Stimulation of H2O2 generation by calcium in brain mitochondria respiring on α-glycerophosphate
Abstract
It has been reported recently (Tretter et al., 2007b) that in isolated guinea pig brain mitochondria supported by α-glycerophosphate (α-GP) reactive oxygen species (ROS) are produced through the reverse electron transport (RET) in the respiratory chain and by α-glycerophosphate dehydrogenase (α-GPDH). We studied the effect of calcium on the generation of H2O2 as measured by the Amplex Red fluorescent assay in this model. H2O2 production in α-GP-supported mitochondria was increased significantly in the presence of 100, 250, and 500 nM Ca2+, respectively. In addition, Ca2+ enhanced the membrane potential, the rate of oxygen consumption, and the NAD(P)H autofluorescence in these mitochondria. Direct measurement of α-GPDH activity showed that Ca2+ stimulated the enzyme by decreasing the Km for α-GP. In those mitochondria where RET was eliminated by the Complex I inhibitor rotenone (2 μM) or due to depolarization by ADP (1 mM), the rate of H2O2 formation was smaller and the stimulation of H2O2 generation by Ca2+ was prevented partly, but the stimulatory effect of Ca2+ was still significant. These data indicate that in α-GP-supported mitochondria activation of α-GPDH by Ca2+ leads to an accelerated RET-mediated ROS generation as well as to a stimulated ROS production by α-GPDH. © 2007 Wiley-Liss, Inc.
Production of reactive oxygen species (ROS) in mitochondria is part of the normal oxidative metabolism in eukaryotic cells but most of the studies dealing with mitochondrial ROS generation have addressed the pathologic aspects of an excessive ROS production. It is well established that diseases such as Parkinson's disease (PD) (Beal, 2003; Gandhi and Wood, 2005) and Alzheimer's disease (AD) (Beal, 2005; Reddy and Beal, 2005) or ischemia-reperfusion syndrome (Siesjo et al., 1999; Starkov et al., 2004a; Christophe and Nicolas, 2006) are associated with an increased ROS production in the brain.
Most of the potentially important, in vitro well characterized mitochondrial sites of ROS generation are associated with dehydrogenase enzymes, either in the respiratory chain (NADH dehydrogenase) (Hansford et al., 1997; Barja and Herrero, 1998; Arnaiz et al., 1999; Gyulkhandanyan and Pennefather, 2004; Adam-Vizi, 2005; Adam-Vizi and Chinopoulos, 2006) or in the matrix (α-ketoglutarate dehydrogenase) (Tretter and Adam-Vizi, 2004; Starkov et al., 2004b; Tretter and Adam-Vizi, 2005). In isolated mitochondria ROS are produced by different mechanisms in the presence of NADH-linked substrates feeding electrons to Complex I or with the Complex II substrate, succinate. The membrane potential of mitochondria (ΔΨm) generated by succinate is sufficient to allow the flow of a fraction of electrons in reverse via Complex I, defined as reverse electron transport (RET) (Azzone et al., 1963; Hinkle et al., 1967), leading to NADH formation (Hansford et al., 1997; Liu et al., 2002; Gyulkhandanyan and Pennefather, 2004) and ROS generation by Complex I (Boveris and Chance, 1973; Korshunov et al., 1997; Votyakova and Reynolds, 2001; Liu et al., 2002). Small decrease in ΔΨm inhibited ROS generation in succinate-supported mitochondria due to the prevention of RET (Korshunov et al., 1997; Tretter et al., 2007a). Electrons derived from NADH-linked substrates generate ROS in the forward electron transport via the respiratory chain mainly by Complex I (Hansford et al., 1997; Votyakova and Reynolds, 2001). See Adam-Vizi (2005) for a review.
The ROS production associated with oxidation of α-GP is relatively neglected, although it could be important potentially. α-GPDH participates in the series of reactions called glycerophosphate shuttle (Klingenberg, 1970), necessary to transport glycolytic NADH into mitochondria. The role of the glycerophosphate shuttle in the brain is controversial, but numerous studies indicate that in neurons it is functional (Cammer et al., 1982; McKenna et al., 1993, 2006; Atlante et al., 1999; Waagepetersen et al., 2001). α-GPDH is localized in the outer surface of the mitochondrial inner membrane (Donnellan et al., 1970; Klingenberg, 1970) and electrons derived from the oxidation of α-GP to dihydroxyacetone phosphate are donated via FADH2 to ubiquinone. Several authors have reported that ROS is produced in α-GP supported mitochondria (Zoccarato et al., 1988; Kwong and Sohal, 1998; Starkov and Fiskum, 2001; Drahota et al., 2002; Miwa et al., 2003; Miwa and Brand, 2005; Chowdhury et al., 2005). The α-GP-associated ROS production has been characterized recently in brain mitochondria, and both RET and α-GPDH have been found to contribute to the generation of ROS (Tretter et al., 2007b). These studies, however, did not address the effect of Ca2+ on ROS generation. Ca2+ has been reported to stimulate α-GPDH in liver mitochondria (Wernette et al., 1981; Beleznai et al., 1988) but there are no data available as to the effect of Ca2+ on ROS formation in α-GP-supported brain mitochondria.
The effects of Ca2+ on ROS formation in isolated mitochondria are controversial. Published data show extreme heterogeneity, possibly originating from the different experimental conditions used e.g., the amount of Ca2+ applied to challenge mitochondria, the state of respiration of mitochondria (state 3 or state 4), the presence or absence of uncouplers, phosphate, ATP, or respiratory inhibitors. An overall conclusion can be formulated that electrophoretic uptake of Ca2+ would decrease ΔΨm-dependent ROS formation, but this rule can be confounded by the opening of the permeability transition pore, by the possible inhibition of respiratory complexes, or by Ca2+-dependent stimulation of Krebs cycle dehydrogenases with a consequent increase in the NAD(P)H level (Brookes et al., 2004; Starkov et al., 2004a; Starkov, 2006).
The present study is an extension of our recent work (Tretter et al., 2007b) on the α-GP-supported ROS generation, addressing the effect of Ca2+ applied in a physiologic concentration range on the H2O2 formation in isolated brain mitochondria. Our main finding is that Ca2+ stimulates α-GP-supported ROS production that is associated with the activation of α-GPDH by Ca2+.
MATERIALS AND METHODS
Preparation of Mitochondria Using a Discontinuous Percoll Gradient
Mitochondria were prepared from the brain of male guinea pigs as described previously (Tretter and Adam-Vizi, 2006) The animals were decapitated by a process being in accordance with the Guidelines for Animal Experiments at Semmelweis University. The brain was homogenized in Buffer A, (225 mM mannitol, 75 mM sucrose, 5 mM HEPES, 1 mM EGTA, pH = 7.4 [KOH]) and centrifuged for 3 min at 1,300× g. The supernatant was centrifuged for 10 min at 20,000× g, and then the pellet was suspended in 15% Percoll and layered on a discontinuous gradient consisting of 40% and 23% Percoll, respectively, and then centrifuged for 8 min at 30,700× g without using brakes. After resuspension of the lowermost fraction in Buffer A, it was centrifuged at 16,600× g for 10 min, the pellet was then resuspended in Buffer A, and centrifuged again at 6,300× g for 10 min. After discharging the supernatant, the pellet was resuspended in Buffer B (225 mM mannitol, 75 mM sucrose, 5 mM HEPES, pH = 7.4 [KOH]) (Sims, 1990).
Incubation Buffer.
Experiments were carried out in an incubation medium containing: 125 mM KCl, 20 mM HEPES, 2 mM K2HPO4, 1 mM MgCl2, 0.1 mM EGTA, pH = 7.0 (KOH) supplemented with fatty acid free BSA (0.025%). To calculate the required amount of Ca2+ the Chelator software was used (Schoenmakers et al., 1992) and checked by free Fura-2 fluorescence (Grynkiewicz et al., 1985).
Measurement of Mitochondrial H2O2 Production
The assay is based on the detection of H2O2 in the medium using the Amplex Red fluorescent dye (Mohanty et al., 1997). The Amplex Red reagent reacts with H2O2 with a 1:1 stoichiometry producing highly fluorescent resorufin, in the presence of horseradish peroxidase. Amplex Red reagent (1 μM) and horseradish peroxidase (5 U/2 ml) were added to the incubation medium described above, and mitochondria (0.05 mg/ml) were added. Fluorescence after H2O2 formation was detected at 37°C in a Deltascan fluorescence spectrophotometer (Photon Technology International, Lawrenceville, NJ). The excitation and emission wavelengths were 550 and 585 nm, respectively. Calibration signals were created with known amounts of H2O2 at the end of each experiment.
Assay of NAD(P)H Fluorescence
The matrix NAD(P)H autofluorescence (344 nm excitation, 460 nm emission wavelengths) was measured in parallel with the Amplex assay exploiting the possibility of double excitation and double emission mode of PTI Deltascan fluorescence spectrophotometer.
Measurement of Mitochondrial Membrane Potential
ΔΨm was detected using the cationic dye safranine O, which is accumulated and quenched inside energized mitochondria (Akerman and Wikstrom, 1976). The excitation and emission wavelengths were 495 and 586 nm, respectively. The dye concentration used was 2 μM. We did not make an attempt to calibrate the safranine O signal.
Measurement of Mitochondrial Oxygen Consumption
Oxygen uptake by mitochondria (0.5 mg/0.5 ml) was measured with a Clark-type oxygen electrode at 37°C using Hansatech Oxygraph Measurement System (Hansatech, Norfolk, UK). The quality of the mitochondrial preparation was estimated with the measurement of the respiratory control ratio (RCR) defined as ADP-stimulated (State 3) respiration divided by resting (State 4) respiration using glutamate–malate (5–5 mM) substrates. State 3 respiration was initiated by addition of ADP (1 mM) to the incubation medium. The effect of ADP was suspended and State 4 respiration was initiated by carboxyatractylate (1 μM), an inhibitor of the ADP/ATP translocator. Only mitochondria showing RCR >12 were used in the experiments.
Measurement of α-GPDH Activity
Enzyme activity was determined in intact mitochondria with a modification of the method of (Wernette et al., 1981). Mitochondria were incubated in 0.4 ml volume containing 25 μg mitochondrial protein. The incubation medium was complemented with no calcium or 100 nM free Ca2+, 1 mM KCN, 0.2% iodonitrotetrazolium chloride, and various amounts of D,L-α-glycerophosphate. The reaction was stopped after 10 min by formaldehyde, Triton X-100, and formic acid as described earlier (Tretter et al., 1987) The formazan was solubilized completely and the absorbance at 490 nm was detected directly.
Statistics
Results are expressed as mean ± SEM values. Statistical significance was calculated using a one-way ANOVA (Sigmastat). Differences were considered significant at a level of P < 0.05.
Materials
Standard laboratory chemicals were obtained from Sigma (St. Louis, MO). The Amplex red reagent and Fura-2 pentapotassium salt were purchased from Invitrogen (Carlsbad, CA).
RESULTS
Stimulation of H2O2 Production by Ca2+
Mitochondrial ROS formation was studied in isolated guinea pig brain mitochondria in the absence of ADP using the Amplex red fluorescent assay. The rate of H2O2 generation was dependent on the concentration of α-GP (Fig. 1A), in agreement with that reported recently by Tretter et al. (2007b). Elevation of extramitochondrial Ca2+ concentration from nominally zero to 100 nM significantly increased the rate of H2O2 formation. The calcium-induced elevation of H2O2 production was most pronounced at low α-GP concentrations 2–10 mM; (Fig. 1A, Table I). At high (40 mM) substrate concentration the effect of Ca2+ on ROS formation was not significant (Table I). It should be noted that having such a high α-GP concentration in in vivo mitochondria is very unlikely. Depolarization of mitochondria by the uncoupler FCCP (250 nM) at the end of each experiment decreased H2O2 production by 75–80%. The decrease in H2O2 formation in response to depolarization indicated that RET could be responsible at least partially for the Ca2+-stimulated H2O2 production in the presence of α-GP.
| α-Glycerophosphate | ||||||
|---|---|---|---|---|---|---|
| 1 mM | 2 mM | 5 mM | 10 mM | 20 mM | 40 mM | |
| Baseline | 58.6 ± 6.6 | 74.8 ± 9.7 | 85.4 ± 12.4 | 95.9 ± 14.1 | 253 ± 35.4 | 602 ± 53.6* |
| Ca2+ 100 nM | 98.7 ± 19.5 | 270 ± 47.1 | 368 ± 61.0 | 435 ± 66.3 | 525 ± 61.7 | 729 ± 63.4* |
| n | 6 | 8 | 8 | 13 | 9 | 7 |
- H2O2 production was measured as described in Materials and Methods. Experiments were carried out in the presence of α-GP (1–40 mM) as shown in Figure 1. After measuring the basal H2O2 formation Ca2+ (100 nM) was added and ROS formation was further followed. H2O2 production is expressed in pmol/min/mg protein ± SEM. Data measured in the presence of Ca2+ were significantly different from the “baseline” data at each α-GP concentration except at 40 mM α-GP indicated by (*). n, number of measurements.
Increase in the NAD(P)H level by Ca2+
Figure 1B shows the NAD(P)H autofluorescence recorded simultaneously with the H2O2 measurement. In agreement with our previous results (Tretter et al., 2007b), addition of α-GP in >10 mM concentrations resulted in an increase in the NAD(P)H fluorescence. Ca2+ given in 100 nM concentration, 200 sec after the addition of α-GP, further increased the NAD(P)H steady state level indicating that the rate of RET was increased by Ca2+. The effect of Ca2+ was already apparent at 2–10 mM α-GP concentrations where the steady level of NAD(P)H showed the lack of NADH formation (lack of RET) in the absence of Ca2+. Depolarization of mitochondria by FCCP caused an immediate drop in the NAD(P)H fluorescence. Addition of Ca2+ (100, 250, or 500 nM) in the absence of α-GP did not induce any increase in ROS production or in the NAD(P)H steady state level.
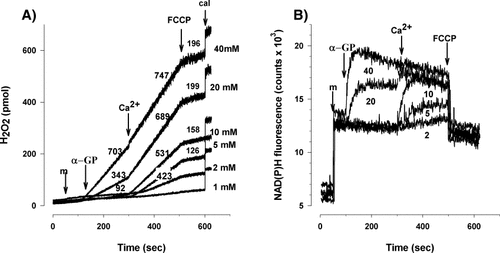
The effect of Ca2+ (100 nM) on H2O2 formation (A) and NAD(P)H autofluorescence (B) in mitochondria supported by α-GP. H2O2 production and NAD(P)H autofluorescence were measured simultaneously as described in Materials and Methods. Mitochondria (m) and α-GP in a concentration indicated (1–40 mM) were added followed by addition of Ca2+ (100 nM) and FCCP (250 nM). At the end of each experiment known amounts of H2O2 was added for the calibration (cal). Numbers indicate in (A) the rate of H2O2 release in pmol/min/mg protein, in (B) the concentrations of α-GP. Traces are representative of four independent experiments.
Increase in ΔΨm by Ca2+
For electrons to be transferred in reverse, high ΔΨm is required. RET has been shown to contribute to the α-GP-induced H2O2 production in Drosophila (Miwa et al., 2003) and in brain mitochondria (Tretter et al., 2007b). We measured the Safranine fluorescence as an indicator of ΔΨm and Figure 2A shows that ΔΨm set at different levels with 10 or 20 mM α-GP was further increased by the addition of Ca2+ (100–500 nM). The highest ΔΨm observed with 40 mM α-GP, was not further hyperpolarized by Ca2+. The Safranine fluorescence method would not allow to quantitate the Ca2+-evoked changes in ΔΨm in mV-s, but showed clearly a hyperpolarizing effect of Ca2+. Figure 2B shows that the stepwise hyperpolarization of ΔΨm induced by consecutive additions of Ca2+ (100, 250, 500 nM, respectively) shown in Figure 2A paralleled a stepwise increase in NAD(P)H fluorescence and H2O2 generation in the presence of 10 mM α-GP.
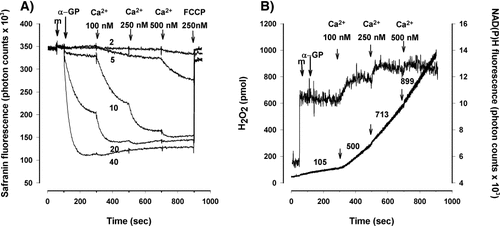
Changes in ΔΨm in α-GP-supported mitochondria by Ca2+ (A). ΔΨm was measured by the Safranin fluorescence assay as described in Materials and Methods. After addition of mitochondria (m) α-GP was given in 2–40 mM concentrations followed by addition of Ca2+ to reach the concentrations indicated. FCCP (250 nM) was added at the end of the measurements. Numbers on the traces indicate the concentrations of α-GP in mM. Traces are representative of three independent experiments. The effect of consecutive additions of Ca2+ (B) added as shown for (A) on the H2O2 production (left axis; lower trace) and on the NAD(P)H steady state level (right axis; upper trace) in mitochondria supported with 10 mM α-GP. Traces are representative of three independent experiments. Numbers on the lower trace indicate the rate of H2O2 generation in pmol/min/mg protein.
Stimulation of the Oxygen Consumption by Ca2+
The effects of Ca2+ should also be manifested in the oxygen consumption. Ca2+-induced increase in ΔΨm indicates a higher proton gradient across the mitochondrial inner membrane that could be associated with an increased flow of electrons and consequently with an increased oxygen consumption. Indeed, addition of Ca2+ (100 nM) to mitochondria respiring on α-GP (10 mM) accelerated the oxygen consumption from 25.2 ± 2.7 to 39.2 ± 2.4 nmol/min/mg protein. Furthermore, addition of Ca2+ to uncoupled (FCCP-treated) mitochondria also resulted in an elevated oxygen consumption (Table II) showing that the stimulation of respiration did not result from a depolarization due to Ca2+ uptake into mitochondria.
| α-Glycerophosphate | |||||
|---|---|---|---|---|---|
| 1 mM | 2 mM | 5 mM | 10 mM | 20 mM | |
| Baseline | 5.9 ± 0.2 | 10.5 ± 2.7 | 18.9 ± 3.9 | 22.2 ± 4.9 | 30.1 ± 8 |
| FCCP | 8.9 ± 0.2 | 13.6 ± 3.5 | 24.7 ± 4.8 | 34.7 ± 9.6 | 49.6 ± 8.3 |
| FCCP + Ca2+ | 17 ± 4.9 | 22.5 ± 7.7 | 35.6 ± 6.9 | 45.8 ± 9.9 | 58.3 ± 10.3 |
- Oxygen consumption was recorded in mitochondria in the presence of α-GP (1–20 mM). After recording the basal oxygen consumption (baseline), FCCP (250 nM) was added followed by the addition of Ca2+ (100 nm). Oxygen consumption is expressed in nmol/min/mg protein ± SEM (n = 4).
Stimulation of α-GPDH by Ca2+
The increased rate of oxygen consumption shown in Table II could result from an activation of α-GPDH by Ca2+. Therefore we compared the activity of α-GPDH measured in the absence or presence of 100 nM Ca2+. In these measurements the final acceptor for the electrons derived from α-GP was not oxygen but a tetrazolium salt, as described in Materials and Methods. Two classical Michaelis-Menten curves were created by plotting the reaction velocity (nitrotetrazolium formazan formation) measured in the presence and in the absence of 100 nM Ca2+ as a function of α-GP concentrations (data not shown). The Km for α-GP was found to be decreased from 10.5 mM to 2 mM in the presence of 100 nM Ca2+. Our results are in agreement with results published earlier on the Ca2+-induced stimulation of rat liver mitochondrial α-GPDH (Wernette et al., 1981).
Prevention of RET by Rotenone or ADP Partially Inhibits H2O2 Generation Stimulated by Ca2+ in α-GP-Supported Mitochondria
Our recent study (Tretter et al., 2007b) as well as results shown in Figure 1 indicated that RET contributed to the H2O2 generation in α-GP-supported mitochondria. To address whether the stimulation of H2O2 formation by Ca2+ is due to an accelerated RET, we studied the effects of Ca2+ in mitochondria where RET was prevented either by the Complex I inhibitor rotenone or ADP. Rotenone added after Ca2+ (500 nM) decreased the rate of H2O2 generation (Fig. 3A, upper trace) supporting the involvement of RET in the Ca2+-stimulated ROS formation. However, when RET was first abolished by rotenone (Fig. 3A, lower trace), Ca2+ was still able to stimulate the production of H2O2, although to a lesser extent than without rotenone (367 vs. 788 pmol/min/mg protein). These experiments were carried out in the presence of 10 mM α-GP and it is clear from the NAD(P)H measurements (Fig. 1B) that at this α-GP concentration no RET can be detected in the absence of Ca2+. The inhibition of the Ca2+-stimulated ROS formation by rotenone (from 788 to 521 pmol/min/mg protein), however, indicates that in the presence of Ca2+ RET is functional even with 10 mM α-GP and is responsible partly for the accelerated H2O2 generation. On the other hand, stimulation of H2O2 formation by Ca2+ in mitochondria where RET was prevented by rotenone (Fig. 3A, lower trace) showed clearly an additional site of H2O2 generation that was stimulated by Ca2+. Figure 3B shows that with succinate as a substrate H2O2 formation is not influenced by Ca2+ either in the presence (Fig. 3B, lower trace) or absence of rotenone (Fig. 3B, upper trace). Using lower concentrations of succinate (0.1 and 0.2 mM) the basal ROS production was smaller and was also unaltered by Ca2+ (data not shown). Because H2O2 is generated largely by RET in well-coupled succinate-supported mitochondria (Korshunov et al., 1997; Votyakova and Reynolds, 2001; Liu et al., 2002; Tretter et al., 2007b), the lack of effect of Ca2+ in these experiments emphasizes the requirement of α-GP as a respiratory substrate for the stimulation of H2O2 formation by Ca2+.
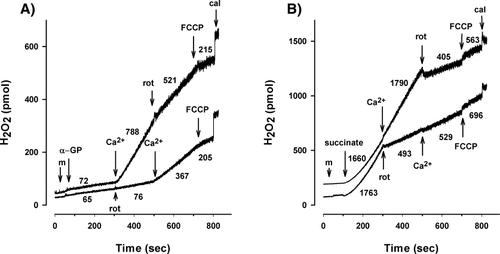
The effect of rotenone on the Ca2+-stimulated H2O2 generation in α-GP-supported (A) and in succinate-supported mitochondria (B). Mitochondria (m), α-GP (10 mM), succinate (5 mM), Ca2+ (500 nM), rotenone (rot; 2 μM), and FCCP (250 nM) were added as shown. At the end of each experiment known amounts of H2O2 was added for the calibration (cal). Numbers indicate the rate of H2O2 release in pmol/min/mg protein. Traces are representative of three independent experiments.
Further support for the involvement of RET in the Ca2+-stimulated H2O2 formation in α-GP-supported mitochondria was obtained from experiments with ADP, which also eliminates RET due to the depolarization of mitochondria. ADP (1 mM) decreased the NAD(P)H level that was elevated previously by addition of Ca2+ (500 nM) and in parallel, decreased the rate of H2O2 generation (Fig. 4A). ADP also induced a robust decrease in the succinate-supported H2O2 generation (Fig. 4B).
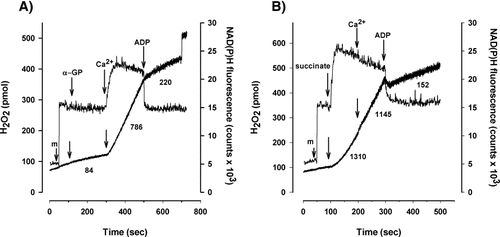
Simultaneous measurements of H2O2 release and NAD(P)H fluorescence in mitochondria supported by 10 mM α-GP (A) or 5 mM succinate (B). The effect of ADP (1 mM) added after Ca2+ (500 nM). Upper traces show NAD(P)H fluorescence, lower traces show H2O2 production. Addition of mitochondria (m) and further additions were as shown. Numbers indicate the rate of H2O2 release in pmol/min/mg protein. Traces are representative of three independent experiments.
DISCUSSION
The present study shows that the production of H2O2 is stimulated by Ca2+ in α-GP-supported isolated guinea pig brain mitochondria. The principal underlying effect of Ca2+ is the stimulation of α-GPDH resulting in an enhanced ROS generation by at least two distinct mechanisms. Stimulation of α-GPDH by Ca2+, on one hand, accelerates the electron influx into the respiratory chain and the higher proton-motive force could promote RET and ROS generation by RET. On the other hand, it involves a stimulated ROS generation by the enzyme itself.
α-GPDH is part of the α-GP shuttle participating in the transport of cytosolic NADH into the mitochondria (Klingenberg, 1970). There are tissue specific and developmental variations in the activity of α-GPDH. Maechler et al. (1997) claimed recently that mitochondrial α-GPDH is a key component of the pancreatic β-cell glucose sensing device, and mutations in the calcium-binding domain of the enzyme have been detected in patients suffering from familial Type-2 diabetes (Novials et al., 1997). The existence of the α-GP shuttle in the brain has been shown in several studies (Cammer et al., 1982; McKenna et al., 1993; Atlante et al., 2001; Waagepetersen et al., 2001) and high rate of α-GP oxidation has been found in brain mitochondria (Beleznai et al., 1988; Zoccarato et al., 1988; Tretter et al., 2007b).
Oxidation of α-GP in mitochondria is associated with generation of ROS (Patole et al., 1986; Zoccarato et al., 1988; Kwong and Sohal, 1998; Starkov and Fiskum, 2001; Drahota et al., 2002; Miwa et al., 2003; Gyulkhandanyan and Pennefather, 2004; Miwa and Brand, 2005; Chowdhury et al., 2005) and we have established recently that in α-GP-supported brain mitochondria ROS is formed through RET and in the reaction catalyzed by α-GPDH (Tretter et al., 2007b). RET-related ROS generation is dominant at higher α-GP concentrations (20 or 40 mM) when the proton-motive force is high enough to support the flow of electrons in reverse resulting in the reduction of NAD+ to NADH (Tretter et al., 2007b). This is also confirmed in the present study showing an increase in the NAD(P)H level in response to the addition of α-GP in 20 or 40 mM concentration (Fig. 1B). The proton-motive force generated by smaller, probably more physiologic concentrations of α-GP (≤10 mM) seems to be insufficient to drive the electrons in reverse and ROS production under this condition is smaller and attributed largely to α-GPDH (Tretter et al., 2007b).
We show that Ca2+ in a physiologic concentration range stimulates H2O2 generation associated with α-GP oxidation and suggest that in this effect the stimulation of α-GPDH by Ca2+ is the crucial underlying mechanism. Ca2+-mediated activation of the enzyme was first detected in insect flight muscle mitochondria (Hansford and Chappell, 1967) and later confirmed for mitochondria deriving from other tissues including the brain (Estabrook and Sacktor, 1958; Fisher et al., 1973; Bukowiecki and Lindberg, 1974; Wernette et al., 1981; Beleznai et al., 1988; Malaisse et al., 1991). Our study shows that activation of α-GPDH by Ca2+ results in a hyperpolarization of the inner membrane and in an increase in the NAD(P)H steady state level (Figs. 1,2) indicating that the activated α-GPDH provide more electrons to the respiratory chain and the higher ΔΨm creates a favorable condition for RET. This was supported clearly by the observation that prevention of RET either by rotenone (Fig. 3A) or ADP (Fig. 4A) decreased the H2O2 generation stimulated by Ca2+. It is worth noting that in the presence of 100 nM Ca2+ RET was induced at small α-GP concentrations (5 or 10 mM), where no RET could be detected without Ca2+ (Figs. 1B,2B).
RET is a phenomenon likely to occur only in isolated mitochondria and with only substrates (succinate or α-GP) that donate electrons to the respiratory chain via ubiquinone by-passing Complex I (Adam-Vizi and Chinopoulos, 2006). There is no supporting evidence for the existence of RET in isolated mitochondria respiring on NADH-linked substrates or in in situ mitochondria where the majority of electrons are also donated to the respiratory chain by NADH. Therefore, the Ca2+-stimulated RET-related component of ROS formation found in α-GP-supported mitochondria might not be applicable to in vivo brain mitochondria.
Stimulation of H2O2 generation by Ca2+ in the presence of rotenone (Fig. 3A) or ADP (Fig. 4A) indicated an additional Ca2+-sensitive mechanism unrelated to RET but attributable to α-GPDH. Similarly to other flavin containing dehydrogenases (Massey, 1994), the FAD+ prosthetic group of α-GPDH could be an immediate source of ROS. The Amplex red fluorescence assay used in our study is able to detect H2O2 with high accuracy and specificity. However, the primary ROS produced by the enzyme could be either superoxide or H2O2 or both. Superoxide produced on the outer surface of the mitochondrial inner membrane can be dismutated by the intermembrane space Cu-Zn SOD to H2O2, thus will be detectable for the Amplex red assay.
There was an apparent difference in the Ca2+-stimulated ROS generation measured in the absence of RET depending on the means by which RET was prevented. In the presence of rotenone, which eliminates RET due to Complex I inhibition without changing the ΔΨm, the rate of ROS formation could be elevated by Ca2+ by five- to six-fold (Fig. 3A, lower trace), whereas in the presence of ADP, which causes depolarization, the remaining Ca2+-stimulated ROS generation after abolishing RET was smaller (Fig. 4A, lower trace). This might suggest that ROS generation by α-GPDH is also dependent on ΔΨm. The result that depolarization of rotenone-treated mitochondria by FCCP decreased the rate of the Ca2+-stimulated ROS generation (Fig. 3A) further supported this suggestion. The latter result is in agreement with that obtained in Drosophila mitochondria (Miwa et al., 2003).
Several authors have reported that massive mitochondrial Ca2+ accumulation promotes ROS generation during ischemia/reperfusion (Brookes et al., 2004; Starkov et al., 2004a) and excitotoxicity (Nicholls and Budd, 1998; Stout et al., 1998). The mechanism by which Ca2+ accumulation leads to accelerated mitochondrial ROS formation, however, is not clear. It is suggested that mitochondrial Ca2+ accumulation triggers cytochrome c release by mitochondrial permeability transition (MPT) dependent and independent pathways, and due to the inhibition of respiration the redox state of redox sites proximal to cytochrome c shifts to a maximally reduced level. Cytochrome c release can be accompanied by an increased ROS production (Starkov et al., 2002), being responsible for the Ca2+-activated, MPT-associated oxidative stress (Kowaltowski et al., 2000; Frantseva et al., 2001).
In the present study, stimulation of ROS generation by Ca2+ was observed at physiologically relevant, small Ca2+ concentrations in mitochondria oxidizing α-GP. These Ca2+ concentrations are below (100 nM) or around (500 nM) the set point of mitochondria (Nicholls, 1978), therefore no depolarization due to electrophoretic Ca2+ uptake into the mitochondria is expected. Consistently, the succinate-supported ROS production, which is highly dependent on ΔΨm in well-coupled mitochondria (Hansford et al., 1997; Korshunov et al., 1997; Votyakova and Reynolds, 2001), was not influenced by Ca2+ applied in this concentration range (Figs. 3B,4B).
Given the presence of α-GPDH and its stimulation by Ca2+ in brain mitochondria, the Ca2+-stimulated α-GPDH-mediated ROS generation presented in this study could be physiologically important. This study is probably the first to show that sub micromolar Ca2+ stimulus is able to promote ROS generation in brain mitochondria.
Acknowledgements
Authors are grateful to K. Takacs and A. Varnagy for the excellent technical assistance. This work was supported by OTKA TS 049851 to V.A-V. and T 46567 to L.T. and by ETT and RET-NKTH and the Hungarian Academy of Sciences to V.A-V.




