Endogenous neurogenesis and neovascularization in the neocortex of the rat after focal cerebral ischemia
Abstract
The present study was designed to examine whether endogenous neurogenesis and neovascularization occur in the neocortex of the ischemic rat brain after unilateral middle cerebral artery occlusion (MCAO). Sprague-Dawley rats were divided into six groups (n = 29): one control group (n = 4) and five groups composed of animals sacrificed at increasing times post-MCAO (2 days and 1, 2, 4, and 8 weeks; n = 5 per group). To determine the presence of neurogenesis and neovascularization in the ischemic brain, nestin, Tuj1, NeuN, GFAP, Tie2, RECA, and 5-bromo-2′-deoxyuridine (BrdU) were analyzed immunohistochemically. In addition, nestin, GFAP, and Tie2 expression was determined by Western blotting. Triple-labeling of nestin, BrdU, and laminin was performed to visualize the interaction between endogenous neurogenesis and neovascularization. The number of BrdU- and nestin-colabeled cells increased markedly in the neocortex and border zone of the ischemic area up to 1 week after MCAO and decreased thereafter. Western blot analysis revealed that the expression of nestin, Tie-2, and GFAP was amplified in the ipsilateral hemisphere 2days after MCAO and peaked 1 week after MCAO, compared with that in the normal brain. After ischemic injury, nestin- and BrdU-colabeled cells were observed in the vicinity of the endothelial cells lining cerebral vessels in the ipsilateral neocortex of the ischemic brain. Endogenous neurogenesis and neovascularization were substantially activated and occurred in close proximity to one other in the ipsilateral neocortex of the ischemic rat brain. © 2007 Wiley-Liss, Inc.
The long-standing assertion, based on the first description by Cajal, that production of neurons in the brain is confined to a discrete period of development is no longer accepted (Allen, 1912; Smart, 1961; Altman and Das, 1965). Two sites well known for endogenous neurogenesis throughout life are the subventricular zone (SVZ) of the lateral ventricles (Altman, 1969; Eriksson et al., 1998) and the subgranular zone (SGZ) of the dentate gyrus (DG) in the hippocampus (Kaplan and Hinds, 1977).
Endogenous neurogenesis can occur in response to different types of central nervous system (CNS) injury, as well as in nonpathological situations. Indeed, various insults, such as transection (Weinstein et al., 1996), seizure (Calza et al., 2001), and ischemic lesions of the brain (Arvidsson et al., 2002; Parent et al., 2002; Jin et al., 2003; Zhang et al., 2004), are supposed to increase SVZ precursor proliferation in the adult rodent forebrain. In ischemia, a significant increase in SVZ progenitors occurs as a result of endogenous neurogenesis, and these progenitors have been demonstrated to selectively migrate to the damaged area of the striatum (Arvidsson et al., 2002; Parent et al., 2002; Zhang et al., 2004) and to express markers of medium spiny neostriatal projection neurons (Arvidsson et al., 2002; Jin et al., 2003). Although the neurogenic potential of adult SVZ cells in ischemia has been investigated extensively, only a few studies have addressed whether endogenous neurogenesis also happens in the neocortex, and as a consequence, little is known about this process. Most previous studies suggested that a small number of neuroblasts appear in the periinfarct neocortex after stroke (Arvidsson et al., 2002; Parent et al., 2002; Jin et al., 2003; Zhang et al., 2004). In contrast, Magavi et al. (2000) proposed that there is a complete absence of constitutive neurogenesis in the murine neocortex. However, the possible existence of cortically located adult multipotent neural precursors has been described in vitro (Marmur et al., 1998; Palmer et al., 1999).
Furthermore, it has been suggested that an abundant pool of mitotically competent neurogenic progenitor cells resides in adult human white matter (Nunes et al., 2003). Nunes et al. (2003) showed that human white matter progenitor cells (WMPCs) could be passaged as neurospheres in vitro and that these cells generated functionally competent neurons and glia both in vitro and after being xenografted to fetal rat brain. Indeed, in a pilot study, we found robust nestin expression in the rodent neocortex after middle cerebral artery occlusion (MCAO). Thus, we hypothesized that endogenous neurogenesis does occur in the neocortex after MCAO.
Meanwhile, it has been suggested that in the hippocampus, endothelial and neuronal progenitor cells interact during development and in brain injury, such as that induced by electroconvulsive seizures (Leventhal et al., 1999; Hellsten et al., 2005). However, there have been no reports about whether or how endogenous neurogenesis is coupled with neovascularization in the adult rat neocortex after ischemic injury.
This study was designed to examine whether endogenous neurogenesis also occurs in the neocortex after ischemic insult and to elucidate whether endogenous neurogenesis is associated with neovascularization in theischemic adult rat brain. In the present study, we showed that endogenous neurogenesis is significantly activated in the neocortex as well as in the SVZ, hippocampus, and striatum and that this neurogenic activity is associated with neovascularization after acute ischemic brain damage.
MATERIALS AND METHODS
Animals
Adult male Sprague-Dawley rats (260–300 g; Samtako, Osan, Korea) were housed in the same animal care facility under a 12-hr light/12-hr dark cycle with food and water available ad libitum. Animal care and surgical procedures were carried out in accordance with guidelines on the ethical use of animals approved by the Experimental Animals Committee of Seoul National University Hospital. All efforts were made to minimize the number of animals used and their suffering.
Overall Experimental Design
Rats that responded poorly or whose injury was inadequate were excluded in this study after magnetic resonance image (MRI). A 1.5 T Advantage Horizon System (GE, Milwaukee, WI) equipped with Wrist coil (GE, Milwaukee, WI), an actively shielded gradient coil, was used in the study. Animals were divided into six groups (n = 29): one control group (n = 4) and five groups of animals sacrificed after increasing durations post-MCAO (2 days and 1, 2, 4, and 8 weeks; n = 5 per group), as shown in Figure 1A. Subsequently, immunofluorescent staining (n = 3 or 4 per control or ischemic group) and Western blotting (n = 1 per group) were conducted. To characterize bromodeoxyuridine (BrdU)-labeled cells in the ischemic brain, immunohistochemical analysis of nestin, glial fibrillary acidic protein (GFAP), NeuN, and Tuj1 expression was carried out. Expression of nestin, GFAP, and Tie-2 was also analyzed by Western blotting using homogenates from both hemispheres. In addition, to examine the possible interaction between endogenous neurogenesis and neovascularization, rat endothelial cell antigen-1 (RECA-1), platelet endothelial cell adhesion molecule-1 (PECAM-1), and laminin (noncollagenous connective tissue glycoprotein) were employed as markers of angiogenesis, and nestin was used to monitor neurogenesis.
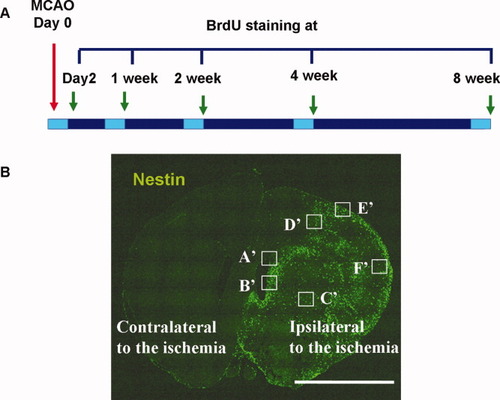
Experimental protocol. A: The six study groups—a control group (n = 4) and five MCAO groups (n = 5 per group) representing animals sacrificed at five different times (2 days and 1, 2, 4, and 8weeks) post-MCAO. All animals received 5-bromo-2′-deoxyuridine (BrdU; 100 mg/kg, i.p.) by intraperitoneal injection for three consecutive days prior to being sacrificed. B: Representative confocal laser microscopic image of brain sections 2 days after MCAO (nestin in green). Spatial and temporal localization of cell-specific markers was determined by confocal laser scanning microscopy on multilabeled tissue sections. ROIs were defined in the SVZ (A′), SEZ (B′), striatum (C′), penumbra (D′), and cortex (E′, F′). Scale bar = 5 mm (B). SVZ, subventricular zone; SEZ, subependymal zone.
BrdU Labeling
BrdU (Sigma Aldrich, Inc., St. Louis, MO) was dissolved in nonpyrogenic 0.9% NaCl at a concentration of 10 μg/μL. To label dividing cells, intraperitoneal injections of BrdU (50 mg/kg) were given twice daily (at 12-hr intervals) as described in the previous study (Arvidsson et al., 2002) for 3 consecutive days preceding the harvest.
Surgical Procedures
Rats were anesthetized by intraperitoneal injection of 1% ketamine (30 mg/kg) and xylazine hydrochloride (4 mg/kg). Rectal temperature was maintained at 37°C using a thermistor-controlled heat blanket. Transient MCAO was induced using an intraluminal vascular occlusion method, as previously described (Longa et al., 1989). Briefly, a 2-cm incision was made at the center of the neck, and the left common carotid artery (CCA), external carotid artery (ECA), and internal carotid artery (ICA) were exposed under an operating microscope. A length of 3/0 monofilament nylon thread whose tip was rounded by heating near a flame was advanced into the lumen of the ICA until it blocked the origin of the MCA. Two hours after MCAO, animals were reanesthetized, and reperfusion was performed by withdrawal of the suture. After recovery from anesthesia, animals were allowed free access to food and water. Rats were kept in air-ventilated cages at 24°C ± 0.5°C for the duration of the experiment.
Tissue Processing
The animals were anesthetized, and intracardiac perfusion with saline followed by ice-cold 4% paraformaldehyde in PBS (pH 7.4) was performed for 10 min. Their brains were removed, postfixed for 1 day at 4°C, and then cryoprotected overnight in the same fixative supplemented with 25% sucrose. The tissues were embedded in OCT compound (Sakura Finetek, Inc., Torrance, CA), and frozen at −70°C with dry ice. Sections were cut with a cryotome (Leica Microsystems, Wetzlar, Germany) to a thickness of 14 μm. To detect BrdU-labeled cells, sections were pretreated for 30 min in 2N HCl at 37°C to denature DNA.
Immunohistochemistry
The sections were washed with 1× PBS, permeabilized with PBS containing 0.1% (v/v) saponin and 4% (v/v) normal goat serum (NGS) for 30 min at 25°C and blocked with PBS containing 0.05% (v/v) saponin and 5% (v/v) NGS for 30 min. Sections were also permeabilized for 30 min with 0.1% (v/v) Triton X. The sections were incubated overnight at 4°C with rat anti-BrdU (1:400; Oxford Biotechnology, Oxfordshire, UK). Subsequently, sections were incubated for 1 hr at room temperature with a fluorescently labeled secondary antibody and mounted with Vectashield medium containing DAPI (Vector Laboratories, Burlingame, CA). For double-labeling procedures, sections were first labeled with rat anti-BrdU (1:400; Oxford Biotechnology, Oxfordshire, UK) to localize BrdU incorporation and then immunostained with the following antibodies: mouse anti-nestin (1:400; Chemicon, Temecula, CA), mouse anti-βIII-tubulin (Tuj1; 1:400; Covance Research Products, Inc., Denver, CO), mouse anti-NeuN (1:400; Chemicon, Temecula, CA), mouse anti-GFAP (1:600; Chemicon, Temecula, CA), mouse anti-PECAM-1 (1:300; Chemicon, Temecula, CA), mouse anti-RECA-1 (1:30; Serotec, Oxford, UK), or rabbit anti-laminin (1:30; Sigma Aldrich, Inc., St. Louis, MO). The sections were incubated with the primary antibody overnight at 4°C. Fluorescence-labeled secondary antibodies raised against the respective hosts of the primary antibodies were used at a dilution of 1:300 and incubated for 1 hr at room temperature. With the exception of Cy5 goat anti-mouse IgG (Vector Laboratories, Burlingame, CA), all secondary antibodies were purchased from Molecular Probes (Invitrogen Co., Carlsbad, CA). They included Alexa 488, Alexa 568 goat anti-rat IgG, goat antimouse IgG, and goat anti-rabbit IgG. All secondary antibody combinations were carefully examined to ensure no cross talk between fluorescent dyes and no cross-reactivity between secondary antibodies (Table I). Naive animals without BrdU injection were used as BrdU-negative controls.
| Primary/secondary antibody | Made in | Dilution | Source | Cell type labeled/conjugate |
|---|---|---|---|---|
| Anti-BrdU (IHC) | Rat | 1:400 | Oxford | Cells in S-Phase |
| Anti-BrdU (IHC) | Mouse | 1:400 | Chemicon | Cells in S-Phase |
| Biotin anti-BrdU (IHC) | Mouse | 1:400 | Chemicon | Cells in S-Phase |
| Anti-nestin (IHC, WB) | Mouse | 1:400 | Chemicon | Neural stem cell |
| Anti-NeuN (IHC) | Mouse | 1:400 | Chemicon | Mature neuron |
| Anti-Tuj1 (IHC) | Mouse | 1:400 | Chemicon | Immature neuron |
| Anti-GFAP (IHC) | Mouse | 1:600 | Chemicon | Astrocyte |
| Anti-GFAP (WB) | Mouse | 1:500 | Santa Cruz | Astrocyte |
| Anti-laminin (IHC) | Rabbit | 1:30 | Sigma Aldrich | Blood vessels |
| Anti-RECA-1 (IHC) | Mouse | 1:30 | Serotec | Endothelial cell |
| Anti-PECAM-1 (IHC) | Mouse | 1:300 | Chemicon | Endothelial cell |
| Anti-Tie2 (WB) | Rabbit | 1:200 | Santa Cruz | Endothelial cell |
| Anti-rat (IHC) | Goat | 1:600 | Molecular Probes | Alexa 568 |
| Anti-mouse (IHC) | Goat | 1:600 | Molecular Probes | Alexa 488, 568 |
| Anti-rabbit (IHC) | Goat | 1:600 | Molecular Probes | Alexa 488, 568 |
| Anti-mouse (IHC) | Goat | 1:600 | Vector laboratory | Cy5 |
| Anti-mouse (WB) | Goat | 1:1,000 | Amersham | Peroxidase |
| Anti-rabbit (WB) | Bovine | 1:1,000 | Amersham | Peroxidase |
- All antibodies made in rat and mouse were monoclonal; all others were polyclonal (IHC, immunohistochemistry; WB, Western blotting).
Confocal Microscopy
Fluorescently immunolabeled sections were analyzed on a Meta confocal microscope (model LSM 510; Carl Zeiss MicroImaging, Inc., Jena, Germany) equipped with four lasers (Diode 405, Argon 488, HeNe 543, and HeNe 633). Each channel was separately scanned using a multitrack PMT configuration to avoid cross talk between fluorescent labels. To visualize labeled structures in relation to other cells, Z sectioning at wider intervals was performed at a magnification of 20× (Plan-Neofluar, multi-immersion, NA = 0.8) for SVZ (A′), subependymal zone (SEZ; B′), striatum (C′), penumbra (D′), and cortex (E′ and F′), as shown in Figure 1B. To evaluate double labeling, confocal Z sectioning was performed at 0.5- to 1.0-μm intervals using Plan-Apochromat 63× oil-immersion (NA = 1.40) objective lenses. It was possible to alter the size of the scan field using a zoom facility, and further magnification up to 1,260× allowed for detailed observations. Images were acquired and three-dimensionally reconstructed using Zeiss LSM software.
Quantitative Analysis
Coronal forebrain sections between the crossing of the corpus callosum and the anterior commissure were analyzed. BrdU+, nestin+, GFAP+, and nestin+/GFAP+ cells were quantified in the SVZ (A′), SEZ (B′), striatum (C′), and cortex (E′ and F′), shown in Figure 1B. Counts were performed in the frontal (primary and secondary motor cortices) and parietal (primary and secondary somatosensory cortices) cortices of the neocortex in coronal forebrain sections between the crossing of the corpus callosum and the anterior commissure. Five regions of interest (ROIs) were randomly selected in the different regions. In addition, five different ROIs were selected in the striatum. A small ROI was defined as a band along the SVZ and SEZ. Each ROI was assessed in six serial sections 180 μm apart. Confocal microscopy was used to scan the entire thickness of each stained section with 4-μm, steps, and the number of BrdU+, nestin+, GFAP+, and nestin+/GFAP+ cells in each region was counted in a low-power field (200×) by a blinded investigator.
The resulting projection image was converted to grayscale using image analysis software. A similar threshold was set for all images, and the area of specific immunoreactivity was measured using an image analyzer (Image-Pro Plus, Media Cybernetics Co., Silver Spring, MD). Immunoreactivity was then expressed as the average number of positive cells per cubic millimeter. Each laminin-immunostained coronal section was used to measure vascular density, which was calculated by dividing the area of laminin-positive vessels by the total area of the ROIs. In addition, the number of colocalized of BrdU+/laminin+ and nestin+/laminin+ cells was quantified in the SEZ (B′), striatum (C′), and cortex (E′ and F′), shown in Figure 1B. Area measurements were performed using the same software.
Western Blot Analysis
The animals were anesthetized and decapitated, and whole brains were cut in 2-mm coronal sections and then rapidly divided into ipsilateral (infarcted) and contralateral hemispheres, following which they were snap-frozen in liquid nitrogen. Brain tissues corresponding to each group (control, 2 days, and 1 week after MCAO) were homogenized in lysis buffer [1× PBS containing 2% CHAPS, 1% DTT, 4.2% urea (7M), 1.52% thiourea (2M)] that included 4% protease inhibitor (Roche Diagnostics, Mannheim, Germany), followed by incubation for 2 hr at room temperature. Samples were centrifuged at 14,000g at for 5 min at 18°C–20°C. Protein concentration was determined using a Bradford assay (Bio-Rad, Hercules, CA) with bovine serum albumin as a standard. Aliquots (total volume 20 μL) of 50 μg of total protein diluted into 5× sample buffer (Pierce, Rockford, IL) were loaded and separated by electrophoresis on 10% or 12% SDS–polyacrylamide gels for nestin or GFAP and tie-2, respectively. Proteins in the gels were then electrotransferred onto poly(vinylidene fluoride) membranes (Pall Co., East Hills, NY). Blocking was performed for 1 hr with 5% nonfat dried milk (NFDM) in TBST [0.5 M Tris-HCl (pH 7.4), 1.5 M NaCl containing 0.1% Tween-20], followed by incubation overnight at 4°C with mouse anti-nestin (1:500; Chemicon, Temecula, CA), mouse anti-GFAP (1:500; Santa Cruz Biotechnology Inc., Santa Cruz, CA), or rabbit anti-Tie-2 (1:200; Santa Cruz Biotechnology Inc., Santa Cruz, CA) diluted in TBST containing 5% NFDM. Membranes were extensively washed with TBST and were then incubated for 2 hrs with horseradish peroxidase–conjugated goat antimouse IgG (Amersham, Little Chalfont, UK) or bovine antirabbit IgG (Amersham, Little Chalfont, UK) diluted 1:1,000 in TBST containing 5% NFDM. Proteins were visualized using an ECL detection system (Amersham, Little Chalfont, UK).
Statistical Analysis
Statistical analysis was performed using SPSS software. Data are expressed as the mean ± SEM of independent experiments. Differences between experimental groups and the control group were analyzed by one-way analysis of variance (ANOVA) followed by Dunnett's multiple comparison test. For the laminin-related data, differences between means were determined by the Student t test. Differences with a P value < 0.05 were considered significant.
RESULTS
Stroke-Induced Unilateral BrdU-Labeled Cells
Ischemic injury gave rise to a large number of BrdU-labeled cells in the ipsilateral SVZ (Fig. 2B,D) but not in the contralateral SVZ (Fig. 2A,C). Similarly, the number of BrdU-labeled cells increased significantly in the ipsilateral neocortex (Fig. 2F), whereas there were significantly fewer BrdU-labeled cells in the contralateral neocortex (Fig. 2E).
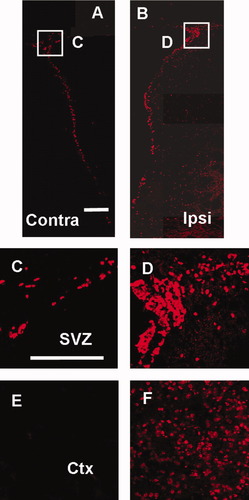
Unilateral increase in the number of BrdU-labeled cells after acute MCAO. Montage of stacked coronal images shows contralateral (A) and ipsilateral (B) SVZ. High-magnification images show contralateral (C) and ipsilateral (D) SVZ and contralateral (E) and ipsilateral (F) cortex. Scale bars = 200 μm (A–F); Contra, contralateral; Ipsi, ipsilateral; SVZ, subventricular zone; Ctx, cortex.
Temporal and Spatial Pattern of BrdU Incorporation after Stroke
To examine spatiotemporal shifts in incorporation of BrdU, immunohistochemical staining was performed. Control sections showed a typical pattern of BrdU staining, predominantly in the SVZ and SEZ (Fig. 3A,B), but not in the neocortex or striatum (Fig, 3C,D). After MCAO, incorporation of BrdU increased markedly in the neocortex (Fig. 3G,K), which does not normally take up BrdU, as well as in the SVZ (Fig. 3E,I), SEZ (Fig. 3F,J), and striatum (Fig. 3H,L). This increased incorporation of BrdU was especially marked after 2 days and peaked 1 week after MCAO. The number of BrdU-positive cells increased substantially until 1 week after MCAO in the neocortex (Fig. 3K) and in the SVZ (Fig. 3I), SEZ (Fig. 3J), and striatum (Fig. 3L) and then decreased gradually (Supplementary Fig. 1). Compared with control animals, the number of BrdU-labeled cells in the SVZ and SEZ (Fig. 3M,N) increased significantly 2 days (P < 0.01), 1 week (P < 0.01), and 2 weeks after MCAO, whereas the number of BrdU-labeled cells in the striatum and cortex (Fig. 3O,P) was significantly increased 2 days (P < 0.01), 1 week (P < 0.01), 2 weeks (P < 0.01), and 4 weeks (P < 0.05) after MCAO.
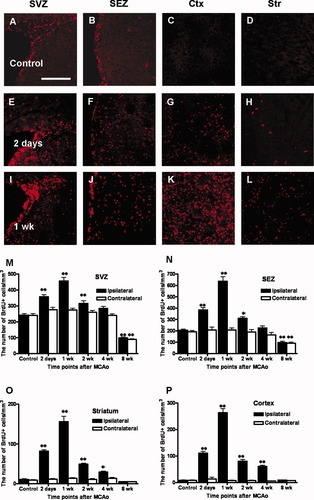
Time-dependent BrdU incorporation after MCAO and quantitative analysis of temporal changes in BrdU-labeled cells in various regions of interest (ROIs). Low-magnification images (200×) show BrdU-labeled cells in the SVZ, SEZ, Ctx, and Str in the control (A–D) and in the ipsilateral hemispheres 2 days after MCAO (E–H) and 1 week after MCAO (I–L). Data are expressed as the mean number (± SEM) of BrdU-labeled cells per cubic millimeter in the SVZ (M), SEZ (N), striatum (O), and neocortex (P). ☆Significant difference at P < 0.05; ☆☆significant difference at P < 0.01. Scale bar = 200 μm (A–L). SVZ, subventricular zone; SEZ, subependymal zone; Ctx, cortex; Str, striatum.
Characterization of BrdU-Labeled Cells
To characterize BrdU-labeled cells, immunohistochemical staining was performed for nestin, Tuj1, NeuN, and GFAP. Both BrdU- and nestin-positive cells were increased on the ipsilateral ischemic side compared with the contralateral, nonischemic side (Fig. 4A–C). Compared with that in the nonischemic control brains (Fig. 4D–G), the number of nestin- and BrdU-colabeled cells peaked 1 week after MCAO in the SVZ, SEZ, neocortex, and striatum (Fig. 4L–O) on the ipsilateral side of the ischemic brains. Notably, 2 days and 1 week after MCAO, considerable coexpression of nestin and BrdU was observed in the neocortex as well as in the SVZ, SEZ, and striatum (Fig. 4H–O) of the ipsilateral ischemic brains. Merged confocal images produced by three-dimensional reconstruction confirmed double labeling of nestin and BrdU in the neocortex 1 week after MCAO (Fig. 4P). A similar pattern of GFAP and BrdU colabeling was confirmed in the SVZ, SEZ, and striatum of the ipsilateral ischemic brains (data not shown). There were very few NeuN+/BrdU+ (Fig. 4Q) or Tuj1+/BrdU+ (Fig. 4R) cells in the SVZ. A montage of stacked coronal images allowed us to visualize the chain migration of neuronal progenitor cells 2 days after MCAO (Fig. 5A). Newborn neural precursors with a migratory morphology appeared to migrate to the striatum from the SVZ along the corpus callosum. Higher magnification revealed newborn neural progenitor cells in the striatum (Fig. 5B–D) and the SVZ (Fig. 5E–G). Meanwhile, most neuroblasts migrating from the SVZ to the olfactory bulb expressed nestin and GFAP 2 days and 1 week after MCAO (Supplementary Fig. 2).
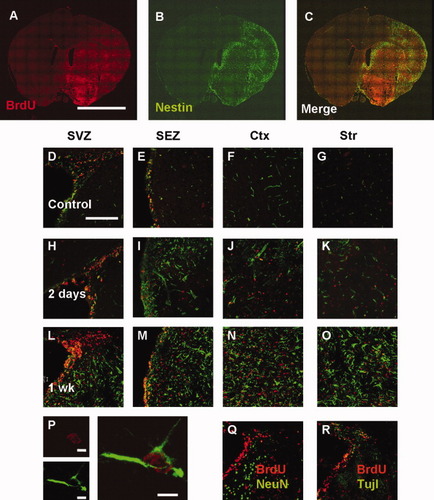
Relationship between BrdU incorporation (red) and nestin expression (green). Montage of stacked coronal images indicates the expression pattern of BrdU+ cells (A), nestin+ cells (B), and merged cells (C) 2 days after MCAO. Low-magnification images (200×) show nestin- and BrdU-expressing cells in the SVZ, SEZ, cortex, and striatum of the nonischemic control brain (D–G) and the ipsilateral side of the ischemic brain 2 days after MCAO (H–K) and 1 week after MCAO (L–O). Higher-magnification (1,260×) images show nestin- and BrdU-colabeled cells in the neocortex 1 week after MCAO (P). Scale bar = 5 mm (A–C), 200 μm (D–O, Q, R), and 5 μm (P). SVZ, subventricular zone; SEZ, subependymal zone; Ctx, cortex; Str, striatum.
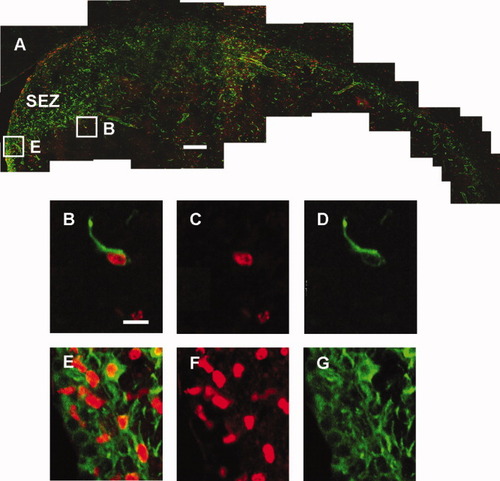
Proliferation and migration of neuronal progenitor cells from SVZ into striatum 2 days after MCAO. Montage of stacked coronal images shows chain migration of nestin (green) and BrdU (red) 2 days after MCAO (A). Higher-magnification images shows newborn neuroprogenitor cells in the striatum (B–D) and in the SVZ (E–G). Scale = 200 μm (A), 5 μm (B–G). SEZ, subependymal zone.
Ischemia-Induced Expression of Nestin and GFAP
Colocalization of nestin and GFAP was confirmed through double fluorescence labeling. A montage of stacked coronal images showed dominant expression of nestin and GFAP in the ipsilateral ischemic brain (Fig. 6A–C). Quantification confirmed asymmetrical expression of nestin and GFAP in the SVZ, SEZ, striatum (Supplementary Fig. 3), and neocortex (Fig. 6N–P). Notably, expression of GFAP was limited to a population of cells in the border zone of the ischemic cortex (Fig. 6D,F), whereas it was not detected in the neocortex ipsilateral to the ischemia. Nestin expression, however, was observed broadly in the neocortex ipsilateral to the ischemia (Fig. 6H,J) as well as in the border zone of the ischemic cortex (Fig. 6D,F). Higher-magnification three-dimensional reconstruction revealed colocalization of nestin and GFAP in the border zone of the ischemic cortex (Fig. 6E,G) and showed the topology of nestin expression in the neocortex (Fig. 6I,K).
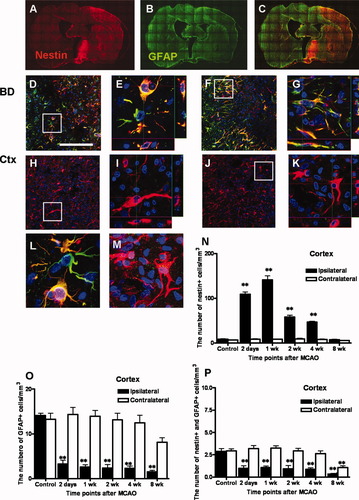
Up-regulation of the astrocyte marker GFAP (green) and the neuronal precursor cell marker nestin (red) on the ipsilateral side of the ischemic brain 2 days and 1 week following MCAO and quantitative analysis of temporal changes in nestin+, GFAP+, and nestin+/GFAP+ cells in the neocortex. Montage of stacked coronal images shows distribution of nestin+ cells (A), GFAP+ cells (B), and merged cells (C) 1 week after MCAO. Low-magnification images (200×) show numerous cells expressing GFAP and nestin in the border zone of the ischemia (D, F) and cells expressing only nestin in the neocortex ipsilateral to the lesion (H, J). Higher-magnification images (1,260×) show colocalization of nestin, GFAP, and DAPI (E, G) and of nestin and DAPI (I, K). Scale bar = 200 μm (D, F, H, and J). Data are expressed as the mean number (± SEM) of nestin+, GFAP+, and nestin+/GFAP+ cells per cubic millimeter in the cortex (N–P). Differences between means were determined by one-way analysis of variance (ANOVA), followed by Dunnett's multiple comparison test (☆significant difference at P < 0.05, ☆☆significant difference at P < 0.01; BD, border zone; Ctx, cortex).
Western Blot Analysis
Western blot analysis (Fig. 7) demonstrated that, compared with that in the normal brain, expression of GFAP and Tie-2 was amplified in the ipsilateral ischemic brains 2 days and 1 week after MCAO. Consistent with the immunohistochemical analysis, the expression of nestin peaked in the ipsilateral hemisphere 1 week after MCAO. In contrast, GFAP expression peaked 2 days after MCAO.
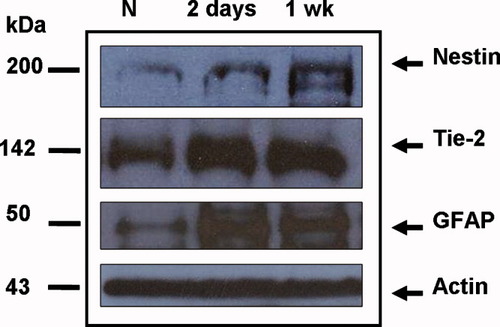
Representative Western blot of nestin, Tie-2, GFAP, and actin in samples from normal (nonischemic) and ischemic brains. Compared with that in the normal brain, expression of nestin, Tie-2, and GFAP was amplified in the ipsilateral ischemic hemisphere after MCAO.
Spatial Correlation of Neurogenesis with Neovascularization
To examine a possible association between endogenous neurogenesis and neovascularization, antibodies for RECA-1, PECAM-1, and laminin were employed to detect endothelial and vessel markers, and an antibody for nestin was used to detect endogenous neurogenesis. Microvessels and proliferating cells were detected by PECAM-1 or RECA-1 overlapping with BrdU (Supplementary Fig. 4). BrdU-labeled cells were detected in the vicinity of PECAM-1 or RECA-1, and some of the BrdU immunopositivity was colocalized with PECAM-1 or RECA-1. Compared with that in the ipsilateral ischemic brains, the number of BrdU-PECAM-1- and BrdU-RECA-1-positive cells increased substantially up to 1 week after MCAO in the neocortex as well as in the striatum and SEZ of the brain, following which it diminished. To elucidate the relationship between neurogenesis and neovascularization, triple-labeling for laminin, nestin, and BrdU was performed in the SEZ (Fig. 8A–D), cortex (Fig. 8E–H), and striatum (Fig. 8I–L). Cells double-labeled with BrdU and nestin were detected in the vicinity of the laminin-positive cells, and some BrdU-labeled cells colocalized with laminin-positive cells.
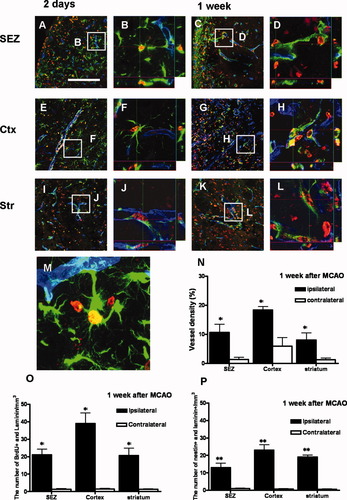
Correlation between neurogenesis and neovascularization. Low magnification (200×) shows triple labeling for laminin (blue), nestin (green), and BrdU (red) in the SEZ, cortex, and striatum 2 days (A, E, I) and 1 week (C, G, K) after MCAO. Confocal three-dimensional reconstruction shows spatial overlap between laminin+ and nestin+ cells in the SEZ, cortex, and striatum on the ipsilateral ischemic side 2 days (B, F, J) and 1 week (D, H, L) after MCAO. Representative image (1,260×) of merged three-dimensional reconstruction shows colabeling of BrdU, laminin, and nestin (M). Vascular density was calculated by dividing the area of laminin-positive vessels by the total volume of the ROIs (N). The number of cells that were BrdU+/laminin+ (O) and nestin+/laminin+ (P) was also quantified in the SEZ, striatum, and cortex. Data are expressed as the mean number (± SEM) of BrdU+/laminin+ and nestin+/laminin+ cells per cubic millimeter. Differences between means were determined by the Student t test (☆significant difference at P < 0.05, ☆☆significant difference at P < 0.01; SEZ, subependymal zone; Ctx, cortex; Str, striatum). Scale bar = 200 μm (A, C, E, G, I, K).
DISCUSSION
Endogenous Neurogenesis in the Cortical Region
In this study, quantitative immunohistochemical analysis revealed that BrdU incorporation peaked in the ipsilateral hemisphere of the ischemic brain 1 week after MCAO. Moreover, a remarkable increase in the number of cells in which nestin and BrdU colocalized was observed in the neocortex, where endogenous neurogenesis was previously thought to occur only rarely. It is unclear whether these cells arise from the SVZ or from intraparenchymal progenitors in the nearby neocortex.
If neuronal precursors can be recruited from their original sites to the site of an ischemic injury, it is possible that ischemic injury increases the migration rate of neuronal precursors from the SVZ to the neocortex. The speed of SVZ cells migrating in the rostral migratory stream (RMS) of the uninjured brain has been estimated at 23 μm/hr (Luskin and Boone, 1994). In this study, however, considerable coexpression of nestin and BrdU was observed in the neocortex of the ipsilateral ischemic brains 2 days after MCAO and 3 days after the rats received a 3-consecutive-day injection of BrdU prior to sacrifice. Three days after the BrdU injections seemed too short a duration for SVZ cells to migrate from the SVZ to the neocortex at an estimated migration speed of 23 μm /hr. Therefore, we speculate that the newborn cells observed in the neocortex 2 days post-MCAO were not derived from the neuronal precursors residing in the SVZ. Several reports have suggested endogenous neurogenesis constitutively occurs, albeit at a low level, in specific regions of the neocortex of adult primates (Gould et al., 1999, 2001), in the visual cortex of adult rats (Kaplan, 1981), and in the rodent neocortex (Dayer et al., 2005). In addition, the possible existence of cortically located adult multipotent neural precursors has been described in vitro (Marmur et al., 1998; Palmer et al., 1999). The results of those studies raise the possibility that after various types of brain injury, there exists a sequence and combination of molecular signals that stimulate endogenous neurogenesis in the adult cerebral cortex. Although several reports have demonstrated that a small number of neuroblasts appear in the periinfarct neocortex after stroke (Parent et al., 2002; Jin et al., 2003; Zhang et al., 2004), Magavi et al. (2000) suggested that constitutively occurring neurogenesis was completely absent in the murine neocortex. However, that assertion appears to reflect the methodological problems with their study. Specifically, because there were fewer nestin-labeled cells in the neocortex than in the SVZ, they were difficult to recognize on the low-power fluorescence microscopy used in the Magavi et al. study (2000). Contrary to their observations, nestin-labeled cells were easily observed in the high-power field examination in the present study.
Also, for several reasons, it is unlikely that those nestin-positive cells in the neocortex were a result of BrdU-labeling of cells undergoing DNA repair or abortive mitosis. First, BrdU immunostaining, using a staining protocol nearly identical to that used in the present study, is very specific for dividing cells or cells with very high levels of DNA repair, as discussed previously (Palmer et al., 2000; Cameron and McKay, 2001; Dayer et al., 2005). Second, if cells undergoing DNA repair incorporate detectable amounts of BrdU, BrdU labeling should have been observed in various types of cells. However, very few cells colabeled with BrdU and NeuN were detectable in either the SVZ or the neocortex, regions in which extensive cell loss occurred.
Taking all these explanations together, nestin-labeled cells in the neocortex might arise from neural progenitor cells that reside in the cortex itself. It is interesting that the expression of nestin was up-regulated 1 week post-MCAO in the ipsilateral neocortex of ischemic brains. The number of BrdU+/nestin+ cells increased markedly until 1 week after MCAO not only in the SVZ, hippocampus, and striatum of the brain, which have all been described in previous studies, but also in the neocortex. It is noteworthy that GFAP expression was limited to a population of cells in the border zone of the ischemic area, whereas nestin expression was observed broadly in the neocortex.
Characterization of BrdU-Labeled Cells
In the neocortex, SVZ, SEZ, and striatum, most BrdU-positive cells were also immunopositive for nestin, implying that endogenous neurogenesis was induced by MCAO in all these regions. Within the SVZ, some of these cells were also Tuj1 positive, confirming their neuronal identity. Nestin is expressed in both embryonic and adult CNS stem cells. In the embryonic CNS, nestin expression is closely associated, both spatially and temporally, with the proliferation of neuronal progenitor cells (Lendahl et al., 1990) and is also expressed in adult stem cells both in vivo and in vitro under nondifferentiating conditions (Johe et al., 1996). Although there were a few BrdU+/NeuN+ cells in the SVZ, SEZ, and SGZ of the hippocampus, no detectable BrdU-NeuN-colabeled cells were found in the neocortex of the ischemic brain. In agreement with previous reports, BrdU-positive cells were often observed close to NeuN-positive cells (Arvidsson et al., 2002). It might be speculated that the survival time of 3 days after BrdU injection is not sufficient for neural progenitor cells to differentiate into mature neurons. Another report described observing BrdU+/NeuN+ neurons in the brain a much longer time after BrdU injection, that is, 4 or 5 weeks (Dayer et al., 2005).
In the SVZ, SEZ, and SGZ of the hippocampus, most BrdU-labeled cells were colocalized with GFAP, confirming their astrocytic identity, whereas in the neocortex, most BrdU-labeled cells were colocalized with nestin but not with GFAP. The GFAP-expressing cells we observed in the SVZ, SEZ, and SGZ of the hippocampus might be neuronal progenitor cells, as described in previous studies (Seri et al., 2001; Garcia et al., 2004). Newly generated GFAP-positive neuronal precursor cells have gained significant attention both in the SVZ and the SGZ of the hippocampus (Seri et al., 2001; Garcia et al., 2004). In addition, it has been found that coculturing adult-derived precursor cells with astrocytes induced their development into electrically active neurons that formed networks in vitro (Song et al., 2002). However, the possibility that GFAP-expressing cells in the border zone of the ischemic cortex are reactive astrocytes cannot be excluded.
Endogenous Neurogenesis and Neovascularization
The existence of a spatial overlap between proliferating cells and microvessels was revealed by immunohistochemical staining and confocal microscopy. Confocal three-dimensional stacks confirmed the spatial arrangement of laminin-positive and nestin-positive cells. The present results correspond with those of other recent studies reporting that neural stem cells lie close to blood vessels in the hippocampus (Palmer et al., 2000), in the SVZ (Capela and Temple, 2002), and in the higher vocal center of songbirds (Louissaint et al., 2002). In addition, endothelial cells have been identified as critical components of the neural stem cell niche, as they secrete soluble factors that maintain CNS stem cell self-renewal and neurogenic potential, whereas the maintaining of blood flow to these injured areas may be enough to allow these newly born cells to survive. Furthermore, vascular cells lie close to CNS germinal zones throughout life, and it has been suggested that they form a niche for neural stem cells (Shen et al., 2004). Consistent with previous reports, our results demonstrated that nestin-positive neural stem cells lie in the vicinity of the endothelial cells lining cerebral vessels in the process of neovascularization.
However, further studies are warranted to further clarify the coupling and cross talk between endogenous neurogenesis and neovascularization in the neocortex of the ischemic brain.




