and buildup of the upstream intermediate(s) such that the concentration of brain gangliosides stays the same despite the change in glycan profile. For example, B4galnt1 null mice, which lack the N-acetylgalactosaminyltransferase responsible for ganglioside extension, lack all of the major brain gangliosides (including GD1a and GT1b) but instead express an equivalent total amount of the simpler structures GD3 and GM3. MAG does not bind to GD3 and binds poorly to GM3 (Collins et al., 1997). The phenotype of B4galnt1 null mice indicates a problem in axon–myelin interactions. Although the mice are born in Mendelian ratios and are grossly normal (Takamiya et al., 1996), as they age they display peripheral and central axon degeneration, reduced neurofilament spacing, reduced axon caliber, and progressive motor behavioral deficits (Sheikh et al., 1999; Chiavegatto et al., 2000). A direct comparison of Mag null, B4galnt1 null, and double null mice bred on the same strain background revealed striking quantitative similarities in their phenotypes, consistent with a similar biological role for MAG and the MAG-binding ganglioside terminal sequence in axon–myelin stability and axon cytoarchitecture (Pan et al., 2005). Studies using an independently derived strain of B4galnt1 null mice demonstrated that, as with Mag null mice, the distribution of ion channels and adhesion molecules was disrupted at nodes of Ranvier. Paranodal and juxtaparanodal markers failed to segregate completely, and sodium channel clusters were broadened. Consistently with these molecular anatomical observations, nerve conduction velocity was also impaired in B4galnt1 null mice (Takamiya et al., 1996; Susuki et al., 2007). Notably, MAG protein levels fell progressively and selectively in the brains of B4galnt1 null mice (Sun et al., 2004), such that they were reduced to half of wild-type levels at 6 months of age despite the presence of equivalent MAG mRNA levels and equivalent levels of other myelin proteins. These data provide indirect evidence for a physical/functional relationship between MAG and the MAG-binding glycan terminus on gangliosides in vivo. They are consistent with the hypothesis that MAG on myelin binds to ganglioside receptors on axons to initiate some of its biological effects.
The phenotypes of mice null for other ganglioside glycosyltransferases provide additional support for the hypothesis that MAG function is related to ganglioside structure. Mice that retain MAG-binding gangliosides have a normal axon–myelin phenotype, whereas those lacking MAG-binding gangliosides do not. A striking example is mice with a disrupted St3gal5 gene (Yamashita et al., 2003), a mutation intended to knock out brain ganglioside biosynthesis by blocking the addition of the first sialic acid to lactosylceramide to make GM3 (see Fig. 1). Instead, these mice expressed brain gangliosides bearing rare glycan structures, such as GD1α (Fig. 1), an excellent MAG-binding ganglioside (Collins et al., 1999). These mice had normal axon–myelin interactions. In contrast, St2gal5/B4galnt1 double null mice, which lacked all MAG-binding brain gangliosides, displayed axon degeneration and severely disrupted myelin–axon interactions (Yamashita et al., 2005).
Although mice engineered to lack NgR1 (Kim et al., 2004; Zheng et al., 2005) and NgR2 (Williams et al., 2008) have been generated, thorough studies of axon–myelin stability in these mice has not yet been reported. Gross brain and myelin morphology appeared normal in Ngr1 null mice. They displayed mild open-field behavior differences and a modest but significant decrease in motor coordination compared with wild-type mice. Whether this reflects a role for NgR in establishment or maintenance of axon–myelin interactions awaits further analysis of axon structure and stability, as well as independent neuronal phenotypes (Lee et al., 2008), in Ngr mutant mice.
GANGLIOSIDES AS MAG RECEPTORS IN AXON REGENERATION
When MAG was identified as an axon regeneration inhibitor (McKerracher et al., 1994; Mukhopadhyay et al., 1994), attention turned to identification of its functional receptors on axons, with the aim of revealing new targets for therapeutic intervention. The goal of finding a single receptor or single pathway for MAG inhibition has proved elusive, generating what at times appear to be conflicting data. In part, this may be due to the use of different tools. The intention of this Review is to analyze the data from a unifying perspective that incorporates the complexity of the interaction between MAG and its neuronal receptors.
Based on MAG's characteristics as a Siglec family member, sialoglycans were evaluated as MAG receptors. Enzymatic desialylation partially reversed MAG inhibition of axon outgrowth when cerebellar granule neurons (CGN) were plated on a monolayer of fibroblasts engineered to express MAG (DeBellard et al., 1996). Adhesion of various nerve cell types to microwells coated with a chimeric soluble form of MAG (MAG-Fc) was also reversed by desialylation and, depending on the cell type, either partially or nearly completely reversed by protease (DeBellard et al., 1996). This was interpreted to implicate a sialoglycoprotein as a functional MAG receptor but, in the light of more recent data, might also have indicated the presence of multiple receptors, sialoglycans, and proteins. The differential effects of protease on MAG-mediated adhesion by CGN (partial reversal) and dorsal root ganglion neurons (DRGN; near-total reversal) might have been an early indication that different nerve cell types use different MAG receptors. Subsequent studies by the same group concluded that desialylation of CGNs blocked inhibition of axon outgrowth induced by MAG-Fc (Tang et al., 1997b). These data implicated sialoglycans as functional MAG receptors in axon outgrowth inhibition. If this is the case, modifying MAG to impair its sialic acid binding would block its ability to inhibit axon outgrowth. The results of these experiments, reported by two independent groups, were more complex than anticipated (Tang et al., 1997a; Vinson et al., 2001).
Siglec family members share sequence similarity in their N-terminal Ig-like domains, with conservation of an arginine residue that engages the sialic acid (Varki and Angata, 2006). When that arginine was mutated in MAG-Fc (R118A), the resulting soluble chimeric MAG neither bound CGNs nor inhibited their axon outgrowth (Tang et al., 1997a). However, the same MAG mutant expressed on fibroblasts or Schwann cells inhibited axon outgrowth (Tang et al., 1997a). These and related data led the authors to propose that MAG had a sialic acid binding site on its N-terminal Ig-like domains and a separate axon inhibition site that required its C-terminal Ig-like domains. Whereas a soluble form of MAG required the sialic acid binding domains to engage neurons, the authors concluded, the membrane-bound form did not. This interpretation was complicated by the subsequent finding (Vinson et al., 2001) that the same R118A mutant of MAG-Fc retained some of its axon outgrowth inhibitory activity when tested on hippocampal neurons (HNs) and that the residual inhibition was reversed by sialoglycan inhibitors, including gangliosides GD1a and GT1b. This opened the prior data to an alternate interpretation, that R118A MAG retained reduced sialic acid binding affinity and, when expressed at high density on cell surfaces, inhibited axon outgrowth through a sialic acid binding mechanism.
A potential functional role for gangliosides as MAG receptors in axon outgrowth inhibition was supported by the finding that cross-linking GD1a and GT1b on HNs and CGNs inhibited axon outgrowth and that this inhibition was upstream of RhoA activation (Vinson et al., 2001). These findings were confirmed and extended by using genetic, biochemical, and biosynthetic methods to reduce ganglioside expression in CGNs, each of which impaired or blocked MAG inhibition of axon outgrowth (Vyas et al., 2002). Although these results support a role for gangliosides as functional receptors for MAG-mediated axon outgrowth inhibition, they did not explain growing evidence, from other studies, for MAG receptors that had properties inconsistent with those of gangliosides.
NOGO RECEPTORS AS MAG RECEPTORS IN AXON REGENERATION
Studies from two groups reported that the Nogo receptor NgR1 is a MAG receptor mediating inhibition of neurite outgrowth (Domeniconi et al., 2002; Liu et al., 2002). MAG binds to NgR1 with remarkably high affinity (KD ∼10 nM), and MAG binding to NgR1 was insensitive to treatment with sialidase but was sensitive to treatment with phosphatidylinositol-specific phospholipase C (PI-PLC), which releases GPI-linked proteins. MAG-mediated inhibition of neurite outgrowth was sensitive to PI-PLC and anti-NgR antibody. A third axon regeneration inhibitor, oligodendrocyte-myelin glycoprotein (OMgp), was also found to bind to NgR1 and block axon outgrowth (Wang et al., 2002), leading to a unifying hypothesis that implicated a single receptor, NgR1, as the functional target for three different axon regeneration inhibitors (McGee and Strittmatter, 2003).
Subsequent studies challenged the single-NgR1-pathway axon outgrowth-inhibition model. Myelin-mediated axon outgrowth inhibition was not reduced in CGN and DRGN isolated from mice genetically engineered to lack NgR1 (Zheng et al., 2005). The single-NgR1-pathway model also appears to conflict with data that established a role for sialoglycans, and especially gangliosides GD1a and GT1b, in axon outgrowth inhibition (Vyas et al., 2002). Integrating these different data sets remains a challenge. In an effort toward this goal, evidence for a direct association of ganglioside GT1b and NgR1 was reported (Williams et al., 2008). In contrast to prior studies (Domeniconi et al., 2002; Liu et al., 2002), MAG binding to NgR1 on cells was found to be sialidase sensitive (Williams et al., 2008), and biophysical methods demonstrated a direct GT1b-NgR1 association. Addition of NgR1-related peptides or use of neurons from NgR1 null mice attenuated axon outgrowth inhibition induced by cross-linking GT1b or by MAG-Fc. Taken together, these data support a model in which GT1b and NgR1 cooperate to support MAG-mediated axon outgrowth inhibition. This model is consistent with some axon outgrowth-inhibition studies but is not supported by other studies in which independent rather that cooperative effects of agents that disrupt gangliosides and NgRs were found (see below). Adding a further level of complexity to MAG-mediated axon inhibition, NgR2 was found to bind to MAG with comparable affinity as NgR1 (Lauren et al., 2007) and in a sialic acid-dependent manner (Venkatesh et al., 2005). The relative roles of NgR1 and NgR2 in MAG-mediated inhibition have yet to be fully established. In one study, CGN from NgR1 null mice were less sensitive to axon outgrowth inhibition by MAG-Fc, whereas CGN from NgR2 null mice retained MAG sensitivity equal to that of wild-type CGN (Williams et al., 2008). In another study, however, CGN from NgR1 null mice were equally sensitive to inhibition by MAG (expressed on fibroblasts), and RNAi knockdown of NgR1 in cortical neurons did not release them from MAG-mediated inhibition (Chivatakarn et al., 2007).
The question of whether sialoglycans or NgRs are capable of or required for MAG-mediated axon outgrowth inhibition remains contentious. Different groups report either complete or no sialidase sensitivity of MAG-mediated axon inhibition (Domeniconi et al., 2002; Liu et al., 2002; Vyas et al., 2002). Site-directed mutagenesis and domain swap studies argue that the sialic acid binding site of MAG is not responsible for its axon outgrowth inhibition (Tang et al., 1997a; Cao et al., 2007), but other groups argue that mutant forms of MAG retain sialic acid-dependent inhibition (Vinson et al., 2001). Some groups find that NgR family member binding is sialic acid dependent, whereas others do not (Domeniconi et al., 2002; Liu et al., 2002; Venkatesh et al., 2005; Williams et al., 2008), and cells from mice lacking NgRs either do or do not retain MAG-mediated inhibition (Zheng et al., 2005; Williams et al., 2008). How can these different data sets be resolved? Although the answer is not yet clear, some recent studies begin to address this question.
MULTIPLE MAG RECEPTORS INHIBIT AXON OUTGROWTH
Apparent conflicting data concerning MAG receptors may, in part, be due to the use of different nerve cells, different forms of MAG, or different outcome measures. To the extent that these can be teased apart and resolved, a more complete model of MAG-mediated axon outgrowth receptors may emerge. Recent studies have begun to do just that.
In one set of studies (Venkatesh et al., 2007), MAG-mediated axon outgrowth inhibition of CGNs grown on MAG-expressing fibroblasts was found to be attenuated by sialidase, whereas inhibition of retinal ganglion neuron (RGN) outgrowth was sialidase insensitive. However, when RGNs from NgR1 null mice were used, sialidase significantly reversed MAG-mediated outgrowth inhibition. These findings imply that different cells may use different MAG receptors and that the same cells may use redundant MAG receptors. These findings were confirmed and extended in studies that dissected out the role of NgRs and gangliosides in MAG-mediated axon outgrowth inhibition of CGN, DRGN, and hippocampal neurons (Mehta et al., 2007). By using surfaces adsorbed with native MAG to inhibit outgrowth, MAG-mediated inhibition of CGN axon outgrowth was completely reversed by sialidase or by an inhibitor of ganglioside biosynthesis (P4) but was unaffected by PI-PLC or a peptide inhibitor of MAG-NgR binding (NEP1-40). In contrast, DRGN outgrowth inhibition was largely reversed by PI-PLC and NEP1–40, only modestly reversed by sialidase and P4, and completely reversed by combinations of sialidase and PI-PLC or NEP1–40 and P4 in an additive manner. Inhibition of DRGN outgrowth by a soluble form of native MAG (dMAG) was completely reversed by PI-PLC or NEP1–40, whereas dMAG inhibition of CGN outgrowth was completely reversed by sialidase or P4. Hippocampal neuron inhibition was robustly reversed by both sialidase and PI-PLC. Together these data imply that the NgR and ganglioside pathways can act independently and additively and are differentially used by different nerve cell types and different forms of MAG (Fig. 3). Whether the two pathways interact directly, as suggested in other studies (Williams et al., 2008), has yet to be fully explored.
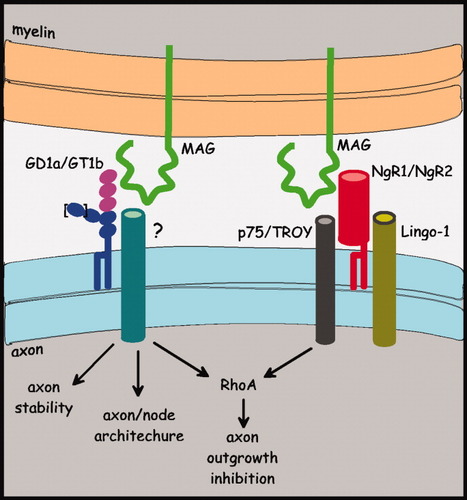
Dual MAG receptor model. MAG is envisioned as interacting independently with gangliosides (GD1a or GT1b, MAG-binding determinant highlighted) and with NgRs (NgR1 or NgR2). In each pathway, MAG engagement results in transmembrane signals. For example, NgR1 interacts with transmembrane signaling molecules p75NTR and Lingo-1 to transduce a signal that results in RhoA activation and inhibition of axon outgrowth. Gangliosides may signal via some of the same components or via as yet undefined components as shown, to activate RhoA, modulate axon and node of Ranvier structures, and stabilize axons. The proximal ganglioside transmembrane signal transducing molecules responsible for MAG's different biological effects may be shared (as shown) or independent. Additional modulating factors (cAMP, calcium, PKC) and potential pathway cross-talk are not shown (see Yiu and He, 2003).
MAG INHIBITION SIGNALING PATHWAYS
How do gangliosides and GPI-anchored NgR proteins, which reside on the extracellular leaflet of the plasma membrane, transmit signals into the neuron to control the axon cytoskeleton? The intracellular cytoskeletal regulator RhoA appears to be a common downstream target of both receptor systems, in that axon outgrowth inhibition by MAG and other axon regeneration inhibitors is reversed by blocking RhoA or its downstream effector molecules, such as the RhoA-activated kinase ROCK (Lehmann et al., 1999; McKerracher and Higuchi, 2006). The signaling molecules that lead from MAG engagement of its cell surface receptors to RhoA have not been fully elucidated. Two general mechanisms are worth considering. Transmembrane transducers may directly associate with gangliosides and/or NgRs on the outer leaflet of the plasma membrane and also with signal-transducing molecules on the inner leaflet. MAG binding to its receptors could then lead to intracellular signaling. Alternatively, both gangliosides and GPI-anchored proteins segregate on cell membranes in lipid rafts, local membrane environments that also segregate transmembrane or inner leaflet-associated signaling molecules. When lipid rafts are clustered by extracellular engagement of MAG receptors, intracellular signaling cascades may be initiated.
Because MAG has multiple receptors, it may signal from the cell surface to RhoA via multiple independent or interacting pathways. Components of such pathways have been discovered. The “NgR1–p75NTR/TROY–Lingo-1” pathway is exemplary.
The transmembrane proteins p75NTR and LINGO-1 form a physical complex with NgR1 and mediate signal transduction downstream of Nogo, MAG, and OMgp (for review see Yiu and He, 2006). p75NTR engages a Rho-GDI (GDP dissociation inhibitor), resulting in RhoA activation (Yamashita and Tohyama, 2003), completing the link from cell surface binding of axon regeneration inhibitors, including MAG, and RhoA activation. Direct physical associations have also been reported between the MAG-binding ganglioside GT1b and both p75NTR and NgR1, providing a potential common pathway for RhoA activation via MAG binding to NgR1 and gangliosides (Yamashita et al., 2002; Fujitani et al., 2005; Williams et al., 2008). Because p75NTR is not expressed in all neurons that respond to axon regeneration inhibitors, a search for related transducers with wider neuronal distribution revealed TROY (Park et al., 2005; Shao et al., 2005), leading to the “NgR1–p75NTR/TROY–Lingo-1” receptor hypothesis (Yiu and He, 2006).
Although the single pathway is appealing as a unifying hypothesis, its universality remains in question. Retinal and cerebellar neurons from p75NTR null and TROY null mice remain fully susceptible to MAG-mediated axon outgrowth inhibition (Venkatesh et al., 2007). In other studies, p75NTR null CGN remained fully sensitive to myelin inhibitors, whereas p75NTR null DRGN did not (Zheng et al., 2005). Consistent with these findings, addition of NgR1 or p75NTR blockers failed to reduce MAG inhibition of CGN axon outgrowth, whereas the same blockers reversed MAG inhibition of outgrowth from DRGN (Mehta et al., 2007). These data imply multiple independent MAG signaling pathways (Fig. 3). Redundancy in the system (NgR1/NgR2, p75NTR/TROY) has not yet been fully explored, and the existence of as yet undefined signaling pathways and components seems likely.
MAG RECEPTORS: AN EMBARRASSMENT OF RICHES
Evidence implicates at least two families of molecules, gangliosides (GD1a and GT1b) and NgRs (NgR1 and NgR2), as functional MAG receptors, and the list is likely to grow. These receptors, in turn, appear to link to multiple downstream signaling pathways, each of which may have additional modulators in a complex signaling network that includes cyclic AMP, intracellular calcium, and protein kinase C (Sandvig et al., 2004; Yiu and He, 2006). A current experimental challenge is to integrate these discoveries into a consistent model for MAG's multiple functions in stabilizing axons, regulating the axon cytoskeleton, contributing to membrane structures at nodes of Ranvier, and inhibiting axon regeneration. Which receptor(s) mediates which MAG functions (Quarles, 2008)? Do gangliosides and NgRs act independently, cooperatively, or in both modes (Quarles, 2007)? What factors (physiological and experimental) result in systems that use one or another of MAG's multiple receptors and/or signaling pathways? Answering these questions may enhance our understanding and provide new ways to mitigate axon instability and poor axon regeneration that limit recovery from nerve injury and disease. Toward this goal, each MAG receptor and each MAG signaling pathway is worthy of vigorous experimental exploration. As multiple MAG receptors and signaling pathways are revealed, efforts must turn toward integrating them into a unified view of the complexity and versatility of MAG signaling.
NOTE ADDED IN PROOF: After this Review was submitted, papers appeared that implicate the axonal surface molecules β1-integrin (Goh et al., 2008) and PirB (Atwal et al., 2008) as additional functional receptors for MAG. Investigating the relationships of these additional MAG receptors to gangliosides and NgRs, and thier roles in MAG's multiple functions, is a compelling matter for further research.




