Mitochondrial dysfunction disrupts trafficking of Kir4.1 in spiral ganglion satellite cells
Abstract
The inward-rectifier K+ channel Kir4.1 is responsible for maintaining cochlear homeostasis and restoring neural excitability. The large-conductance calcium-activated K+ channel (BKCa) plays a key role in phase locking signals in the mammalian inner ear. To evaluate the influence of mitochondrial dysfunction on the expression and subcellular localization of these channels, 3-nitropropionic acid (3-NP) was administered to rat round window membranes for 30 min. Auditory brainstem response was measured both before and 2 hr after 3-NP administration. Immunofluorescent confocal microscopy was used to measure the expression and subcellular localization of Kir4.1 and BKCa. Alexa Fluor 568-labeled bovine serum albumin (BSA) was applied to round window membranes as a tracer to explore the cochlear distribution of drug delivery and was detected in the lateral wall, spiral ganglion, cochlear nerve, and organ of Corti. Hearing loss of 23 (±4.4 SE) and 58 (±6.7 SE) dB developed in rats treated with 0.3 and 0.5 mol/liter of 3-NP, respectively. BKCa was visualized in the cellular membrane and cytoplasm in the upper and middle region of inner hair cells, and it was not affected by 3-NP. Kir4.1 was detected in intermediate cells of the stria, Deiter's cells, and spiral ganglion satellite cells. Kir4.1 failed to reach the perineural cytoplasm of the satellite cells after 3-NP treatment. The results of this study suggest that mitochondrial dysfunction disrupts trafficking of Kir4.1 in spiral ganglion satellite cells. © 2008 Wiley-Liss, Inc.
Potassium (K+) channels are a diverse and ubiquitous family of membrane proteins that are present in both excitable and nonexcitable cells and have high potassium selectivity (Jenkinson, 2006). This characteristic is based on a selectivity filter, the structure of which is probably similar in all K+ channels. Apart from the central pore of the channel, K+ channels vary in membrane topology (composed of two, four, or six or seven transmembrane domains), subunit composition, and mechanisms of activation and inactivation (Yellen, 2002). Depending on their function, K+ channels are also classified as voltage-gated K+ channels or inward-rectifier K+ channels (Kir). Voltage-gated K+ channels, including Shaker-related channels, human ether-a-go-go-related K+ channels, calcium-activated K+ channels, and KCNQ channels, are activated by depolarization. Kirs conduct K+ ions more strongly for inward compared with outward currents, and they are important in setting the resting membrane potential (Shieh et al., 2000). Members of this channel family play critical roles in cellular signaling processes, regulating neurotransmitter release, heart rate, insulin secretion, neuronal excitability, epithelial electrolyte transport, smooth muscle contraction, and cell volume regulation.
K+ is the main electrolyte in the endolymph of the cochlea. It enters hair cells through the mechanosensitive nonselective cationic channel at the top of their cilia and excites the hair cells upon auditory stimulation. This channel is likely to be a transient receptor potential channel vanilloid (Eldredge and Miller, 1971; Hudspeth, 1989, 1997; Kim et al., 2003; O'Neil and Heller, 2005; Tabuchi et al., 2005; Takumida et al., 2005; Wangemann, 2006). K+ is removed from the excited hair cells to the perilymph via two voltage-gated K+ channels, the large-conductance calcium-activated K+ channel (BKCa) and KCNQ4, both of which are located in the hair cells, and the inward-rectifier K+ channel Kir4.1 (KCNJ10), which is located in Deiter's cells below the outer hair cells (OHCs; Hibino et al., 1997; Hibino and Kurachi, 2006). The hair cells are then repolarized, and the accumulated K+ is carried back to the endolymph by a connective-tissue gap-junction network in the basilar membrane, spiral ligament, and stria vascularis. Here, the K+ interacts with the Na+/K+-ATPase, Na+/K+/2Cl− cotransporter (NKCC1), Kir4.1 (KCNJ10), Kir5.1, and KCNQ1/KKCNE1 (Hibino and Kurachi, 2006). The expression of Kir4.1 in the satellite cells of the cochlear ganglia might be responsible for buffering the K+ extruded from the spiral ganglion cells (SGCs) during excitation (Hibino et al., 1999; Jin et al., 2006). Although BKCa is responsible for cochlear tuning to different frequencies in vertebrates (Jones et al., 1999; Ramanathan et al., 1999; Ramanathan and Fuchs, 2002), it plays a key role in phase locking signals in the mammalian inner ear (Oliver et al., 2006).
Recent research in protein trafficking has revealed that K+ channels are dynamic entities (Stockklausner and Klocker, 2003; Leonoudakis et al., 2004; Kim et al., 2007; Steele et al., 2007). ATP, which is synthesized during mitochondrial respiration, is important for both protein synthesis and trafficking, and it provides the energy necessary to drive translation, combined transcription and translation processes, and the dynamics of transport vesicles. Mitochondrial disease or dysfunction that causes electron transport failure and ATP deficiency underlies the pathogenesis of sensorineural hearing loss (Wallace et al., 1988; Gold and Rapin, 1994; Seidman et al., 1999; Hutchin and Cortopassi, 2000; Fischel-Ghodsian et al., 2004). An animal model of sensorineural hearing loss was generated by administering a mitochondrial toxin, 3-nitropropionic acid (3-NP), to inhibit succinate dehydrogenase irreversibly (Hoya et al., 2004; Okamoto et al., 2005). Using this animal model, we explored the effect of mitochondrial dysfunction, which induces ATP deficiency, on potassium channel expression and its trafficking-related subcellular localization. In the present study, we focus on two representative K+ channels in the cochlea: the inward rectifier K+ channel Kir4.1 and the voltage-gated K+ channel BKCa. The acetylcholine receptor subunit α9 (AChRα9) was selected to characterize the cochlear inner hair cell (IHC; Elgoyhen et al., 1994).
MATERIALS AND METHODS
Twelve Sprague Dawley rats were included in the study. Nine male rats (250–300 g) with normal Pryer's reflex chosen for 3-NP delivery were provided by the animal laboratory of the Affiliated Sixth People's Hospital of Shanghai Jiaotong University. The study protocol was reviewed and approved by the Animal Experiment Committee of Shanghai Jiaotong University School of Medicine. Three rats were randomly selected for each of the three groups: a phosphate-buffered saline (PBS), a low-dosage, and a high-dosage group. Three additional male Sprague Dawley rats (400–420 g) with normal Pryer's reflex were supplied by the experimental animal unit of the University of Tampere with permission from the Ethical Committee of the University (permission No. 985/2003). Animal care and experimental procedures were conducted in accordance with European legislation. All experiments and procedures were performed with animals under general anesthesia maintained with xylazine (16 mg/kg) and ketamine (60 mg/kg). The auditory brainstem response (ABR) was recorded both before the operation and 2 hr after drug delivery.
3-NP or PBS Delivery
The round window membrane was used for inner ear exposure to 3-NP or PBS. 3-NP (Sigma-Aldrich, St. Louis, MO) was prepared in high-dosage (0.5 mol/liter) and low-dosage (0.3 mol/liter) solutions. 3-NP solutions were sterilized by filtration through a 0.22-μm filter, and PBS (0.01 mol/liter, pH 7.3) was sterilized in an autoclave. The local skin around the left ear was shaved and cleaned, and the eyes were protected with a topical erythromycin ointment. In addition to general anesthesia, the wound area was treated with local anesthetic agents (lidocaine, 1%). Under an operating microscope, the bulla was opened using a ventral approach; the round window niche was then identified. A small piece of gelfoam sponge (8 mm3) that had been rinsed in 6 μl of either 3-NP solution or PBS was placed on the round window membrane and left in place for 30 min. The gelfoam was then removed and the bulla packed with muscle before wound closure.
ABR Measurement
To assess auditory thresholds, we used BioSig32 (Tucker Davis Technologies) to record ABR thresholds. The ABR was recorded with subcutaneous platinum needle electrodes placed at the vertex (noninverting input), left mastoid prominence (inverting input), and right mastoid prominence (indifferent site). A click with a duration of 50 μsec and a repetition rate of 21.1 Hz was used for stimulation. Responses from 512 sweeps were averaged, with a gain of 100k at each intensity level, using a filter ranging from 0.1 to 3 kHz. Thresholds were judged by a visible, repeatable wave II.
Immunofluorescent Confocal Microscopy
Ninety minutes after removal of the gelfoam, the cochleae from nine animals were fixed with 4% formaldehyde by cardiac perfusion after exsanguination with 0.01 mol/liter PBS (pH 7.4). The bulla was removed and incubated overnight in the same fixative solution. After washing with 0.1 M PBS, decalcification with 10% EDTA was performed at room temperature for 4 weeks. A standard paraffin-embedding procedure was used, and the samples were sectioned at a thickness of 4 μm. For immunofluorescent staining, two to four sections from each animal were selected, deparaffinized with xylene, and passed through an ethanol gradient and final PBS wash. The sections were digested with 0.1% trypsin at 37°C for 30 min, washed with PBS-T (0.1% Tween-20), and incubated with 1:20 preimmunized goat serum at room temperature for 30 min. Samples were incubated overnight at 4°C with the rabbit polyclonal antibody to Kir4.1, 1:100 (Alomone Laboratories, Jerusalem, Israel); the rabbit polyclonal antibody to BKCa channel α-subunit (Slo, KCNMA), 1:100 (Alomone Laboratories); and the goat polyclonal antibody to AChRα9, 1:200 (Santa Cruz Biotechnology, Santa Cruz, CA); and then washed with PBS-T. As a negative control for Kir4.1, the primary antibody was saturated overnight with Kir4.1 peptide at a concentration of 1 μg peptide/μg antibody-containing 1% preinoculated goat serum. As a negative control for BKCa, the primary antibody was saturated overnight with BKCa fusion protein at concentration of 3 μg fusion protein/μg antibody-containing 1% preinoculated goat serum. As negative controls, AChRα9 primary antibody was omitted. For double staining, slices were incubated with fluorescein isothiocyanate (FITC)-conjugated monoclonal mouse anti-goat IgG antibody (1:400; Sigma-Aldrich) at room temperature for 60 min, washed with PBS-T, incubated with Alexa Fluor 568-conjugated goat antiserum against rabbit IgG (1:400; Invitrogen, Carlsbad, CA) for 60 min, and finally incubated with 4′,6-diamidino-2-phenylindole (DAPI; 10 ng/ml; Sigma-Aldrich) for 10 min. The slides were mounted with Gel Mount Aqueous Mounting Medium (Sigma-Aldrich) after washing with PBS-T. For single staining with Kir4.1 or BKCa, slices were incubated with Alexa Fluor 568-conjugated goat antiserum against rabbit IgG and DAPI and then mounted. For F-actin counterstaining, slices were incubated with FITC-conjugated phalloidin (17 μl/ml; Sigma-Aldrich) for 30 min before DAPI staining and were then washed with PBS-T.
Immunofluorescence slides were observed using an Olympus IX70 microscope installed with ANDOR IQ. Alexa Fluor 568 labels were detected with a 568-nm excitation filter and 607/45-nm emission filter. The FITC signal was detected with a 488-nm excitation filter and 525/50-nm emission filter. An Ar-Kr laser was used as the excitation source. DAPI was excited with a 340–380-nm filter and detected using a 500 LP filter.
BSA Labelling and Cochlear Distribution
According to the Alexa Fluor 568 Protein Kit manual (Molecular Probes, Eugene, OR; A10238), 1 mg BSA (Sigma-Aldrich) was dissolved in 0.5 ml sodium bicarbonate (0.1 mol/liter, pH 8.3) and mixed with 0.05 ml of 1 mol/liter sodium bicarbonate (pH 8.3). The protein solution was incubated with the reactive dye by stirring for 1 hr at room temperature. The labeled BSA was purified by passing the reaction mixture through Bio-Rad Gel P-30 Fine size-exclusion purification resin, which was designed to separate free dye from proteins with an MW > 40 kD (MW of BSA = 66 kD). The eluate containing labeled BSA was collected by checking the illumination of the band. Alexa Fluor 568-labeled BSA (6 μl, 0.4 mmol/liter) was placed on the rat round window membrane for 30 min exactly as 3-NP was administered. Bullae were collected immediately, 30 min, or 90 min after removing the gelfoam. To avoid potential contamination of BSA from the middle ear, the following procedure was performed: the bulla was taken, thoroughly washed with tap water, and fixed by immersing in 4% formaldehyde for 30 min after decapitating the animals; the bulla was washed again with tap water, opened by breaking the lateral wall, rinsed with PBS for 3 × 5 min, incubated with FITC-conjugated phalloidin (50 μg/ml) for 20 min, incubated with DAPI (10 ng/ml) for 10 min, and again washed with PBS. Both Triton X-100 and Tween-20 treatments were avoided to minimize BSA loss during staining. The lateral wall, the bony lamina connected with basilar membrane, and the cochlear nerve were isolated under a Zeiss Stemi 2000-C stereomicroscope and mounted with Gel Mount Aqueous Mounting Medium. The cochlear nerve was transversely cut into slices for mounting. The samples were observed under a confocal microscope with the same parameters used for K+ channel measurement.
In our experience with nanoparticles, most of the target signal will be lost after immunohistochemical staining and penetration of the antibody into the bony spiral ganglion area (Zou et al., 2008). Neurofilament staining for SGC verification was not possible in the present study. As an alternative, one paraffin-embedded slice of a PBS-treated cochlea was selected to demonstrate an overview of the cochlear structure by standard hematoxylin and eosin (HE) staining. The diameter of the SGC nuclei was measured and applied as a reference to verify the nuclei of suspected SGCs by confocal microscopy.
Data Analysis
Quantification was completed with ImageJ 1.32j software by importing the original confocal microscopy TIFF files. Only the image frame excited at 568 nm, which represents the positive BKCa or Kir4.1 signal, was chosen for quantification. The entire area containing intermediate cells, OHCs, and SGC satellite cells showing positive staining in the basal turn were selected. The gray scale of the selected area, representing the fluorescence intensity, was automatically measured. The mean values of the ABR threshold shift and potassium channel protein signal intensities (gray scale) were compared between groups using the nonparametric Kruskal-Wallis test. For Kir4.1 expression, signal intensities were also compared between the basal turn and the second turn of the cochlea among the 3-NP treatment groups using the surface plot function in the ImageJ 1.32 software, which showed the fluorescence intensity of each positive spot.
RESULTS
Cochlear Distribution of BSA After Transient Round Window Membrane Administration
The overall structure of the cochlea, including the lateral wall, organ of Corti, SGCs, and auditory nerve, was demonstrated with an HE-stained slide (Fig. 1A). The round nuclei of SGCs, with diameters of 8.1 ± 0.5 μm (mean ± SD), are the largest nuclei among those of the cells present in the cochlea. The typical nucleus of a Schwann cell of the auditory nerve, basal cell of the stria vascularis, or spiral ligament fibrocyte is represented by a rod-like structure (Fig. 1A). With a confocal microscope (×100 objective lens), BSA was detected in the spiral ligament, stria vascularis, spiral ganglion area, auditory nerve, and organ of Corti in cochleae collected immediately after round window membrane administration, after 30 min, and at other time points (Fig. 1). The nuclei of suspected SGCs in whole-mount samples were the same size as those from paraffin-embedded samples. However, there was a decrease in the distribution of BSA in F-actin-stained cochleae compared with unstained cochleae, in accordance with our nanoparticle study (Zou et al., 2008).

Cochlear distribution of drug after round window membrane administration visualized using Alexa Fluor 568-conjugated BSA as a tracer. The overall structure of the cochlea, including the lateral wall, organ of Corti, SGCs, and auditory nerve, was demonstrated by paraffin embedding and HE staining (A). The SGCs possess typical round nuclei with a mean diameter of 8.1 ± 0.5 μm and are the largest nuclei in the cochlea. The rod-like appearance is typical of the nuclei of the auditory nerve, basal cells of the stria vascularis, and spiral ligament fibrocytes. The distance from the IHC nucleus to the medial SGC is 197.6 μm. By confocal microscopy, BSA (red dots; indicated by arrow) was detected in the lateral wall, including the stria vascularis and spiral ligament, SGCs, auditory nerve, and organ of Corti in cochleae collected immediately after round window membrane administration, 30 min (F: cryosectioning slices) and 90 min after drug delivery (B–G,I,J: whole-mount samples stained for F-actin; H,K: whole-mount samples without F-actin staining). B: Lateral view of the three-dimensional reconstruction image of the stria vascularis. C: Front view of the same three-dimensional reconstruction image as in B. D: Two-dimensional image showing near intercellular junction localization of marginal cells. E: Two-dimensional image showing spiral ligament localization. F: Cryosection showing a more intense distribution of BSA in the lateral wall, including the stria vascularis (StrV) and spiral ligament (SL). G: IHC localization of BSA. H: BSA was preserved in the OHC region of the sample without F-actin staining. I: Overview of the bony spiral lamina containing the SGCs, the region 197.6 μm away from the IHC indicates the SGC area. J: BSA was found in SGCs (typical nuclei as shown in A with diameters of 8 μm). K: Abundant BSA was visualized in the cochlear nerve (typical rod-like nuclei). AN, auditory nerve; OC, organ of Corti; PC, pillar cells; SL, spiral ligament; SLimb, spiral limb; StrV, stria vascularis. The numbers in G indicate OHC row 1, row 2, and row 3. The enlarged windows in A were taken at ×40 magnification. Scale bars = 200 μm in A; 8 μm in D–H,J,K; 80 μm in I. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
3-NP-Induced Hearing Loss
The delivery of PBS onto the round window membrane did not cause any threshold shift. However, 0.3 mol/liter of 3-NP induced an average threshold shift of 23 (±4.4 SE) dB, and 0.5 mol/liter of 3-NP resulted in 58 (±6.7 SE) dB of hearing loss (Fig. 2). This difference was statistically significant between the groups (P < 0.05).
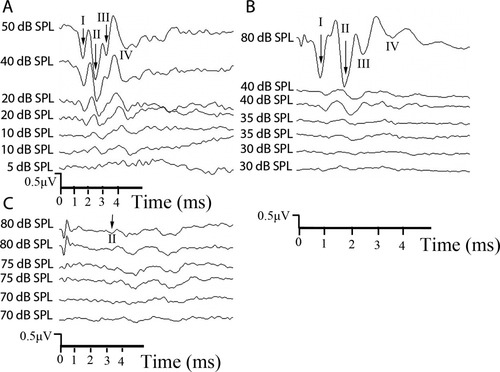
Representative ABR wave form from PBS-treated and 3-NP-treated rats. In the 3-NP-treated rat, the threshold was 10 dB SPL (the same as before the experiment; A). However, the threshold changed from 20 to 35 dB SPL after the animal was treated with 0.3 mol/liter of 3-NP (B). The threshold changed from 35 to 80 dB SPL in the animal treated with 0.5 mol/liter of 3-NP (C).
Neither BKCa Expression Nor Its Subcellular Localization Was Affected by 3-NP in the Organ of Corti
No positive staining was found in slices incubated with the preabsorbed primary antibody (Fig. 3E). In both the PBS control and the 3-NP treated groups, significant amounts of the BKCa channel α-subunit protein were detected in the apical neck region (below the cuticular plate) and middle region of the IHCs (Fig. 3A,B). The IHC localization of BKCa was confirmed by double staining for BKCa and AChRα9 (Fig. 3F). The OHCs and SGCs were negative for BKCa channel α-subunit protein. There was no difference in intensity observed between groups (see Fig. 6; P = ns). The subcellular localization of the BKCa channel α-subunit protein in IHCs was not altered by 3-NP treatment (Fig. 3).
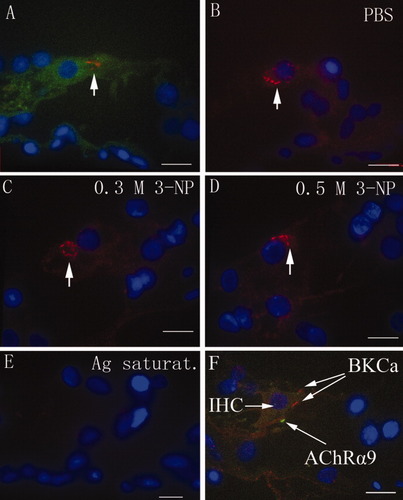
BKCa channel α-subunit protein expression in IHCs was visualized using confocal microscopy. BKCa (red) was present in both the apical neck region (below the cuticular plate) and the middle region of IHCs (arrow). F-actin (green), probed with FITC-labeled phalloidin, and the nucleus (blue), counterstained with DAPI, were stained for comparison (A). There was no difference in BKCa expression in IHCs among rats treated with PBS (B), 0.3 mol/liter of 3-NP (C), or 0.5 mol/liter of 3-NP (D; arrow). There was no positive staining in the cochlea when the primary antibody was saturated with BKCa fusion protein (E). IHC localization of BKCa was confirmed by dual staining for BKCa (red) and AChRα9 (green; F). 0.3 M, 0.3 mol/liter; Ag saturat., antigen saturation. Scale bars = 9 μm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
3-NP Treatment Altered the Trafficking of Kir4.1 in the Spiral Ganglion Satellite but Not in the Stria Vascularis
No positive staining was visualized in slices incubated with the preabsorbed primary antibody (Figs. 4B, 5G,H). Abundant Kir4.1 protein expression was detected in the intermediate cells of the stria vascularis, Deiter's cells of the organ of Corti, and SGC satellite cells (Figs. 4, 5). Because the basolateral “membrane infolding” of the marginal cells lies parallel to the cytosol of the intermediate cells, it is possible to distinguish the cytosol of intermediate cells by examining the staining adjacent to the intermediate cell nuclei. Thus, positive staining for Kir4.1 in connection with the intermediate cell nuclei indicates its localization in intermediate cells, but not in marginal cells (Fig. 4C). SGC satellite cells with myelin extensions surrounding the SGCs showed Kir4.1 staining, but SGCs did not exhibit Kir4.1 staining (Fig. 5D). There were no differences in the intensity of intermediate cells, Deiter's cells, or SGC satellite cells between the control group and 3-NP treatment groups (Figs. 4-6; P = ns). Kir4.1 staining, however, was more condensed in the proximal cellular body of SGC satellite cells in both the low- and the high-dosage 3-NP groups compared with the PBS control group (Figs. 5, 7). This phenomenon was more distinct in the basal turn than in the second turn. Higher concentrations of 3-NP produced a more obvious condensation of Kir4.1 in the proximal cellular body (Fig. 7). Kir4.1 did not extend into the myelin surrounding SGCs in cochleae treated with 0.5 mol/liter 3-NP, but some cells exhibited sparse, dot-like staining (Figs. 5D,F, 7).
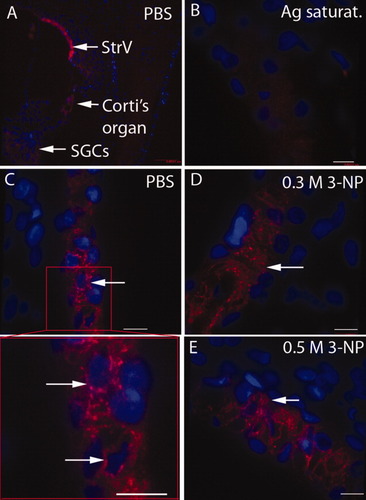
Kir4.1 expression in the stria vascularis. An overview of Kir4.1 expression (red) in the rat cochlea using low-magnification confocal microscopy shows the highest intensity in the stria vascularis (A). However, no staining was observed when the primary antibody was saturated with Kir4.1 peptide (B). The intermediate cell (IC) localization of Kir4.1 was indicated by demonstrating positive perinuclear staining (blue) in ICs (C). Kir4.1 expression was not abrogated by either 0.3 mol/liter (D) or 0.5 mol/liter (E) of 3-NP treatment. Ag saturat., antigen saturation. Scale bars = 9 μm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
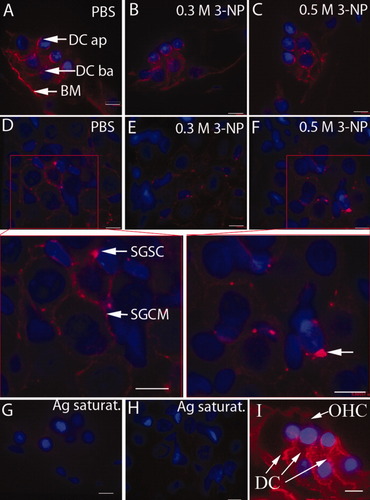
Expression of Kir4.1 in the organ of Corti and spiral ganglion. Kir4.1 was detected in the apical and basolateral membranes of Deiter's cells as well as at the border between Deiter's cells and the basilar membrane in PBS-treated cochlea (A,I). This expression pattern was not altered by either 0.3 mol/liter (B) or 0.5 mol/liter (C) of 3-NP treatment. In the spiral ganglion of PBS-treated cochleae, Kir4.1 was expressed in the cytoplasm of SGC satellite cells and their extensions surrounding SGCs (D). After treatment with both a low concentration (E) and a high concentration (F) of 3-NP, Kir4.1 expression was limited to the cytoplasm of SGC satellite cells and disappeared from their extension structures. The Kir4.1 signal was absent in the organ of Corti (G) and spiral ganglion (H) when the primary antibody was saturated with Kir4.1 peptide. Ag saturat., antigen saturation; BM, basilar membrane; DC, Deiter's cell; DC ap, apical membrane of DC; DC ba, basolateral membrane of DC; SGCM, SGC myelin; SGSC, SGC satellite cell. Scale bars = 9 μm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
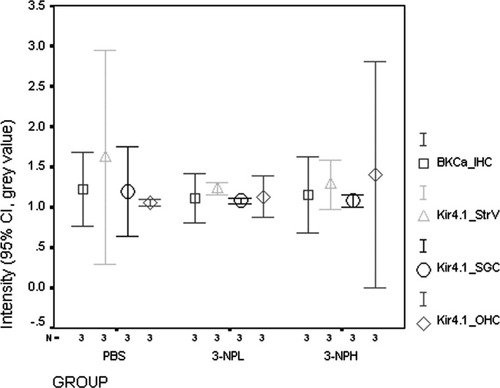
Quantification of BKCa and Kir4.1 in the cochlear cellular population. Although there was a trend toward reduced Kir4.1 intensity in the stria vascularis after treatment with 3-NP, this change was not statistically significant. There was no difference in BKCa in IHCs or Kir4.1 in Deiter's cells and SGC satellite cells. 3-NPL, 0.3 mol/liter 3-NP; 3-NPH: 0.5 mol/liter 3-NP; BKCa_IHC, BKCa intensity in IHCs; Kir4.1_StrV, Kir4.1 intensity in the stria vascularis; Kir4.1_SGSC, Kir4.1 intensity in SGC satellite cells; Kir4.1_OHC, Kir4.1 intensity in OHCs.
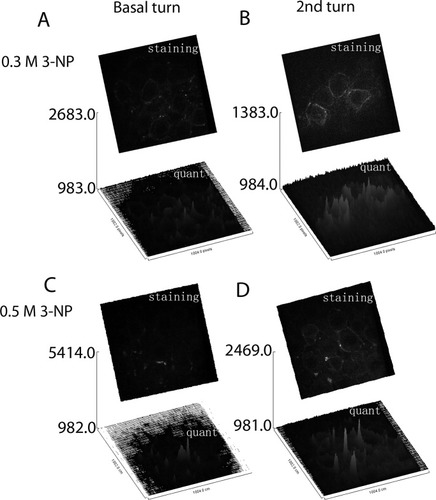
Surface plot of Kir4.1 intensity in spiral ganglion satellite cells as measured from original confocal microscopy TIFF files using Image J 1.32j software. Kir4.1 trafficking in the SGC satellite cells of cochleae treated with 3-NP was more severely affected in the basal turn (A,C) than in the second turn (B,D), as evidenced by cytosol spot staining. This influence was more pronounced in cochleae treated with higher concentrations of 3-NP (C vs. A; D vs. B). The gray value is presented as arbitrary units. Staining, Kir4.1 positive staining; quant, quantification of the positive staining.
DISCUSSION
The main finding of the present study was that mitochondrial dysfunction caused significant hearing loss but did not affect the expression of either Kir4.1 or BKCa in the rat cochlea. The 3-NP treatment, however, altered the subcellular localization of Kir4.1 in SGC satellite cells. The distribution of Kir4.1, especially with regard to staining in the stria vascularis, has been a subject of controversy in the literature (Hibino et al., 1997; Ando and Takeuchi, 1999). Although both immunofluorescence microscopy and immunoelectron microscopy were performed in prior studies, positive staining for Kir4.1 has been shown only in isolated intermediate cells and not in isolated marginal cells (Ando and Takeuchi, 1999). By counterstaining the nucleus with DAPI, our study provides evidence of intermediate cell localization. The positive staining for Kir4.1 besides the nuclei of intermediate cells observed in the present study supports the findings of Ando and Takeuchi (1999).
Our data showed different trafficking properties of Kir4.1 in intermediate cells, Deiter's cells, and SGC satellite cells with regard to its dependence on mitochondrial function. After synthesis in the endoplasmic reticulum, ion channels are carried to the Golgi complex; from there, they are ultimately transported to the plasma membrane. Having reached the cell surface, they undergo endocytosis and either are recycled back to the plasma membrane or are diverted for degradation. These membrane trafficking events are mediated by protein-coated vesicular or tubular containers, into which transported cargo is sorted and concentrated (Rothman, 2002; Altan-Bonnet et al., 2004; Bonifacino and Glick, 2004). Efforts to understand the molecular mechanisms underlying membrane trafficking have identified several proteins crucial to the formation of coated vesicles. These include coat proteins and Arf1, a GTP-binding protein (Donaldson and Lippincott-Schwartz, 2000; Spang, 2002; Bonifacino and Glick, 2004). The asymmetrical structure of SGC satellite cells, in which the nucleus is far from the myelin sheath, may cause Kir4.1 trafficking to be particularly susceptible to ATP deficiency as a result of mitochondrial dysfunction. This long-distance trafficking has a much higher energy demand than the corresponding processes in intermediate cells and Deiter's cells. The distribution of Kir4.1 in the proximal cytosol of SGC satellite cells likely is incapable of pumping K+ from extracellular regions during neuronal activity, because “K+ spatial buffering” or “K+ siphoning” occurs when the channels are in contact with K+ (Butt and Kalsi, 2006; Fig. 8). Failure to remove K+ from the extracellular region of the SGC may partially contribute to hearing loss by reducing neural excitability (Hibino et al., 1999).
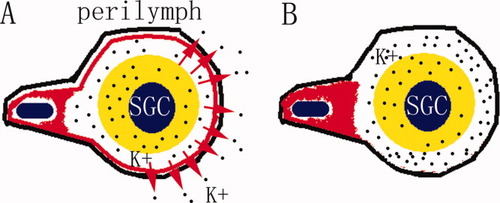
Illustration of the modified Kir4.1 trafficking in SGC satellite cells leading to a failure to pump K+ from the extra-SGC space to the perilymph. The red ring and handle indicate Kir4.1 in a SGC satellite cell. The black line represents the cellular membrane of the SGC satellite cell. In the normal SGC satellite cell, Kir4.1 circles between the proximal cellular body and the distal peri-SGC ring, pumping K+ from the extra-SGC fluid to the perilymph, which is located outside the cytoplasm of the SGC satellite cell (A). After treatment with 3-NP, Kir4.1 accumulates in the proximal cellular body, and K+ accumulates in the extra-SGC space in the absence of Kir4.1 (B). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
A dosage-dependent effect was observed, in that higher concentrations of 3-NP more profoundly interrupted trafficking in SGC satellite cells (Fig. 7). The winding difference in Kir4.1 trafficking disruption, i.e., that the basal turn showed greater alternation than the second turn, can be explained by the drug concentration gradient that is induced in the cochlea upon round window administration and is related to cochlear fluid dynamics (Zou et al., 2005). To prove that the observed trafficking disturbances are due to 3-NP treatment, visualization of drug distribution in the spiral ganglion area upon round window delivery is necessary. Because of difficulty in fluorescently labeling 3-NP, Alexa Fluor 568-conjugated BSA was designed in the present study as a mimic to explore the cochlear distribution of 3-NP after round window membrane drug delivery. BSA appeared in the spiral ligament, stria vascularis, spiral ganglion region, auditory nerve, and organ of Corti after administration using the same protocol used for 3-NP. Because it is a much smaller molecule, there is no doubt that 3-NP can easily reach the spiral ganglion area within the time observed.
Although BKCa was expressed in adult guinea pig IHCs and SGCs (Skinner et al., 2003) and adult mouse IHCs, SGCs, and spiral prominence epithelium (Hafidi et al., 2005), the strongest staining was seen in IHCs. BKCa mRNA transcription was shown in different cell types of the rat cochlea, including IHCs at P12 (Langer et al., 2003), but there are no reports available in the literature concerning adult rat cochleae. We analyzed the BKCa α-subunit because it forms the main core of the channel, including the pore structure for K+ transport, and is related to trafficking (Park et al., 2004; Brainard et al., 2005; Ghatta et al., 2006). We found that it was expressed only in the upper and middle region of IHCs in the adult rat cochlea. Its cell-specific localization indicates that ion channel expression is more unique in adult rat cochleae. This finding supports the hypothesis that IHCs provide a phase locking function with the precise timing necessary for cochlear signaling (Oliver et al., 2006). In mammalian IHCs, the fast potassium currents produced by BKCa may be modulated in a voltage-dependent manner by the entrance of Ca2+ from a source other than L-type Ca2+ channels (Marcotti et al., 2004). Because of this, it is not necessary for BKCa α-subunit protein to be expressed in IHC synaptic basal regions, as shown in the mouse (Brandt et al., 2005; Hafidi et al., 2005).
Most BKCa channels contain both a C-terminal endoplasmic reticulum export signal (DLIFCL) and a nearly adjacent sorting motif (NAGQSRA), both of which are required for selective expression of Slo1 (α subunits) on the apical surface of polarized epithelial cells (Wang et al., 2003; Kwon and Guggino, 2004). Moreover, the C-terminus of the α-subunit represents a rich area for protein–protein interactions, including interactions with proteins that could potentially regulate trafficking (Park et al., 2004; Brainard et al., 2005), modulation (Tian et al., 2006; Ma et al., 2007), and degradation of BKCa (Jo et al., 2005). These processes are energy dependent, so we induced ATP deficiency in the cochlea by treating cells with 3-NP. We observed the effects of this manipulation on the subcellular localization of BKCa Slo gene products. The expression of the BKCa α-subunit in IHCs was not changed in 3-NP-treated cochleae, suggesting a relatively steady state of BKCa in the rat cochlea. This is in accordance with the fast response of IHCs to sound, which cannot work with a fast dynamic turnover of BKCa, allowing for the precise timing of high-frequency cochlear signaling (Oliver et al., 2006).
In summary, mitochondrial dysfunction significantly disturbs the trafficking of Kir4.1 in SGC satellite cells. The failure of Kir4.1 to reach the perineural region may ablate the K+ gradient between the intracellular and the extracellular spaces and reduce SGC excitability. We speculate that this disturbance partially contributes to the acute hearing loss induced by mitochondrial dysfunction, although other direct disruptions of hair cell function and cochlear homeostasis may occur. BKCa expression was restricted to IHCs in the adult rat cochlea, differing from its expression in the newborn rat and mouse. However, its trafficking was not influenced by mitochondrial dysfunction.
Acknowledgements
We acknowledge Dr. Jingchun He (The Affiliated Sixth People's Hospital, Otolaryngology Institute of Shanghai Jiaotong University) for assisting with the ABR measurement.




